Abstract
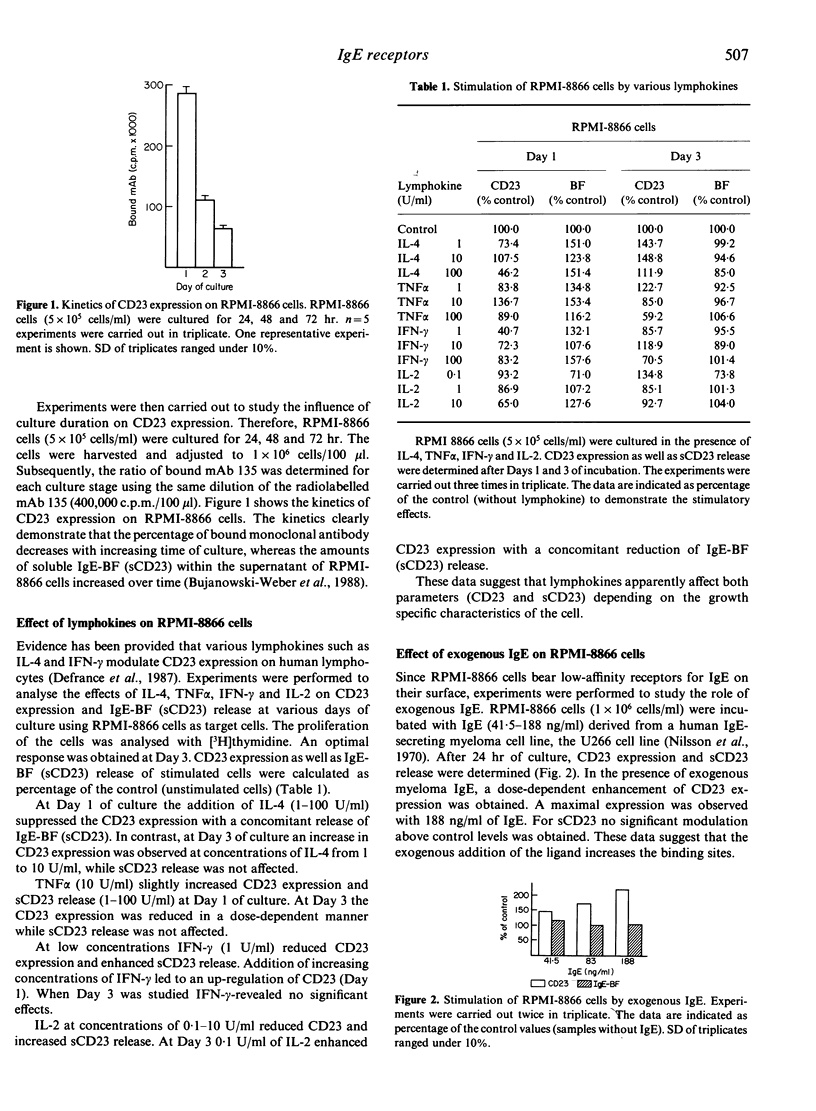
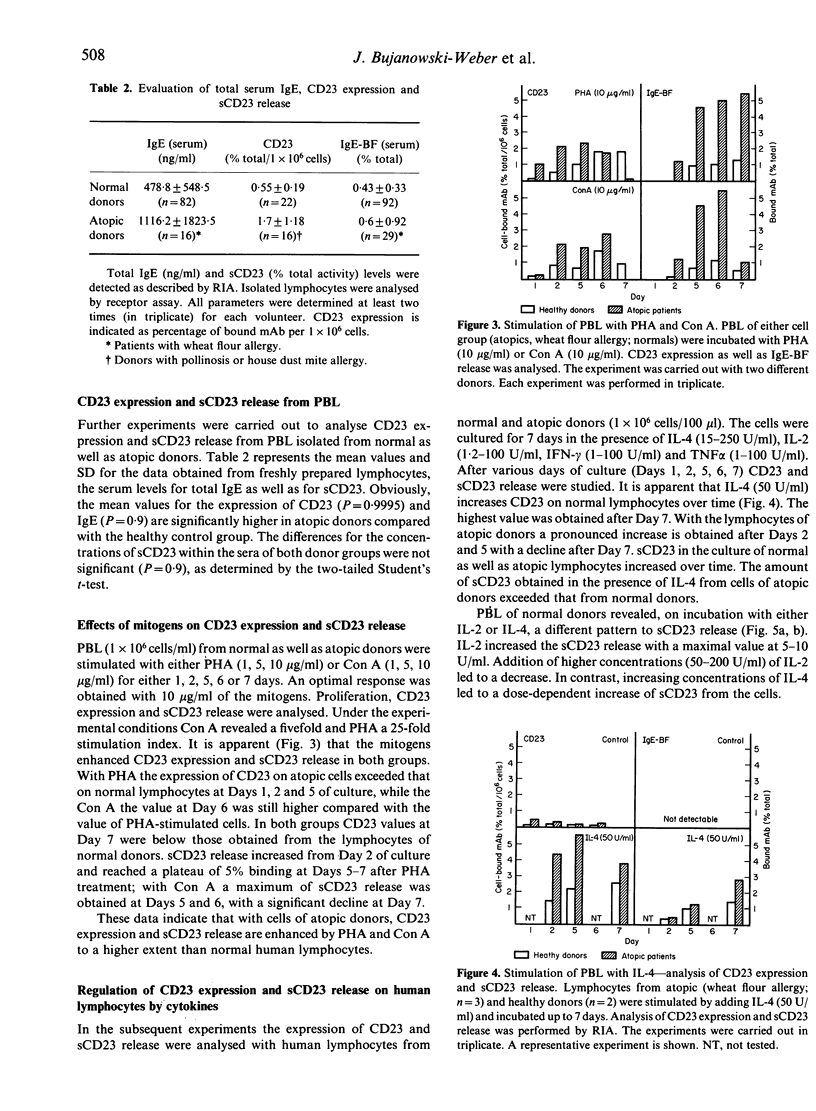
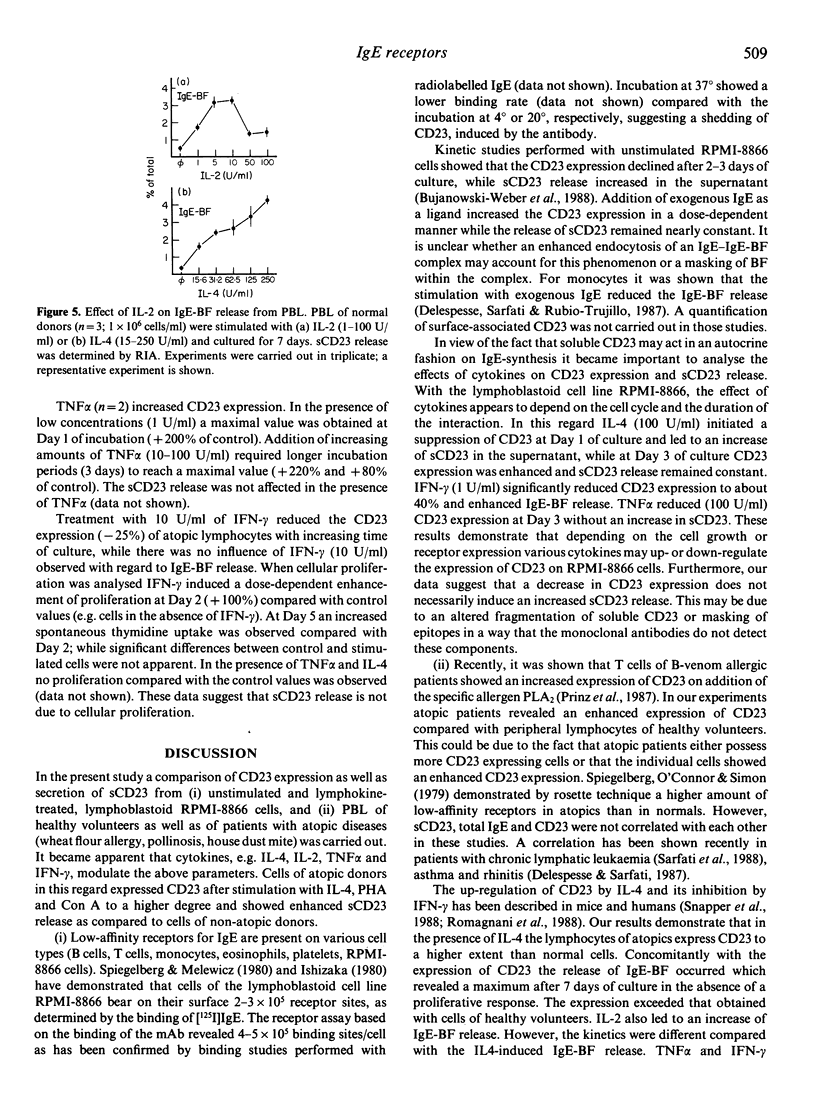
The low-affinity receptor for IgE (CD23) as well as the soluble IgE-binding factors (IgE-BF, sCD23) are important factors in IgE antibody regulation. The CD23 expression and the concomitant release of CD23 were analysed from the lymphoblastoid B-cell line RPMI-8866 and from peripheral blood lymphocytes (PBL) of healthy volunteers as well as atopic patients. CD23 expression and sCD23 release of RPMI-8866 cells were dependent on the stage of culture. While CD23 expression decreased with increasing time of culture (Day 1-3), the sCD23 release was enhanced during the culture period. Cytokines such as IL-4, IL-2, TNF alpha and IFN-gamma exerted various effects on the target cells depending on the culture period. CD23 expression on normal lymphocytes was lower compared with the expression on atopic cells. Lymphokines (IL-2, IL-4) as well as mitogens (PHA, Con A) enhanced CD23 expression and IgE-BF (sCD23) release. The degree of enhancement was always higher with atopic cells compared with the results obtained with cells of normal donors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonnefoy J. Y., Aubry J. P., Peronne C., Wijdenes J., Banchereau J. Production and characterization of a monoclonal antibody specific for the human lymphocyte low affinity receptor for IgE: CD 23 is a low affinity receptor for IgE. J Immunol. 1987 May 1;138(9):2970–2978. [PubMed] [Google Scholar]
- Bonnefoy J. Y., Defrance T., Peronne C., Menetrier C., Rousset F., Pène J., De Vries J. E., Banchereau J. Human recombinant interleukin 4 induces normal B cells to produce soluble CD23/IgE-binding factor analogous to that spontaneously released by lymphoblastoid B cell lines. Eur J Immunol. 1988 Jan;18(1):117–122. doi: 10.1002/eji.1830180118. [DOI] [PubMed] [Google Scholar]
- Capron M., Capron A., Dessaint J. P., Torpier G., Johansson S. G., Prin L. Fc receptors for IgE on human and rat eosinophils. J Immunol. 1981 Jun;126(6):2087–2092. [PubMed] [Google Scholar]
- Defrance T., Aubry J. P., Rousset F., Vanbervliet B., Bonnefoy J. Y., Arai N., Takebe Y., Yokota T., Lee F., Arai K. Human recombinant interleukin 4 induces Fc epsilon receptors (CD23) on normal human B lymphocytes. J Exp Med. 1987 Jun 1;165(6):1459–1467. doi: 10.1084/jem.165.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi H., Suemura M., Ishizaka A., Ozaki Y., Kishimoto S., Yamamura Y., Kishimoto T. IgE class-specific suppressor T cells and factors in humans. J Immunol. 1983 Dec;131(6):2751–2756. [PubMed] [Google Scholar]
- Gonzalez-Molina A., Spiegelberg H. L. A subpopulation of normal human peripheral B lymphcytes that bind IgE. J Clin Invest. 1977 Apr;59(4):616–624. doi: 10.1172/JCI108679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J., Webb A. J., Walker L., Guy G. R., Rowe M. Evidence for an association between CD23 and the receptor for a low molecular weight B cell growth factor. Eur J Immunol. 1986 Dec;16(12):1627–1630. doi: 10.1002/eji.1830161225. [DOI] [PubMed] [Google Scholar]
- Huff T. F., Ishizaka K. Formation of IgE-binding factors by human T-cell hybridomas. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1514–1518. doi: 10.1073/pnas.81.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K. Immunoglobulin E (IgE). Methods Enzymol. 1985;116:76–94. doi: 10.1016/s0076-6879(85)16006-4. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Sandberg K. Formation of IgE binding factors by human T lymphocytes. J Immunol. 1981 May;126(5):1692–1696. [PubMed] [Google Scholar]
- Joseph M., Capron A., Ameisen J. C., Capron M., Vorng H., Pancré V., Kusnierz J. P., Auriault C. The receptor for IgE on blood platelets. Eur J Immunol. 1986 Mar;16(3):306–312. doi: 10.1002/eji.1830160318. [DOI] [PubMed] [Google Scholar]
- Kikutani H., Suemura M., Owaki H., Nakamura H., Sato R., Yamasaki K., Barsumian E. L., Hardy R. R., Kishimoto T. Fc epsilon receptor, a specific differentiation marker transiently expressed on mature B cells before isotype switching. J Exp Med. 1986 Nov 1;164(5):1455–1469. doi: 10.1084/jem.164.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinman N. R., Taylor R. B. General methods for the study of cells and serum during the immune response: the response to dinitrophenyl in mice. Clin Exp Immunol. 1969 Apr;4(4):473–487. [PMC free article] [PubMed] [Google Scholar]
- Leung D. Y., Frankel R., Wood N., Geha R. S. Potentiation of human immunoglobulin E synthesis by plasma immunoglobulin E binding factors from patients with the hyperimmunoglobulin E syndrome. J Clin Invest. 1986 Mar;77(3):952–957. doi: 10.1172/JCI112395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüdin C., Hofstetter H., Sarfati M., Levy C. A., Suter U., Alaimo D., Kilchherr E., Frost H., Delespesse G. Cloning and expression of the cDNA coding for a human lymphocyte IgE receptor. EMBO J. 1987 Jan;6(1):109–114. doi: 10.1002/j.1460-2075.1987.tb04726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson K., Bennich H., Johansson S. G., Pontén J. Established immunoglobulin producing myeloma (IgE) and lymphoblastoid (IgG) cell lines from an IgE myeloma patient. Clin Exp Immunol. 1970 Oct;7(4):477–489. [PMC free article] [PubMed] [Google Scholar]
- Prinz J. C., Endres N., Rank G., Ring J., Rieber E. P. Expression of Fc epsilon receptors on activated human T lymphocytes. Eur J Immunol. 1987 Jun;17(6):757–761. doi: 10.1002/eji.1830170604. [DOI] [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Wideman J., Bonnefoy J. Y., De Vries J. E. Interleukin 5 enhances interleukin 4-induced IgE production by normal human B cells. The role of soluble CD23 antigen. Eur J Immunol. 1988 Jun;18(6):929–935. doi: 10.1002/eji.1830180615. [DOI] [PubMed] [Google Scholar]
- Rosenthal H. E. A graphic method for the determination and presentation of binding parameters in a complex system. Anal Biochem. 1967 Sep;20(3):525–532. doi: 10.1016/0003-2697(67)90297-7. [DOI] [PubMed] [Google Scholar]
- Sarfati M., Nutman T., Fonteyn C., Delespesse G. Presence of antigenic determinants common to Fc IgE receptors on human macrophages, T and B lymphocytes and IgE-binding factors. Immunology. 1986 Dec;59(4):569–575. [PMC free article] [PubMed] [Google Scholar]
- Sarfati M., Rector E., Rubio-Trujillo M., Wong K., Sehon A. H., Delespesse G. In vitro synthesis of IgE by human lymphocytes. III. IgE-potentiating activity of culture supernatants from Epstein-Barr virus (EBV) transformed B cells. Immunology. 1984 Oct;53(2):207–214. [PMC free article] [PubMed] [Google Scholar]
- Sarfati M., Rector E., Wong K., Rubio-Trujillo M., Sehon A. H., Delespesse G. In vitro synthesis of IgE by human lymphocytes. II. Enhancement of the spontaneous IgE synthesis by IgE-binding factors secreted by RPMI 8866 lymphoblastoid B cells. Immunology. 1984 Oct;53(2):197–205. [PMC free article] [PubMed] [Google Scholar]
- Sarfati M., Rubio-Trujillo M., Wong K., Rector E., Sehon A. H., Delespesse G. In vitro synthesis of IgE by human lymphocytes. I. The spontaneous secretion of IgE by B lymphocytes from allergic individuals: a model to investigate the regulation of human IgE synthesis. Immunology. 1984 Oct;53(2):187–196. [PMC free article] [PubMed] [Google Scholar]
- Snapper C. M., Finkelman F. D., Paul W. E. Differential regulation of IgG1 and IgE synthesis by interleukin 4. J Exp Med. 1988 Jan 1;167(1):183–196. doi: 10.1084/jem.167.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelberg H. L., Melewicz F. M. Fc receptors specific for IgE on subpopulations of human lymphocytes and monocytes. Clin Immunol Immunopathol. 1980 Mar;15(3):424–433. doi: 10.1016/0090-1229(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Spiegelberg H. L., O'Connor R. D., Simon R. A., Mathison D. A. Lymphocytes with immunoglobulin E Fc receptors in patients with atopic disorders. J Clin Invest. 1979 Sep;64(3):714–720. doi: 10.1172/JCI109514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. F., Mellon M. H., Zeiger R. S., Spiegelberg H. L. Characterization with monoclonal antibodies of T lymphocytes bearing Fc receptors for IgE (T epsilon cells) and IgG (T gamma cells) in atopic patients. J Immunol. 1983 Dec;131(6):2772–2776. [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Mann K. P. Early events in Epstein-Barr virus infection provide a model for B cell activation. J Exp Med. 1985 Jul 1;162(1):45–59. doi: 10.1084/jem.162.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. C., Leung D. Y., Geha R. S. Production of IgE-potentiating factor in man by T cell lines bearing Fc receptors for IgE. Eur J Immunol. 1984 Oct;14(10):871–878. doi: 10.1002/eji.1830141003. [DOI] [PubMed] [Google Scholar]
- Yukawa K., Kikutani H., Owaki H., Yamasaki K., Yokota A., Nakamura H., Barsumian E. L., Hardy R. R., Suemura M., Kishimoto T. A B cell-specific differentiation antigen, CD23, is a receptor for IgE (Fc epsilon R) on lymphocytes. J Immunol. 1987 Apr 15;138(8):2576–2580. [PubMed] [Google Scholar]


