Abstract
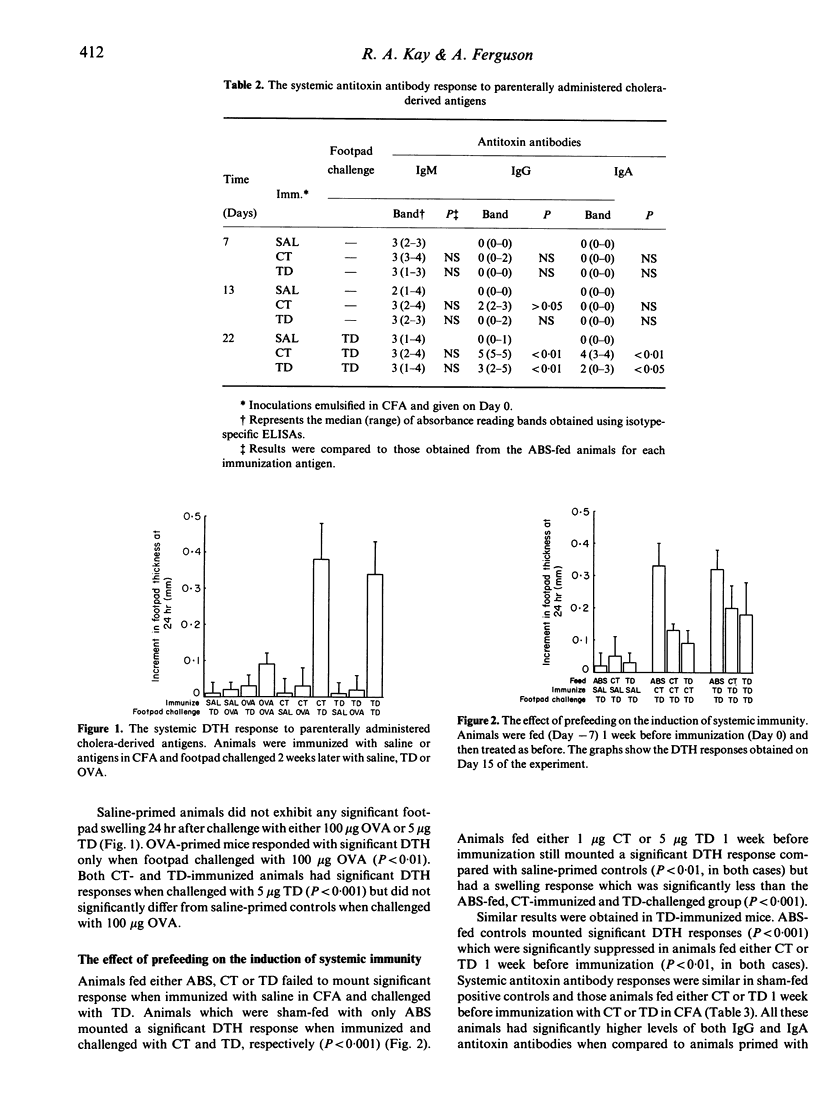
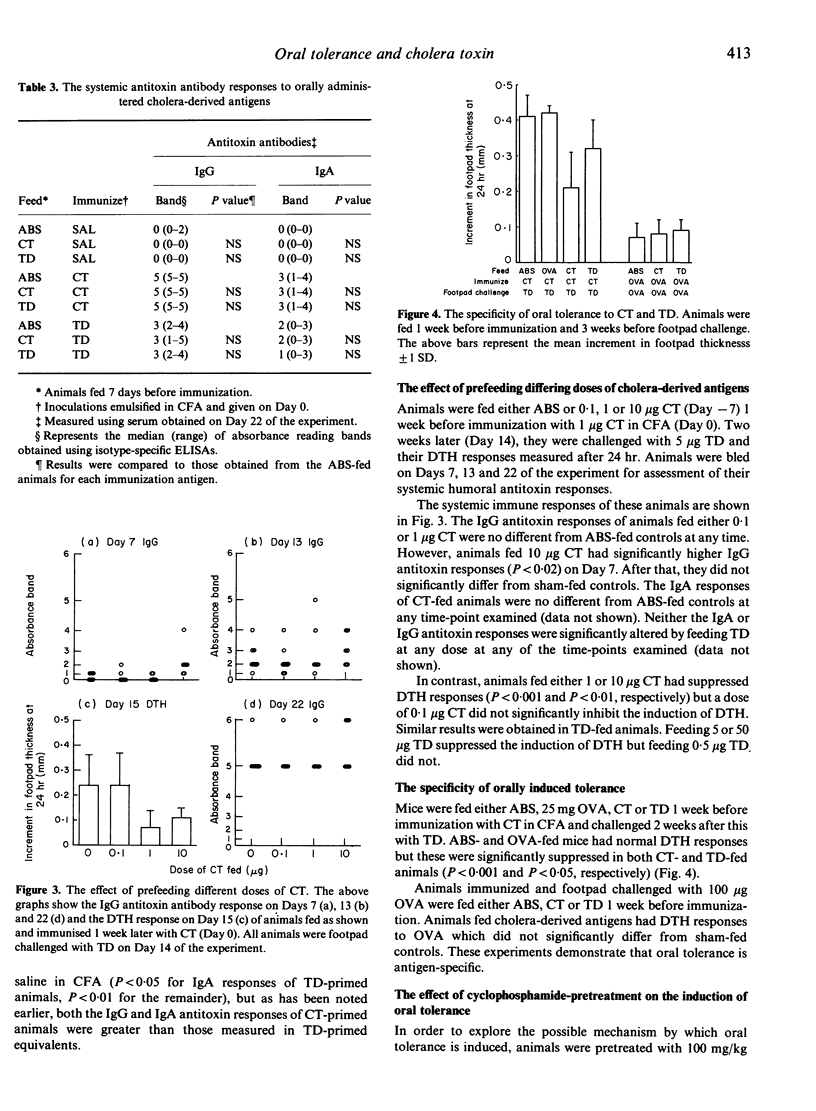
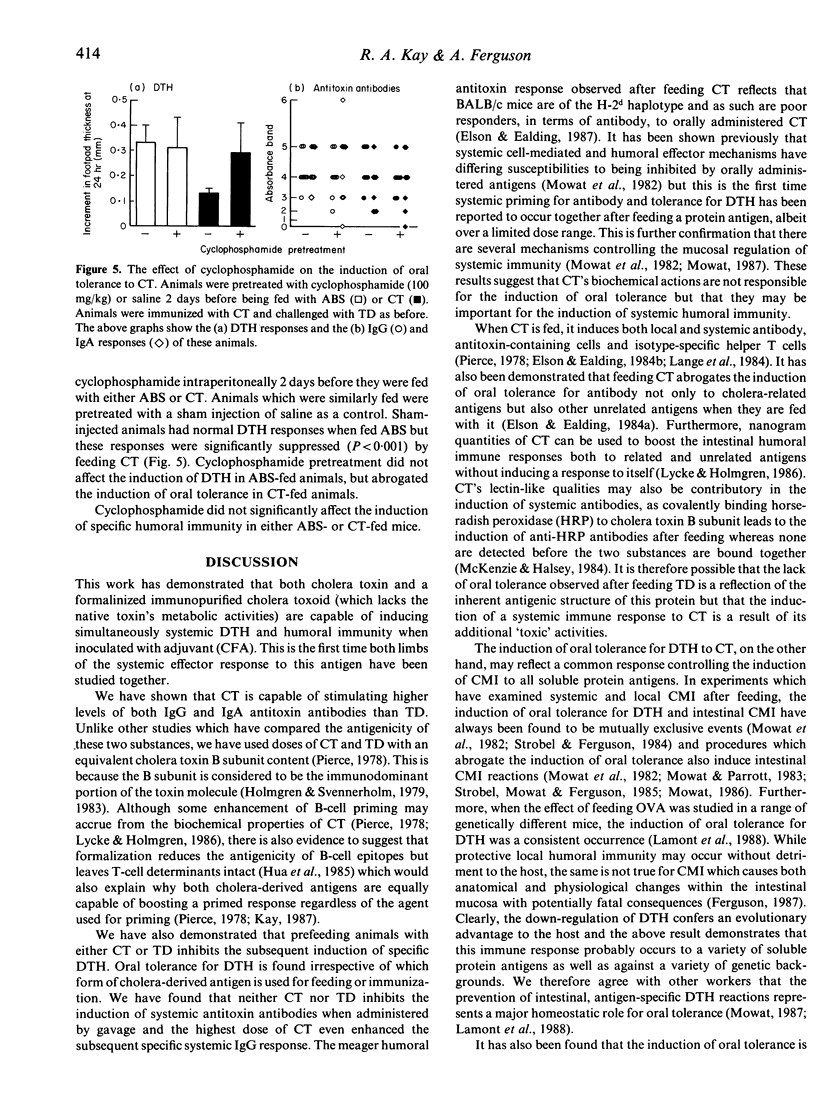
Immunization of adult BALB/c mice with 1 microgram cholera toxin (CT) in complete Freund's adjuvant (CFA) induced both humoral (IgG and IgA) and cell-mediated (DTH) immunity. Although an immunopurified, formalinized, cholera toxoid (TD) in CFA was inferior to the native holotoxin at inducing antitoxin antibodies, both cholera-derived antigens were equally immunogenic for specific DTH. When mice were fed either 1 microgram CT or 5 microgram TD 1 week before immunization, the induction of DTH was inhibited but the development of specific antibody was the same as in sham-fed controls. A feed of 10 micrograms CT not only suppressed the induction of DTH but also enhanced the IgG antitoxin responses measured 1 week after immunization. A dose of TD (50 micrograms), with a similar cholera toxin B subunit content, also induced oral tolerance for DTH but had no effect on the subsequent development of humoral immunity. The smallest doses of CT or TD fed (0.1 microgram and 0.5 microgram, respectively) failed to affect the development of either limb of the systemic immune response. These results suggest that oral tolerance for DTH is not consequent upon the metabolic actions of CT but that stimulation of systemic antibodies after enteric administration may be. Pretreating mice with cyclophosphamide (Cy) (100 mg/kg) before feeding CT abrogated the induction of oral tolerance for DTH but had no effect on humoral immunity, suggesting that suppressor T cells may be responsible for the induction of oral tolerance in these animals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attallah A. M., Ahmed A., Sell K. W. In vivo induction of carrier-specific cyclophosphamide-sensitive suppressor cells for cell-mediated immunity in mice. Int Arch Allergy Appl Immunol. 1979;60(2):178–185. doi: 10.1159/000232340. [DOI] [PubMed] [Google Scholar]
- Diamantstein T., Klos M., Hahn H., Kaufmann S. H. Direct in vitro evidence for different susceptibilities to 4-hydroperoxycyclophosphamide of antigen-primed T cells regulating humoral and cell-mediated immune responses to sheep erythrocytes: a possible explanation for the inverse action of cyclophosphamide on humoral and cell-mediated immune responses. J Immunol. 1981 May;126(5):1717–1719. [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984 Dec;133(6):2892–2897. [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Ir gene control of the murine secretory IgA response to cholera toxin. Eur J Immunol. 1987 Mar;17(3):425–428. doi: 10.1002/eji.1830170320. [DOI] [PubMed] [Google Scholar]
- Fuhrman J. A., Cebra J. J. Special features of the priming process for a secretory IgA response. B cell priming with cholera toxin. J Exp Med. 1981 Mar 1;153(3):534–544. doi: 10.1084/jem.153.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M. Cholera and the immune response. Prog Allergy. 1983;33:106–119. [PubMed] [Google Scholar]
- Hua C., Langlet C., Buferne M., Schmitt-Verhulst A. M. Selective destruction by formaldehyde fixation of an H-2Kb serological determinant involving lysine 89 without loss of T-cell reactivity. Immunogenetics. 1985;21(3):227–234. doi: 10.1007/BF00375375. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H., Diamantstein T. Relative susceptibilities of T cell subsets involved in delayed-type hypersensitivity to sheep red blood cells to the in vitro action of 4-hydroperoxycyclophosphamide. J Immunol. 1980 Sep;125(3):1104–1108. [PubMed] [Google Scholar]
- Lamont A. G., Mowat A. M., Browning M. J., Parrott D. M. Genetic control of oral tolerance to ovalbumin in mice. Immunology. 1988 Apr;63(4):737–739. [PMC free article] [PubMed] [Google Scholar]
- Lange S., Lönnroth I., Nygren H. Protection against experimental cholera in the rat. A study on the formation of antibodies against cholera toxin and desensitization of adenylate cyclase after immunization with cholera toxin. Int Arch Allergy Appl Immunol. 1984;75(2):143–148. [PubMed] [Google Scholar]
- Lindholm L., Holmgren J., Lange S., Lönnroth I. Interaction of cholera toxin and toxin derivatives with lymphocytes. II. Modulating effects of cholera toxin on in vivo humoral and cellular immune responses. Int Arch Allergy Appl Immunol. 1976;50(5):555–573. doi: 10.1159/000231560. [DOI] [PubMed] [Google Scholar]
- Lycke N., Holmgren J. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology. 1986 Oct;59(2):301–308. [PMC free article] [PubMed] [Google Scholar]
- McKenzie S. J., Halsey J. F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984 Oct;133(4):1818–1824. [PubMed] [Google Scholar]
- Miller S. D., Hanson D. G. Inhibition of specific immune responses by feeding protein antigens. IV. Evidence for tolerance and specific active suppression of cell-mediated immune responses to ovalbumin. J Immunol. 1979 Nov;123(5):2344–2350. [PubMed] [Google Scholar]
- Mowat A. M. Depletion of suppressor T cells by 2'-deoxyguanosine abrogates tolerance in mice fed ovalbumin and permits the induction of intestinal delayed-type hypersensitivity. Immunology. 1986 Jun;58(2):179–184. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Parrot D. M. Immunological responses to fed protein antigens in mice. IV. Effects of stimulating the reticuloendothelial system on oral tolerance and intestinal immunity to ovalbumin. Immunology. 1983 Dec;50(4):547–554. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Strobel S., Drummond H. E., Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982 Jan;45(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel S., Ferguson A. Immune responses to fed protein antigens in mice. 3. Systemic tolerance or priming is related to age at which antigen is first encountered. Pediatr Res. 1984 Jul;18(7):588–594. doi: 10.1203/00006450-198407000-00004. [DOI] [PubMed] [Google Scholar]
- Strobel S., Mowat A. M., Drummond H. E., Pickering M. G., Ferguson A. Immunological responses to fed protein antigens in mice. II. Oral tolerance for CMI is due to activation of cyclophosphamide-sensitive cells by gut-processed antigen. Immunology. 1983 Jul;49(3):451–456. [PMC free article] [PubMed] [Google Scholar]


