Abstract
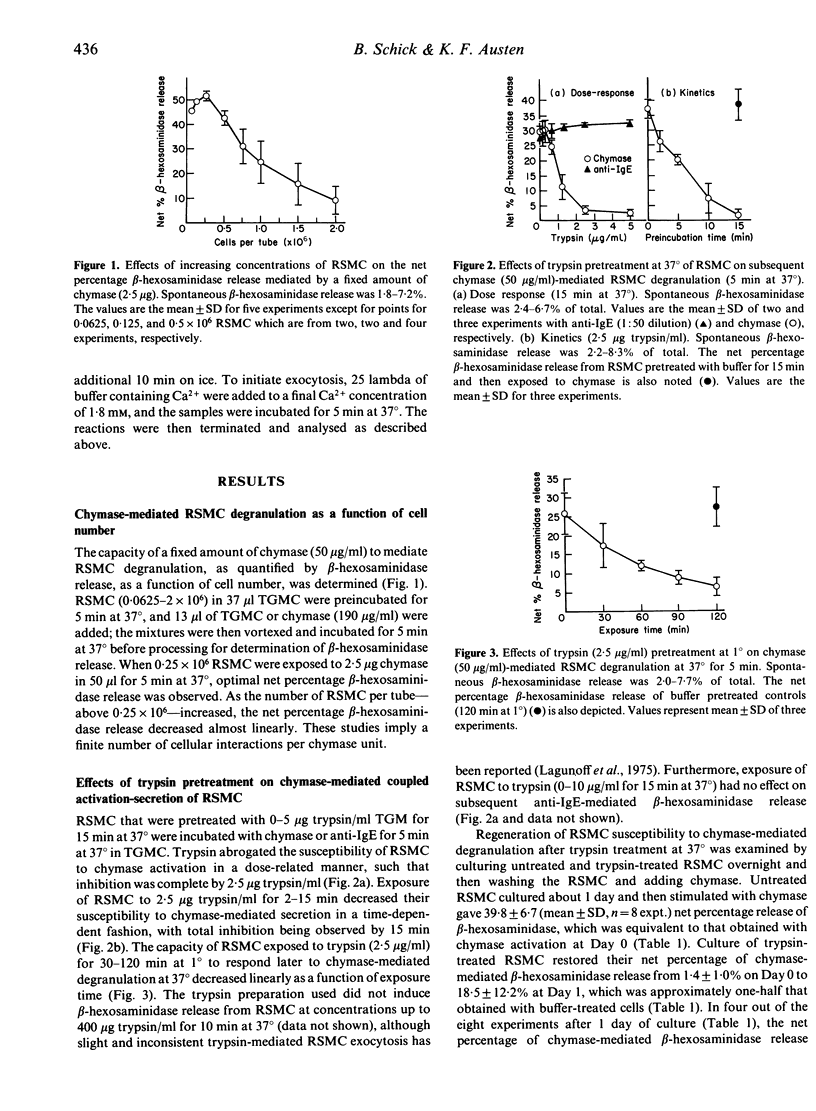
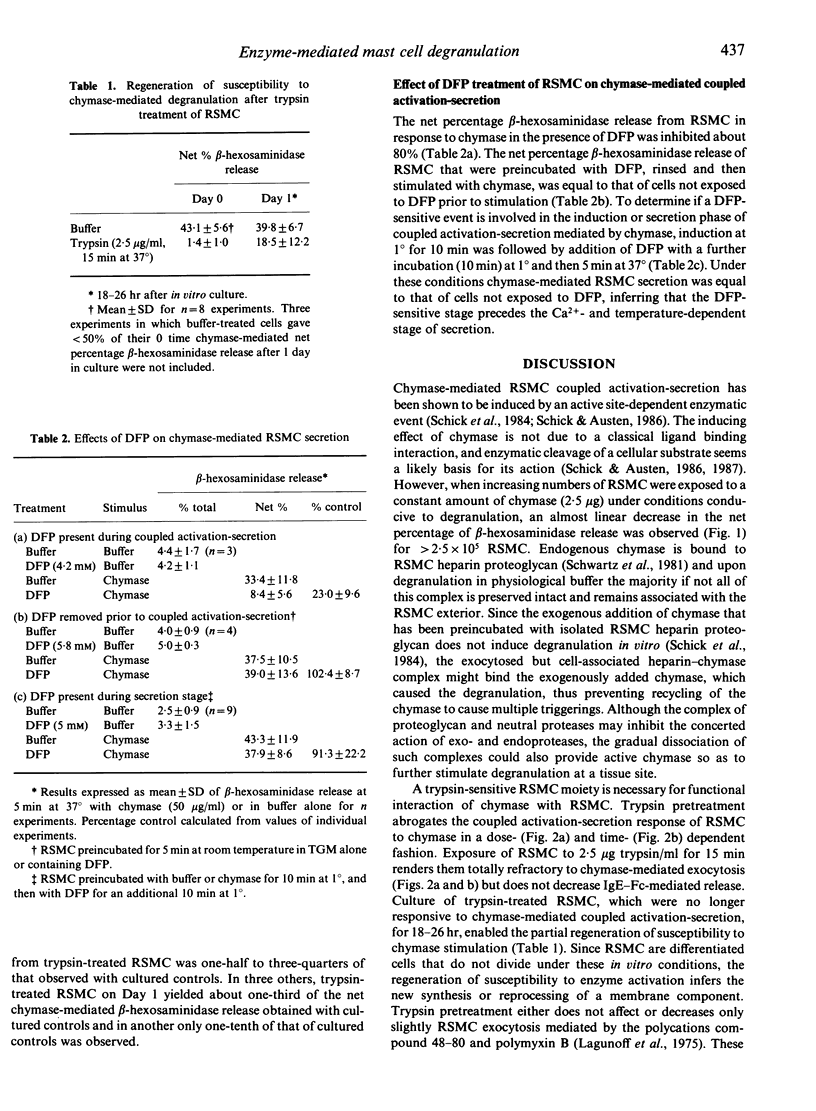
Exposure of rat serosal mast cells (RSMC) to chymase, an endogenous secretory granule serine protease, at 37 degrees results in exocytosis, as determined by beta-hexosaminidase release. As the number of RSMC is increased with a set amount of chymase, the net percentage beta-hexosaminidase release decreases linearly, implying a finite set of cellular interactions per chymase unit. Pretreatment of RSMC with trypsin at 37 degrees renders them refractory to subsequent exocytosis mediated by chymase in a dose- and time-dependent fashion, with complete refractiveness occurring by 15 min at 37 degrees with 2.5 micrograms trypsin/ml. Anti-IgE-mediated coupled activation-secretion of RSMC is not affected by the same trypsin pretreatment. When RSMC are pretreated with trypsin (2.5 micrograms/ml) for 0-120 min at 1 degree a progressive loss of sensitivity to activation by chymase at 37 degrees occurs. RSMC susceptibility to chymase-mediated degranulation after trypsin pretreatment can be partially regenerated by culturing the RSMC for about 24 hr in medium at 37 degrees. These findings suggest that a trypsin-sensitive constituent, possibly a receptor or substrate, is necessary for the functional interaction of chymase with RSMC. When added with diisopropyl fluorophosphate (DFP), chymase does not induce RSMC degranulation at 37 degrees. However, if the DFP is removed before addition of chymase at 37 degrees or is added after the chymase-priming event occurs at 1 degree, subsequent degranulation at 37 degrees is not inhibited. Thus, the induction and not the secretion phase is DFP-inhibitable in chymase-induced activation-secretion. In addition, the priming but not the exocytosis phase of chymase-initiated RSMC activation-secretion, which is not dependent on temperature and calcium ion concentration, involves a cellular trypsin-sensitive protein.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDITT E. P., ARASE M. An enzyme in mast cells with properties like chymotrypsin. J Exp Med. 1959 Sep 1;110:451–460. doi: 10.1084/jem.110.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker E. L., Austen K. F. Mechanisms of immunologic injury of rat peritoneal mast cells. I. The effect of phosphonate inhibitors on the homocytotropic antibody-mediated histamine release and the first component of rat complement. J Exp Med. 1966 Sep 1;124(3):379–395. doi: 10.1084/jem.124.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Evidence for a role of phosphatidylinositol turnover in stimulus-secretion coupling. Studies with rat peritoneal mast cells. Biochem J. 1979 Mar 15;178(3):681–687. doi: 10.1042/bj1780681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt M. T., Neurath H. Purification and partial characterization of an alpha-chymotrypsin-like protease of rat peritoneal mast cells. Biochimie. 1979;61(5-6):653–662. doi: 10.1016/s0300-9084(79)80163-7. [DOI] [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy. 1984;34:188–235. [PubMed] [Google Scholar]
- Kaliner M., Austen K. F. A sequence of biochemical events in the antigen-induced release of chemical mediators from sensitized human lung tissue. J Exp Med. 1973 Nov 1;138(5):1077–1094. doi: 10.1084/jem.138.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawiak J., Vensel W. H., Komender J., Barnard E. A. Non-pancreatic proteases of the chymotrypsin family. I. A chymotrypsin-like protease from rat mast cells. Biochim Biophys Acta. 1971 Apr 14;235(1):172–187. doi: 10.1016/0005-2744(71)90045-3. [DOI] [PubMed] [Google Scholar]
- Lagunoff D., Chi E. Y., Wan H. Effects of chymotrypsin and trypsin on rat peritoneal mast cells. Biochem Pharmacol. 1975 Sep 1;24(17):1573–1578. doi: 10.1016/0006-2952(75)90081-7. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Soter N. A., Diamond P. T., Austen K. F., Oates J. A., Roberts L. J., 2nd Prostaglandin D2 generation after activation of rat and human mast cells with anti-IgE. J Immunol. 1982 Oct;129(4):1627–1631. [PubMed] [Google Scholar]
- Nials A. T., Vardey C. J., Skidmore I. F. The release of histamine from rat mast cells by chymotrypsin. Int Arch Allergy Appl Immunol. 1983;70(1):83–87. doi: 10.1159/000233278. [DOI] [PubMed] [Google Scholar]
- Okuno-Kaneda S., Saito T., Kawasaki Y., Ichikawa A., Tomita K. Properties of protease in mast cell granules. Biochem Pharmacol. 1980 Jun 15;29(12):1715–1722. doi: 10.1016/0006-2952(80)90130-6. [DOI] [PubMed] [Google Scholar]
- Pruzansky J. J., Patterson R. The diisopropylfluorophosphate inhibitable step in antigen-induced histamine release from human leukocytes. J Immunol. 1975 Mar;114(3):939–943. [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J. Mechanism of histamine release by alpha-chymotrypsin from isolated rat mast cells. Jpn J Pharmacol. 1975 Jun;25(3):311–324. doi: 10.1254/jjp.25.311. [DOI] [PubMed] [Google Scholar]
- Schick B., Austen K. F. Pharmacological modulation of activation-secretion of rat serosal mast cells by chymase, an endogenous secretory granule protease. Immunology. 1985 Nov;56(3):513–522. [PMC free article] [PubMed] [Google Scholar]
- Schick B., Austen K. F. Rat serosal mast cell degranulation mediated by chymase, an endogenous secretory granule protease: active site-dependent initiation at 1 degree C. J Immunol. 1986 May 15;136(10):3812–3818. [PubMed] [Google Scholar]
- Schick B., Austen K. F., Schwartz L. B. Activation of rat serosal mast cells by chymase, an endogenous secretory granule protease. J Immunol. 1984 May;132(5):2571–2577. [PubMed] [Google Scholar]
- Schwartz L. B., Austen K. F., Wasserman S. I. Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J Immunol. 1979 Oct;123(4):1445–1450. [PubMed] [Google Scholar]
- Schwartz L. B., Riedel C., Caulfield J. P., Wasserman S. I., Austen K. F. Cell association of complexes of chymase, heparin proteoglycan, and protein after degranulation by rat mast cells. J Immunol. 1981 Jun;126(6):2071–2078. [PubMed] [Google Scholar]
- Seppä H. E., Järvinen M. Rat skin main neutral protease: purification and properties. J Invest Dermatol. 1978 Feb;70(2):84–89. doi: 10.1111/1523-1747.ep12541221. [DOI] [PubMed] [Google Scholar]
- UVNAES B., ANTONSSON J. TRIGGERING ACTION OF PHOSPHATIDASE A AND CHYMOTRYPSINS ON DEGRANULATION OF RAT MESENTERY MAST CELLS. Biochem Pharmacol. 1963 Aug;12:867–873. doi: 10.1016/0006-2952(63)90117-5. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Everitt M. T., Neurath H., Woodbury R. G., Powers J. C. Substrate specificity of two chymotrypsin-like proteases from rat mast cells. Studies with peptide 4-nitroanilides and comparison with cathepsin G. Biochemistry. 1980 Dec 9;19(25):5799–5804. doi: 10.1021/bi00566a021. [DOI] [PubMed] [Google Scholar]
- Yurt R., Austen K. F. Preparative purification of the rat mast cell chymase: characterization and interaction with granule components. J Exp Med. 1977 Nov 1;146(5):1405–1419. doi: 10.1084/jem.146.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]


