Abstract
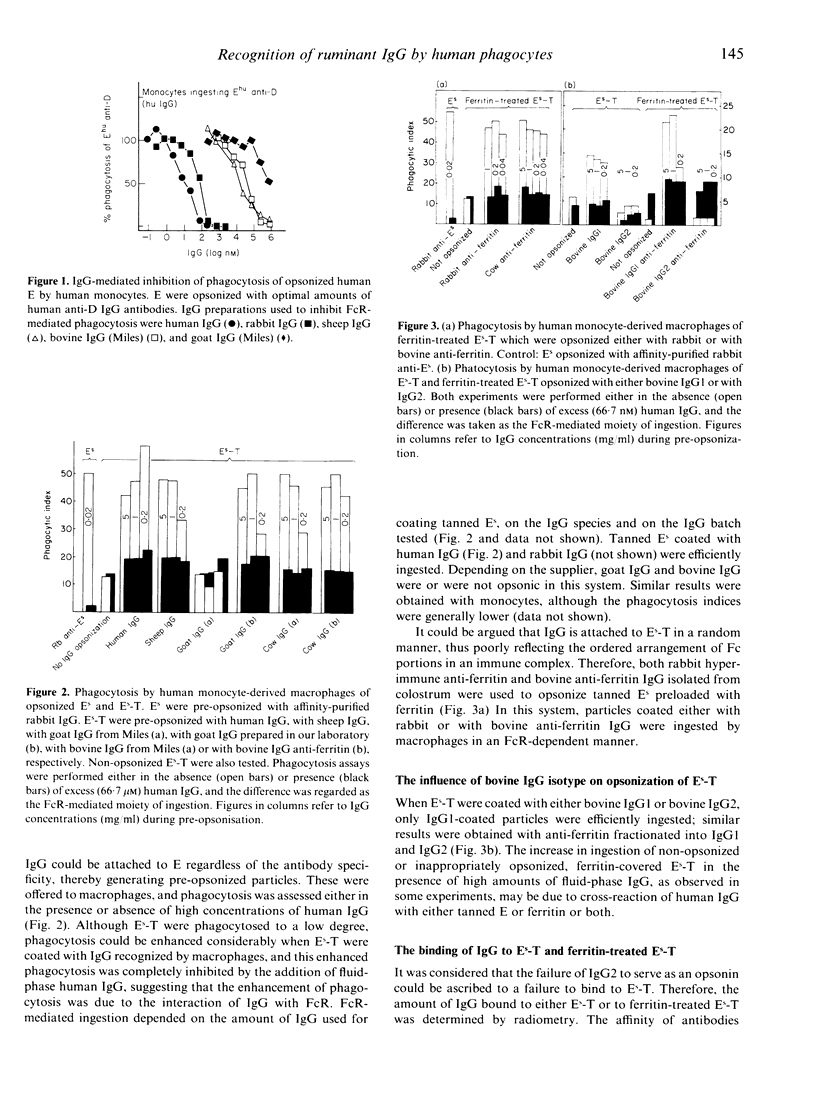
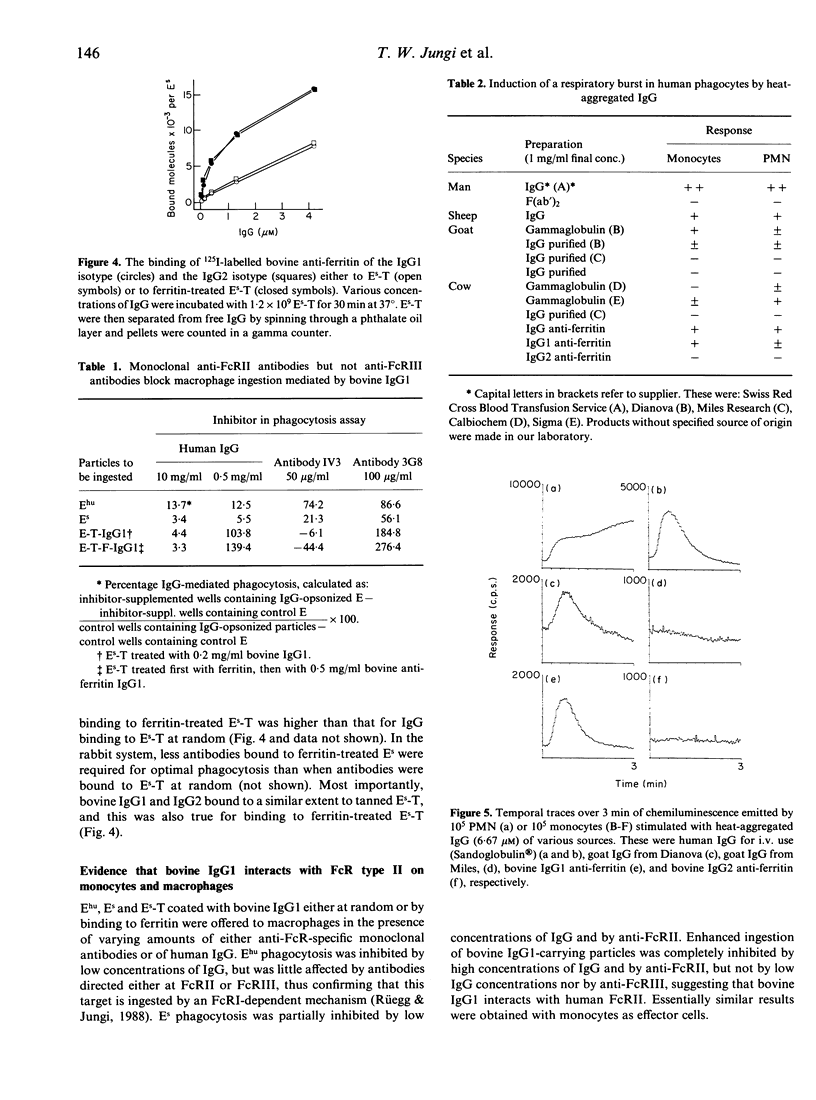
The interaction of ruminant IgG with human phagocytes was assessed using Fc receptor (FcR)-mediated ingestion and the triggering of a respiratory burst as effector functions indicative of receptor-specific interaction. In monomeric form, ruminant IgG was three to five orders of magnitude less potent than homologous IgG in inhibiting FcR-specific phagocytosis by monocytes. However, when attached to tanned sheep erythrocytes (Es-T), ruminant IgG was opsonic, as it promoted enhanced phagocytosis of Es-T, comparable to ingestion of rabbit IgG-coated Es. This phagocytosis was inhibitable by high concentrations of human IgG in the fluid phase. Moreover, Es-T precoated with ferritin could be opsonized to a similar degree by anti-ferritin IgG from rabbit and cow. However, only bovine IgG1, but not IgG2, was opsonic. Bovine and goat IgG of some, but not other, suppliers were inactive. Similar results were obtained by measuring the respiratory burst triggered by heat-aggregated IgG, using a luminol-enhanced chemiluminescence assay. Thus, human IgG and ruminant IgG stimulated monocytes and, to a lesser extent, polymorphonuclear leucocytes (PMN), to generate CL. Depending on the manufacturer, some preparations of bovine and goat IgG were inactive, and bovine IgG2 failed to induce CL. These findings prove that certain ruminant IgG preparations, including bovine IgG1 interacting weakly with homologous PMN and monocytes, do interact with human PMN, monocytes and macrophages in a FcR-specific manner when offered in complexed form. Inhibition studies suggest that bovine IgG1 interacts mainly with human FcR type II. In contrast, bovine IgG2, regarded as cytophilic for homologous PMN, fails to interact with human PMN, monocytes and macrophages.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander E. L., Sanders S. K. F(ab')2 reagents are not required if goat, rather than rabbit, antibodies are used to detect human surface immunoglobulin. J Immunol. 1977 Sep;119(3):1084–1088. [PubMed] [Google Scholar]
- Biegel D., Rabinovitch M. Measurement of phagocytosis utilizing 51Cr-labeled tannic acid treated erythrocytes. J Immunol Methods. 1983 Mar 11;58(1-2):19–23. doi: 10.1016/0022-1759(83)90259-4. [DOI] [PubMed] [Google Scholar]
- Burton D. R. Immunoglobulin G: functional sites. Mol Immunol. 1985 Mar;22(3):161–206. doi: 10.1016/0161-5890(85)90151-8. [DOI] [PubMed] [Google Scholar]
- Butler J. E. Bovine immunoglobulins: an augmented review. Vet Immunol Immunopathol. 1983 Mar;4(1-2):43–152. doi: 10.1016/0165-2427(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Connell P. A., Seehra M. S., Leslie R. G., Reeves W. G. Chemiluminescence of guinea pig peritoneal macrophages stimulated by immune precipitates and soluble immune complexes. Eur J Immunol. 1980 Dec;10(12):966–968. doi: 10.1002/eji.1830101214. [DOI] [PubMed] [Google Scholar]
- Fey H., Bütler R., Marti F. The production in the pregnant cow of anti-human immunoglobulin to be used for the antiglobulin test. Vox Sang. 1973 Sep;25(3):245–253. doi: 10.1111/j.1423-0410.1973.tb04369.x. [DOI] [PubMed] [Google Scholar]
- Fey H., Pfister H., Messerli J., Sturzenegger N., Grolimund F. Methods of isolation, purification and quantitation of bovine immunoglobulins: a technical review. Zentralbl Veterinarmed B. 1976 May;23(4):269–300. doi: 10.1111/j.1439-0450.1976.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Fleit H. B., Wright S. D., Unkeless J. C. Human neutrophil Fc gamma receptor distribution and structure. Proc Natl Acad Sci U S A. 1982 May;79(10):3275–3279. doi: 10.1073/pnas.79.10.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard C. J., Taylor G., Brownlie J. Surface receptors for immunoglobulin on bovine polymorphonuclear neutrophils and macrophages. Res Vet Sci. 1980 Jul;29(1):128–130. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Lehmeyer J. E., Guthrie L. A. Generation of superoxide anion and chemiluminescence by human monocytes during phagocytosis and on contact with surface-bound immunoglobulin G. J Exp Med. 1976 Jun 1;143(6):1551–1556. doi: 10.1084/jem.143.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungi T. W., Barandun S. Estimation of the degree of opsonization of homologous erythrocytes by IgG for intravenous and intramuscular use. Vox Sang. 1985;49(1):9–19. doi: 10.1111/j.1423-0410.1985.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Jungi T. W., Hafner S. Quantitative assessment of Fc receptor expression and function during in vitro differentiation of human monocytes to macrophages. Immunology. 1986 May;58(1):131–137. [PMC free article] [PubMed] [Google Scholar]
- Keller H. U., Gerber H., Hess M. W., Cottier H. Studies on the regulation of the neutrophil chemotactic response using a rapid and reliable method for measuring random migration and chemotaxis of neutrophil granulocytes. Agents Actions. 1976 Feb;6(1-3):326–339. doi: 10.1007/BF01972250. [DOI] [PubMed] [Google Scholar]
- Lubeck M. D., Steplewski Z., Baglia F., Klein M. H., Dorrington K. J., Koprowski H. The interaction of murine IgG subclass proteins with human monocyte Fc receptors. J Immunol. 1985 Aug;135(2):1299–1304. [PubMed] [Google Scholar]
- McGuire T. C., Musoke A. J., Kurtti T. Functional properties of bovine IgG1 and IgG2: interaction with complement, macrophages, neutrophils and skin. Immunology. 1979 Oct;38(2):249–256. [PMC free article] [PubMed] [Google Scholar]
- Mossmann H., Schmitz B., Possart P., Hammer D. K. Antibody-dependent, cell-mediated cytotoxicity in cattle: transfer of IgG subclasses in relation to the protection of the newborn calf. Adv Exp Med Biol. 1981;137:279–292. [PubMed] [Google Scholar]
- Newby T. J., Bourne J. The nature of the local immune system of the bovine mammary gland. J Immunol. 1977 Feb;118(2):461–465. [PubMed] [Google Scholar]
- Rossi C. R., Kiesel G. K. Bovine immunoglobulin G subclass receptor sites on bovine macrophages. Am J Vet Res. 1977 Jul;38(7):1023–1025. [PubMed] [Google Scholar]
- Rouse B. T., Wardley R. C., Babiuk L. A. Antibody-dependent cell-mediated cytotoxicity in cows: comparison of effector cell activity against heterologous erthrocyte and herpesvirus-infected bovine target cells. Infect Immun. 1976 May;13(5):1433–1441. doi: 10.1128/iai.13.5.1433-1441.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüegg S. J., Jungi T. W. Antibody-mediated erythrolysis and erythrophagocytosis by human monocytes, macrophages and activated macrophages. Evidence for distinction between involvement of high-affinity and low-affinity receptors for IgG by using different erythroid target cells. Immunology. 1988 Mar;63(3):513–520. [PMC free article] [PubMed] [Google Scholar]
- Sasaki M., Larson B. L., Nelson D. R. Kinetic analysis of the binding of immunoglobulins IgG1 and IgG2 to bovine mammary cells. Biochim Biophys Acta. 1977 Mar 29;497(1):160–170. doi: 10.1016/0304-4165(77)90149-0. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Fleit H., Mellman I. S. Structural Aspects and Heterogeneity of Immunoglobulin Fc Receptors. Adv Immunol. 1981;31:247–270. doi: 10.1016/s0065-2776(08)60922-0. [DOI] [PubMed] [Google Scholar]
- Watson D. L. The effect of cytophilic IgG2 on phagocytosis by ovine polymorphonuclear leucocytes. Immunology. 1976 Aug;31(2):159–165. [PMC free article] [PubMed] [Google Scholar]
- Weber L., Peterhans E. Stimulation of chemiluminescence in bovine polymorphonuclear leucocytes by virus-antibody complexes and by antibody-coated infected cells. Immunobiology. 1983 May;164(5):333–342. doi: 10.1016/S0171-2985(83)80029-1. [DOI] [PubMed] [Google Scholar]
- Woof J. M., Partridge L. J., Jefferis R., Burton D. R. Localisation of the monocyte-binding region on human immunoglobulin G. Mol Immunol. 1986 Mar;23(3):319–330. doi: 10.1016/0161-5890(86)90059-3. [DOI] [PubMed] [Google Scholar]
- Zuckerman S. H., Douglas S. D. The characterization and functional significance of plasma membrane Fc Receptors. CRC Crit Rev Microbiol. 1978;7(1):1–26. doi: 10.3109/10408417909101176. [DOI] [PubMed] [Google Scholar]
- van de Winkel J. G., van Duijnhoven H. L., van Ommen R., Capel P. J., Tax W. J. Selective modulation of two human monocyte Fc receptors for IgG by immobilized immune complexes. J Immunol. 1988 May 15;140(10):3515–3521. [PubMed] [Google Scholar]


