Abstract
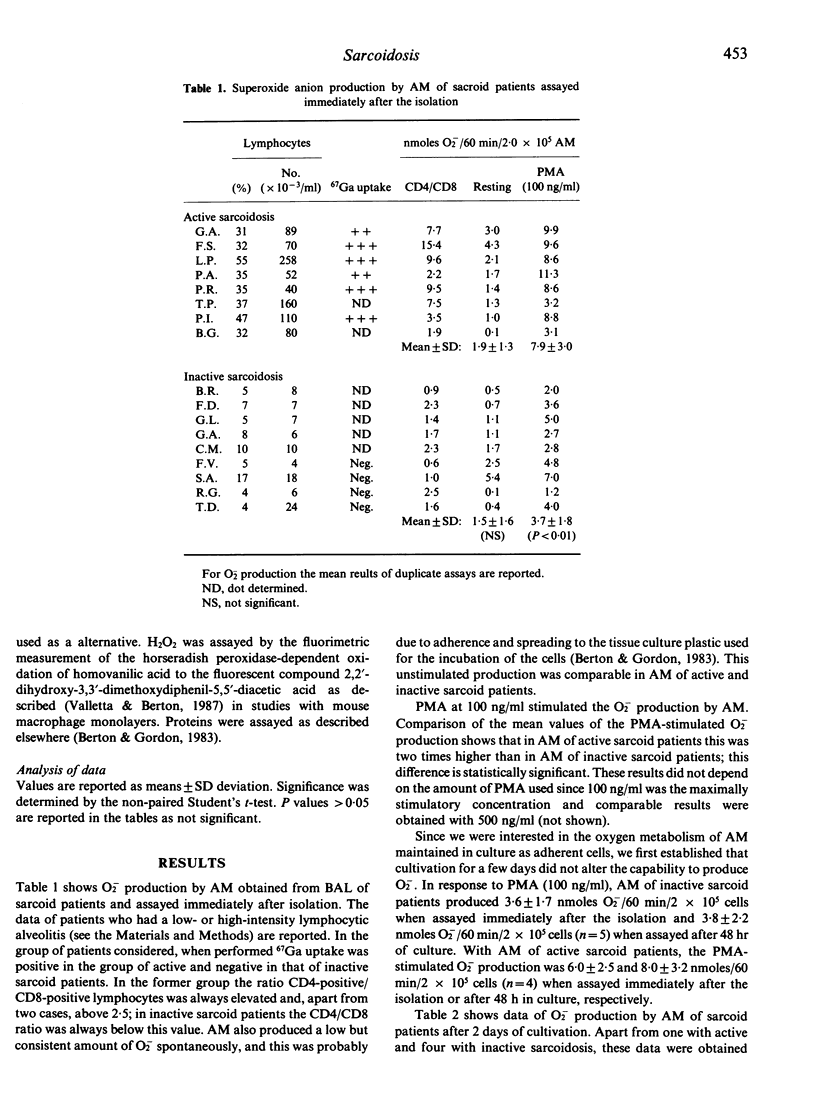
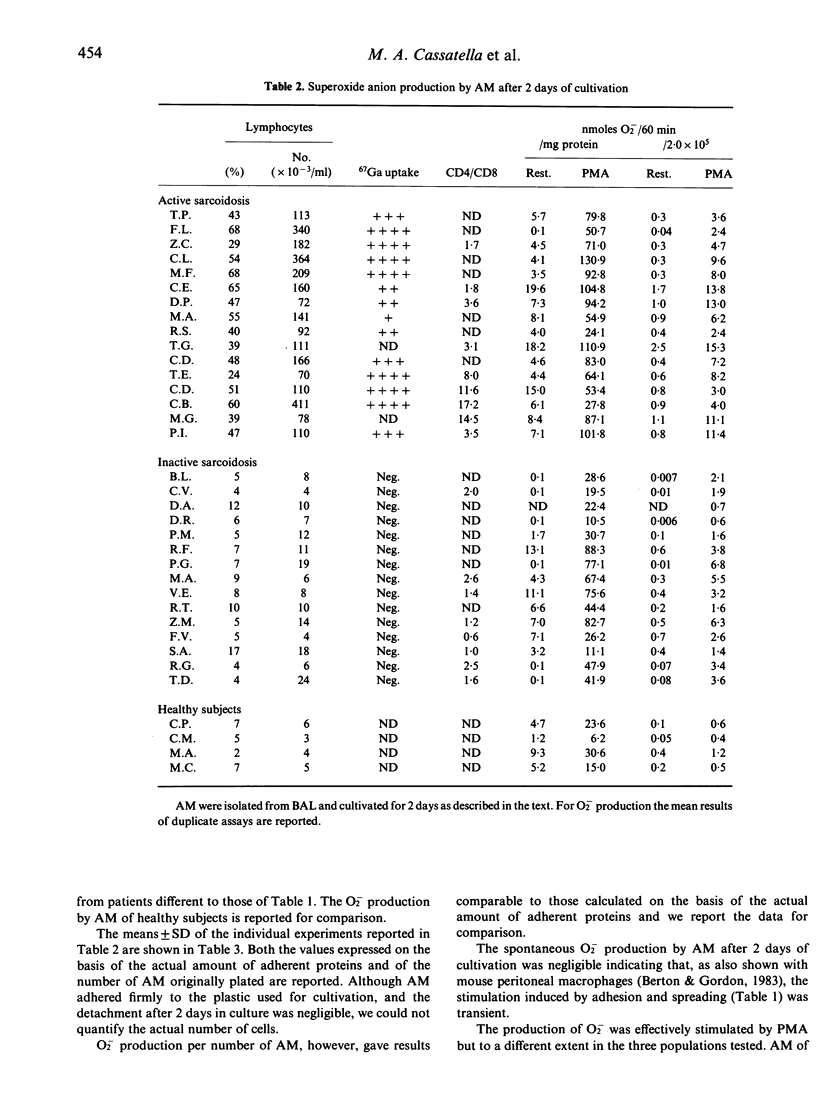
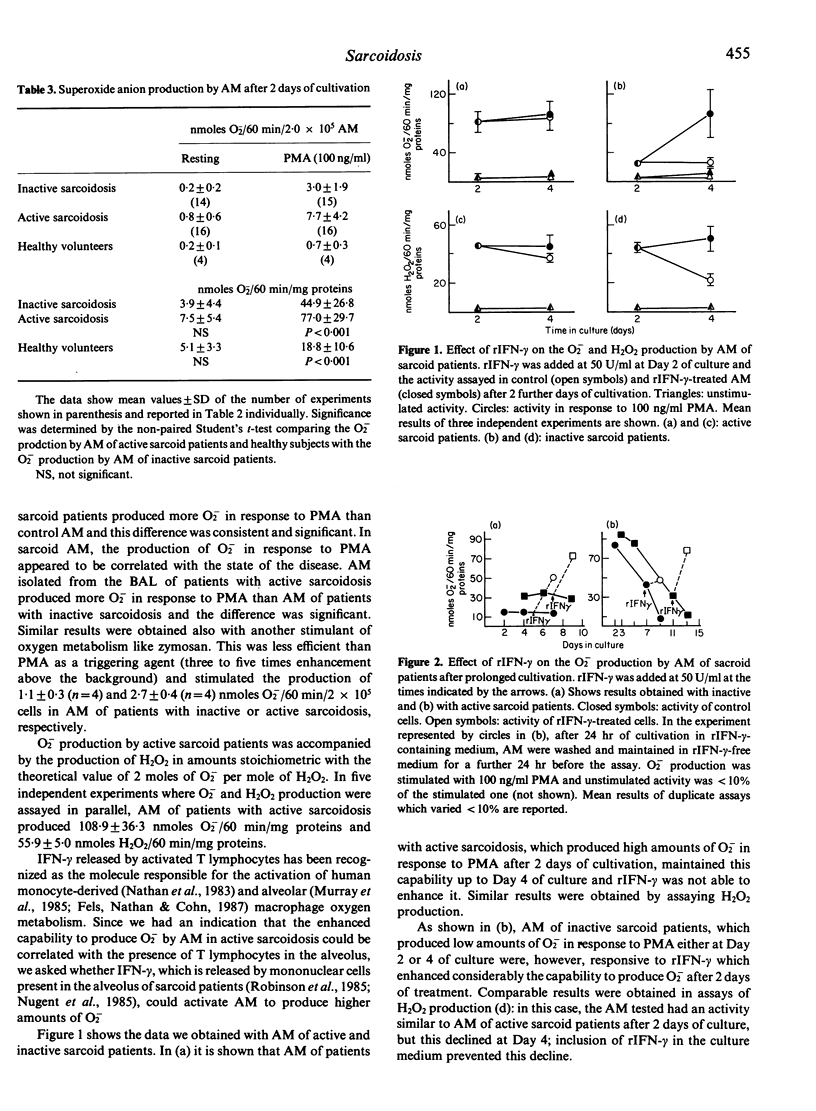
We studied superoxide anion (O2) generation by alveolar macrophages (AM) isolated from bronchoalveolar lavages (BAL) of patients with sarcoidosis, and assayed immediately after the isolation or after maintenance in culture for 2 days. In assays of cells freshly isolated from BAL, AM of patients with active sarcoidosis with a high-intensity lymphocytic alveolitis produced more O2- in response to phorbol myristate acetate than AM of patients with inactive sarcoidosis. Also, after 2 days of cultivation sarcoid AM were heterogeneous in their capability to metabolize oxygen, although both AM of active and inactive sarcoid patients produced higher amounts of O2- than AM of healthy subjects. In vitro treatment with recombinant interferon-gamma (rIFN-gamma) caused an enhancement of the capability of AM of inactive sarcoid patients to produce O2- in response to PMA. AM of patients with active sarcoidosis did not respond to rIFN-gamma when they already produced O2- vigorously. However, they became sensitive to the activating effect of rIFN-gamma after the down-modulation of their capability to produce O2-, that occurred upon prolonged cultivation. Monocytes isolated from blood of sarcoid patients and assayed immediately or after different times of cultivation did not produce more O2- than control monocytes and monocyte-derived macrophages, thus indicating that the activation of AM in sarcoidosis is likely a local phenomenon. These studies strengthen the notion that T lymphocyte-macrophage interaction is a critical event in the pathogenesis of sarcoidosis and establish that the enhanced capability to metabolize oxygen to highly reactive intermediates by AM is one of the consequence of this interaction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aerts C., Wallaert B., Grosbois J. M., Voisin C. Release of superoxide anion by alveolar macrophages in pulmonary sarcoidosis. Ann N Y Acad Sci. 1986;465:193–200. doi: 10.1111/j.1749-6632.1986.tb18495.x. [DOI] [PubMed] [Google Scholar]
- Agostini C., Trentin L., Zambello R., Luca M., Masciarelli M., Cipriani A., Marcer G., Semenzato G. Pulmonary alveolar macrophages in patients with sarcoidosis and hypersensitivity pneumonitis: characterization by monoclonal antibodies. J Clin Immunol. 1987 Jan;7(1):64–70. doi: 10.1007/BF00915427. [DOI] [PubMed] [Google Scholar]
- Babior B. M., Kipnes R. S., Curnutte J. T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J Clin Invest. 1973 Mar;52(3):741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton G., Cassatella M., Cabrini G., Rossi F. Activation of mouse macrophages causes no change in expression and function of phorbol diesters' receptors, but is accompanied by alterations in the activity and kinetic parameters of NADPH oxidase. Immunology. 1985 Feb;54(2):371–379. [PMC free article] [PubMed] [Google Scholar]
- Berton G., Gordon S. Superoxide release by peritoneal and bone marrow-derived mouse macrophages. Modulation by adherence and cell activation. Immunology. 1983 Aug;49(4):693–704. [PMC free article] [PubMed] [Google Scholar]
- Cassatella M. A., Della Bianca V., Berton G., Rossi F. Activation by gamma interferon of human macrophage capability to produce toxic oxygen molecules is accompanied by decreased Km of the superoxide-generating NADPH oxidase. Biochem Biophys Res Commun. 1985 Nov 15;132(3):908–914. doi: 10.1016/0006-291x(85)91893-5. [DOI] [PubMed] [Google Scholar]
- Ewan V. A., Cieplinski W., Hancock W. W., Goldschneider I., Boyd A. W., Rickles F. R. Production and characterization of a monoclonal antibody (A1-3) that binds selectively to activated monocytes and inhibits monocyte procoagulant activity. J Immunol. 1986 Apr 1;136(7):2408–2415. [PubMed] [Google Scholar]
- Fels A. O., Nathan C. F., Cohn Z. A. Hydrogen peroxide release by alveolar macrophages from sarcoid patients and by alveolar macrophages from normals after exposure to recombinant interferons alpha A, beta, and gamma and 1,25-dihydroxyvitamin D3. J Clin Invest. 1987 Aug;80(2):381–386. doi: 10.1172/JCI113083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Douches S., Winchester R. J., Ferrans V. J., Crystal R. G. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol. 1985 Jan;134(1):284–292. [PubMed] [Google Scholar]
- Hancock W. W., Rickles F. R., Ewan V. A., Atkins R. C. Immunohistological studies with A1-3, a monoclonal antibody to activated human monocytes and macrophages. J Immunol. 1986 Apr 1;136(7):2416–2420. [PubMed] [Google Scholar]
- Hunninghake G. W., Garrett K. C., Richerson H. B., Fantone J. C., Ward P. A., Rennard S. I., Bitterman P. B., Crystal R. G. Pathogenesis of the granulomatous lung diseases. Am Rev Respir Dis. 1984 Sep;130(3):476–496. doi: 10.1164/arrd.1984.130.3.476. [DOI] [PubMed] [Google Scholar]
- Murray H. W., Gellene R. A., Libby D. M., Rothermel C. D., Rubin B. Y. Activation of tissue macrophages from AIDS patients: in vitro response of AIDS alveolar macrophages to lymphokines and interferon-gamma. J Immunol. 1985 Oct;135(4):2374–2377. [PubMed] [Google Scholar]
- Nakagawara A., DeSantis N. M., Nogueira N., Nathan C. F. Lymphokines enhance the capacity of human monocytes to secret reactive oxygen intermediates. J Clin Invest. 1982 Nov;70(5):1042–1048. doi: 10.1172/JCI110691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A., Nathan C. F., Cohn Z. A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981 Nov;68(5):1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Horowitz C. R., de la Harpe J., Vadhan-Raj S., Sherwin S. A., Oettgen H. F., Krown S. E. Administration of recombinant interferon gamma to cancer patients enhances monocyte secretion of hydrogen peroxide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8686–8690. doi: 10.1073/pnas.82.24.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Prendergast T. J., Wiebe M. E., Stanley E. R., Platzer E., Remold H. G., Welte K., Rubin B. Y., Murray H. W. Activation of human macrophages. Comparison of other cytokines with interferon-gamma. J Exp Med. 1984 Aug 1;160(2):600–605. doi: 10.1084/jem.160.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent K. M., Glazier J., Monick M. M., Hunninghake G. W. Stimulated human alveolar macrophages secrete interferon. Am Rev Respir Dis. 1985 May;131(5):714–718. doi: 10.1164/arrd.1985.131.5.714. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Hedegaard H. B., Johnston R. B., Jr Cultured human monocytes require exposure to bacterial products to maintain an optimal oxygen radical response. J Immunol. 1982 Jan;128(1):123–128. [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenzato G., Chilosi M., Ossi E., Trentin L., Pizzolo G., Cipriani A., Agostini C., Zambello R., Marcer G., Gasparotto G. Bronchoalveolar lavage and lung histology. Comparative analysis of inflammatory and immunocompetent cells in patients with sarcoidosis and hypersensitivity pneumonitis. Am Rev Respir Dis. 1985 Aug;132(2):400–404. doi: 10.1164/arrd.1985.132.2.400. [DOI] [PubMed] [Google Scholar]
- Valletta E. A., Berton G. Desensitization of macrophage oxygen metabolism on immobilized ligands: different effect of immunoglobulin G and complement. J Immunol. 1987 Jun 15;138(12):4366–4373. [PubMed] [Google Scholar]
- Weinberg J. B., Hobbs M. M., Misukonis M. A. Recombinant human gamma-interferon induces human monocyte polykaryon formation. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4554–4557. doi: 10.1073/pnas.81.14.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]


