Abstract
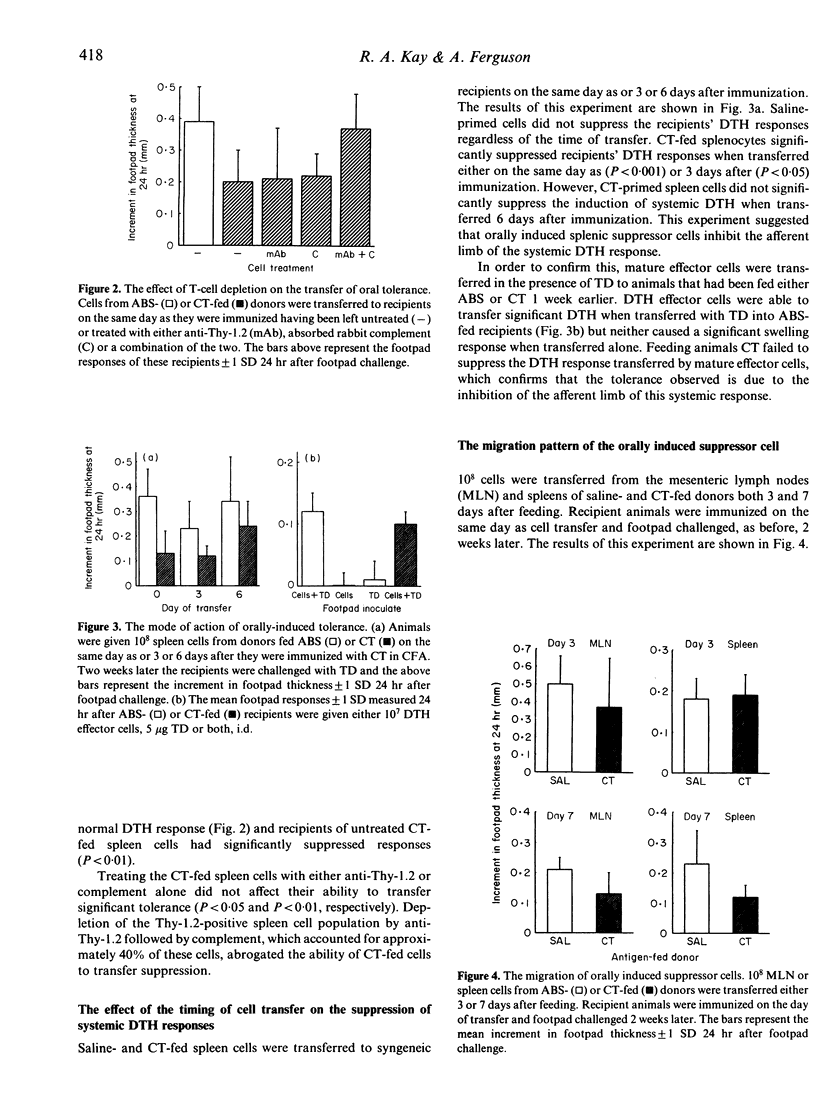
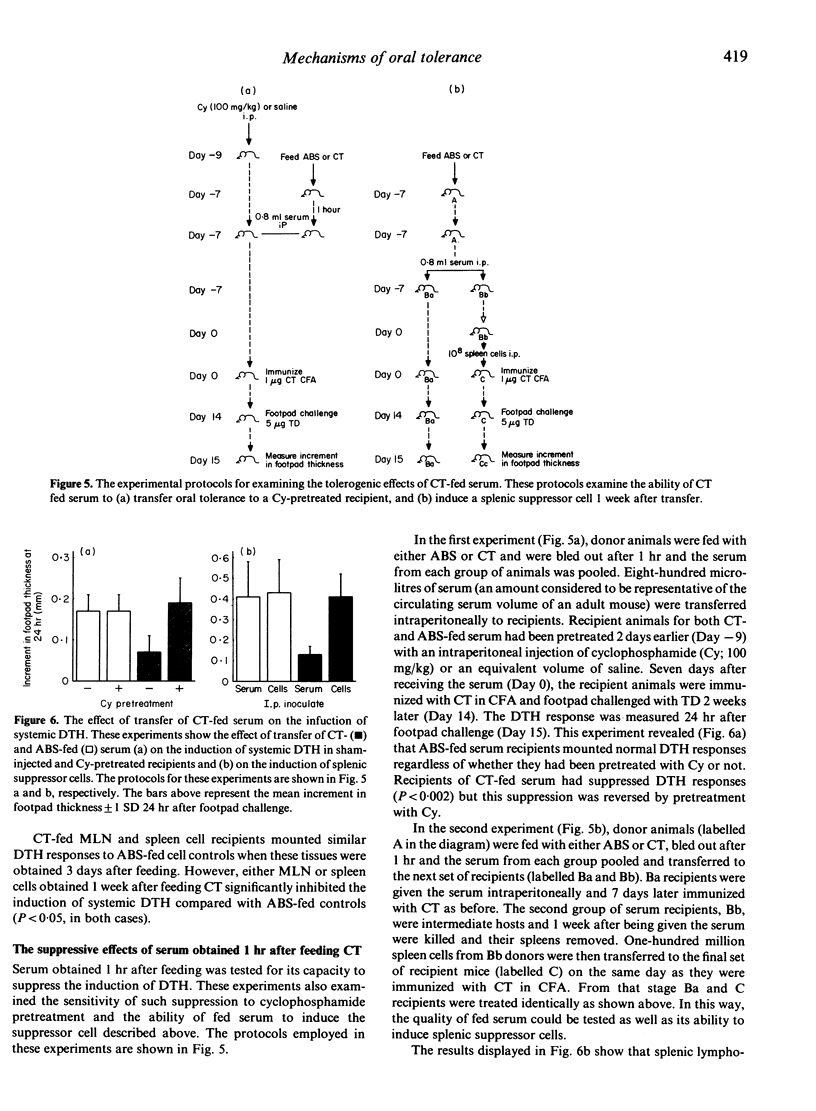
The mechanisms behind the induction of oral tolerance after feeding cholera toxin (CT) were examined using cell and serum transfer protocols. The feeding of CT or cholera toxoid (TD) induced a splenic cell capable of inhibiting the induction of systemic delayed-type hypersensitivity (DTH) but not humoral immunity. Depletion studies showed that this cell was Thy-1.2 positive. Transfer experiments suggested that suppressor cell activity was present in the mesenteric lymph nodes (MLN) and spleens of donor mice 1 week but not 3 days after feeding CT. When spleen cells were transferred to syngeneic recipients at various times after immunization, they were more effective at inhibiting systemic DTH when transferred within a short time of immunization. If the cells were transferred 6 days after immunization they no longer suppressed the development of DTH, which suggested that they inhibit the afferent limb of this immune response. This has been confirmed by the failure of a tolerogenic dose of CT, administered by gavage, to suppress the activity of mature effector TDTH cells. Serum collected 1 hr after feeding CT also suppressed the induction of systemic DTH. However, the tolerogenic activity of CT-fed serum was abrogated by the pretreatment of recipients with cyclophosphamide (Cy) (100 mg/kg), suggesting that this activity is mediated through the induction of suppressor cells. Transfer of fed serum, however, did not induce the splenic suppressor cell described above and we would suggest that several mechanisms may operate in the mucosal regulation of systemic DTH.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., Heremans J. F., Vaerman J. P., Cambiaso C. L. A mechanism for the induction of immunological tolerance by antigen feeding: antigen-antibody complexes. J Exp Med. 1975 Dec 1;142(6):1509–1519. doi: 10.1084/jem.142.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M. G., Ferguson A. Oral tolerance to ovalbumin in mice: studies of chemically modified and 'biologically filtered' antigen. Immunology. 1986 Apr;57(4):627–630. [PMC free article] [PubMed] [Google Scholar]
- Bruce M. G., Strobel S., Hanson D. G., Ferguson A. Irradiated mice lose the capacity to 'process' fed antigen for systemic tolerance of delayed-type hypersensitivity. Clin Exp Immunol. 1987 Dec;70(3):611–618. [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Endres R. O., Grey H. M. Antigen recognition by T cells and B cells: recognition of cross-reactivity between native and denatured forms of globular antigens. Clin Immunol Immunopathol. 1980 Mar;15(3):397–408. doi: 10.1016/0090-1229(80)90051-3. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Cholera toxin feeding did not induce oral tolerance in mice and abrogated oral tolerance to an unrelated protein antigen. J Immunol. 1984 Dec;133(6):2892–2897. [PubMed] [Google Scholar]
- Elson C. O., Ealding W. Generalized systemic and mucosal immunity in mice after mucosal stimulation with cholera toxin. J Immunol. 1984 Jun;132(6):2736–2741. [PubMed] [Google Scholar]
- Feldmann M., Kontiainen S. Suppressor cell induction in vitro. II. Cellular requirements of suppressor cell induction. Eur J Immunol. 1976 Apr;6(4):302–305. doi: 10.1002/eji.1830060413. [DOI] [PubMed] [Google Scholar]
- Habeeb A. F. A study of the antigenicity of formaldehyde- and glutaraldehyde-treated bovine serum albumin and ovalbumin-bovine serum albumin conjugate. J Immunol. 1969 Feb;102(2):457–465. [PubMed] [Google Scholar]
- Hua C., Langlet C., Buferne M., Schmitt-Verhulst A. M. Selective destruction by formaldehyde fixation of an H-2Kb serological determinant involving lysine 89 without loss of T-cell reactivity. Immunogenetics. 1985;21(3):227–234. doi: 10.1007/BF00375375. [DOI] [PubMed] [Google Scholar]
- Kagnoff M. F. Effects of antigen-feeding on intestinal and systemic immune responses. III. Antigen-specific serum-mediated suppression of humoral antibody responses after antigen feeding. Cell Immunol. 1978 Sep 15;40(1):186–203. doi: 10.1016/0008-8749(78)90326-x. [DOI] [PubMed] [Google Scholar]
- Kay R. A., Ferguson A. The immunological consequences of feeding cholera toxin. I. Feeding cholera toxin suppresses the induction of systemic delayed-type hypersensitivity but not humoral immunity. Immunology. 1989 Mar;66(3):410–415. [PMC free article] [PubMed] [Google Scholar]
- Mattingly J. A., Kaplan J. M., Janeway C. A., Jr Two distinct antigen-specific suppressor factors induced by the oral administration of antigen. J Exp Med. 1980 Sep 1;152(3):545–554. doi: 10.1084/jem.152.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly J. A., Waksman B. H. Immunologic suppression after oral administration of antigen. I. Specific suppressor cells formed in rat Peyer's patches after oral administration of sheep erythrocytes and their systemic migration. J Immunol. 1978 Nov;121(5):1878–1883. [PubMed] [Google Scholar]
- McKenzie S. J., Halsey J. F. Cholera toxin B subunit as a carrier protein to stimulate a mucosal immune response. J Immunol. 1984 Oct;133(4):1818–1824. [PubMed] [Google Scholar]
- Miller S. D., Hanson D. G. Inhibition of specific immune responses by feeding protein antigens. IV. Evidence for tolerance and specific active suppression of cell-mediated immune responses to ovalbumin. J Immunol. 1979 Nov;123(5):2344–2350. [PubMed] [Google Scholar]
- Mowat A. M. Depletion of suppressor T cells by 2'-deoxyguanosine abrogates tolerance in mice fed ovalbumin and permits the induction of intestinal delayed-type hypersensitivity. Immunology. 1986 Jun;58(2):179–184. [PMC free article] [PubMed] [Google Scholar]
- Mowat A. M., Strobel S., Drummond H. E., Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982 Jan;45(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Ngan J., Kind L. S. Suppressor T cells for IgE and IgG in Peyer's patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978 Mar;120(3):861–865. [PubMed] [Google Scholar]
- Pancake S. J., Nathenson S. G. Selective loss of H-2 antigenic reactivity after chemical modification. J Immunol. 1973 Oct;111(4):1086–1092. [PubMed] [Google Scholar]
- Pierce N. F. The role of antigen form and function in the primary and secondary intestinal immune responses to cholera toxin and toxoid in rats. J Exp Med. 1978 Jul 1;148(1):195–206. doi: 10.1084/jem.148.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S., Qian J. H., Kokudo S., Ikegami R., Suda T., Hamaoka T., Fujiwara H. Studies on the induction of tolerance to alloantigens. III. Induction of antibodies directed against alloantigen-specific delayed-type hypersensitivity T cells by a single injection of allogeneic lymphocytes via portal venous route. J Immunol. 1988 Feb 1;140(3):717–722. [PubMed] [Google Scholar]
- Strobel S., Mowat A. M., Drummond H. E., Pickering M. G., Ferguson A. Immunological responses to fed protein antigens in mice. II. Oral tolerance for CMI is due to activation of cyclophosphamide-sensitive cells by gut-processed antigen. Immunology. 1983 Jul;49(3):451–456. [PMC free article] [PubMed] [Google Scholar]


