Abstract
We re-investigated the properties of a monoclonal antibody (mAb), 4D11, to human growth hormone (hGH) that showed a very weak affinity, recognizing hGH only when the hormone was solubilized on a solid surface. MAb4D11 did not significantly bind 125I-hGH. It was found that three mAb directed to different hGH epitopes (mAb 3C11, 10C1 and NA71) were able to induce the binding of the soluble antigen to mAb 4D11. The co-operative effect could be demonstrated by the formation of binary complexes (Ag:Ab, 1:2) detected by high-performance liquid chromatography (HPLC) and by the increase of radioactivity found when the synergistic mAb were added to 125I-hGH incubated with mAb 4D11 immobilized on polyvinyl microplates. Other possible explanations, such as the formation of cyclic complexes or the generation of a new epitope in the Fc fragment of the first antibody (Ab), were dismissed because the Fab fragment of one of the enhancing mAb (3C11) gave the same effect as the intact Ab. The data suggest that the hGH molecule undergoes a localized conformational change after binding to mAb 3C11, NA71 or 10C1 and that mAb 4D11 binds with high affinity to the modified region of the hormone. The formation or not of ternary complexes (Ag:Ab, 1:3) was used to localize the 4D11 epitope on the surface of the Ag. It is suggested that mAb 4D11 recognizes a conformational change produced in the region defined by the AE5/AC8 epitopes, which is close to the hGH antigenic domain only expressed when the protein is immobilized on plastic surfaces.
Full text
PDF
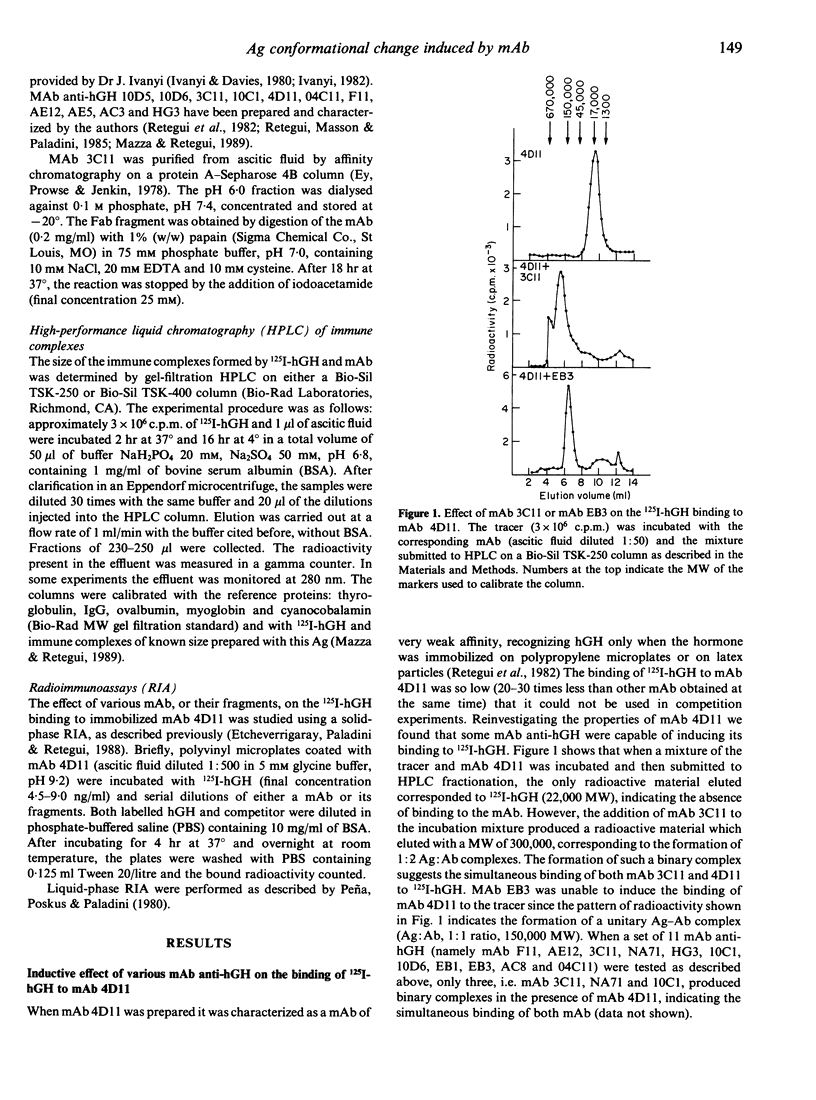
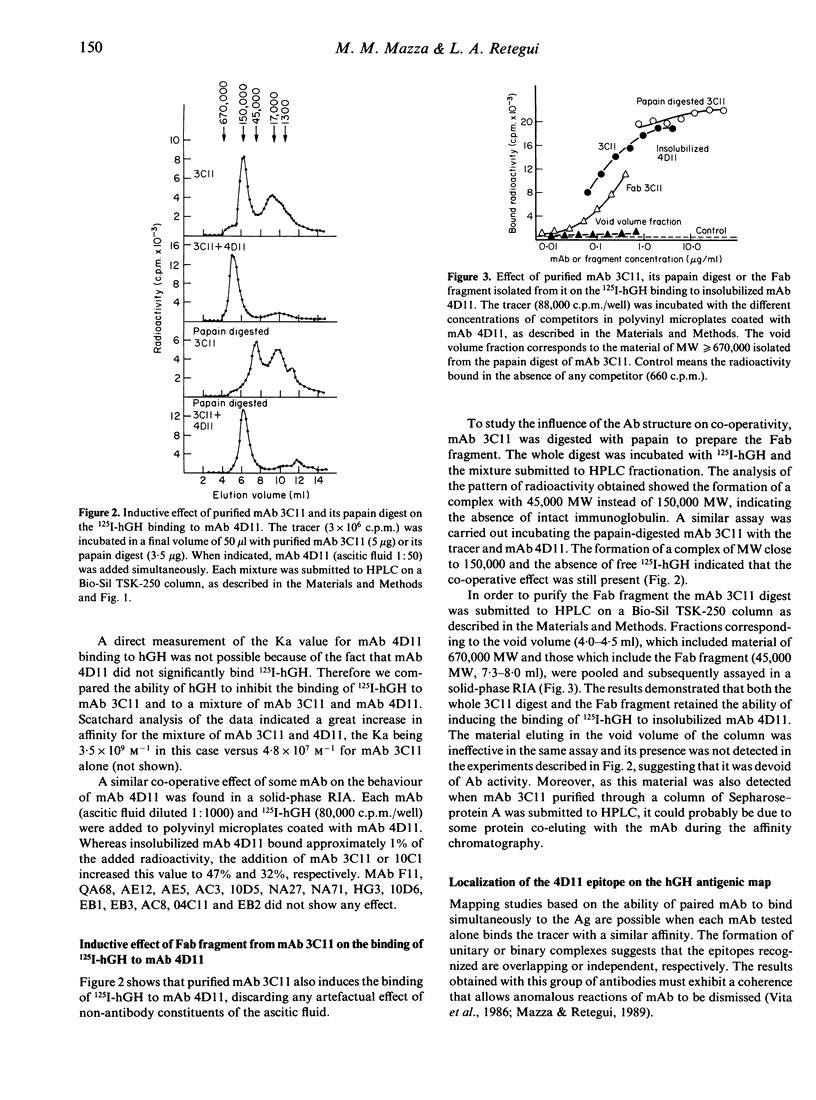
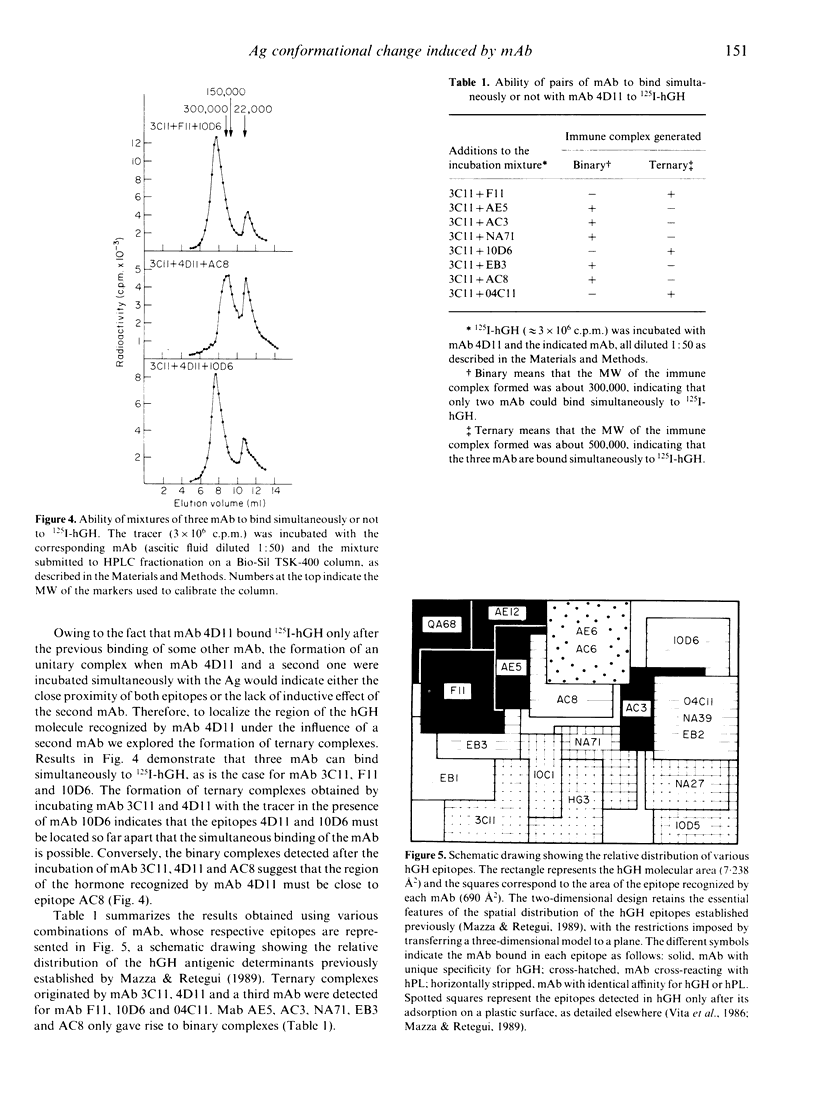


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Meguid S. S., Shieh H. S., Smith W. W., Dayringer H. E., Violand B. N., Bentle L. A. Three-dimensional structure of a genetically engineered variant of porcine growth hormone. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6434–6437. doi: 10.1073/pnas.84.18.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Diamond A. G., Butcher G. W., Howard J. C. Localized conformational changes induced in a class I major histocompatibility antigen by the binding of monoclonal antibodies. J Immunol. 1984 Mar;132(3):1169–1175. [PubMed] [Google Scholar]
- Duncan R. J., Hewitt J., Weston P. D. Activation of beta-galactosidase by monoclonal antibodies. Biochem J. 1982 Jul 1;205(1):219–224. doi: 10.1042/bj2050219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. H., Moyle W. R., Moustafa Z. A., Canfield R. E. Mixing two monoclonal antibodies yields enhanced affinity for antigen. J Immunol. 1982 Jun;128(6):2709–2713. [PubMed] [Google Scholar]
- Etcheverrigaray M., Paladini A. C., Retegui L. A. Stochastic humoral expression of human growth hormone epitopes. Immunology. 1988 Apr;63(4):595–601. [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Friguet B., Djavadi-Ohaniance L., Goldberg M. E. Some monoclonal antibodies raised with a native protein bind preferentially to the denatured antigen. Mol Immunol. 1984 Jul;21(7):673–677. doi: 10.1016/0161-5890(84)90053-1. [DOI] [PubMed] [Google Scholar]
- Harper M., Lema F., Boulot G., Poljak R. J. Antigen specificity and cross-reactivity of monoclonal anti-lysozyme antibodies. Mol Immunol. 1987 Feb;24(2):97–108. doi: 10.1016/0161-5890(87)90081-2. [DOI] [PubMed] [Google Scholar]
- Holmes N. J., Parham P. Enhancement of monoclonal antibodies against HLA-A2 is due to antibody bivalency. J Biol Chem. 1983 Feb 10;258(3):1580–1586. [PubMed] [Google Scholar]
- Ivanyi J., Davies P. Monoclonal antibodies against human growth hormone. Mol Immunol. 1980 Feb;17(2):287–290. doi: 10.1016/0161-5890(80)90082-6. [DOI] [PubMed] [Google Scholar]
- Mazza M. M., Retegui L. A. The antigenic topography of human growth hormone. Mol Immunol. 1989 Mar;26(3):231–240. doi: 10.1016/0161-5890(89)90076-x. [DOI] [PubMed] [Google Scholar]
- Mills J. B., Ashworth R. B., Wilhelmi A. E., Hartree A. S. Improved method for the extraction and purification of human growth hormone. J Clin Endocrinol Metab. 1969 Nov;29(11):1456–1459. doi: 10.1210/jcem-29-11-1456. [DOI] [PubMed] [Google Scholar]
- Milne R. W., Blanchette L., Théolis R., Jr, Weech P. K., Marcel Y. L. Monoclonal antibodies distinguish between lipid-dependent and reversible conformational states of human apolipoprotein B. Mol Immunol. 1987 May;24(5):435–447. doi: 10.1016/0161-5890(87)90017-4. [DOI] [PubMed] [Google Scholar]
- Moyle W. R., Anderson D. M., Ehrlich P. H. A circular antibody-antigen complex is responsible for increased affinity shown by mixtures of monoclonal antibodies to human chorionic gonadotropin. J Immunol. 1983 Oct;131(4):1900–1905. [PubMed] [Google Scholar]
- Nemazee D. A., Sato V. L. Enhancing antibody: a novel component of the immune response. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3828–3832. doi: 10.1073/pnas.79.12.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham P. Changes in conformation with loss of alloantigenic determinants of a histocompatibility antigen (HLA-B7) induced by monoclonal antibodies. J Immunol. 1984 Jun;132(6):2975–2983. [PubMed] [Google Scholar]
- Peña C., Poskus E., Paladini A. C. A relevant antigenic site in human growth hormone localized in sequence 98-128. Mol Immunol. 1980 Dec;17(12):1487–1491. doi: 10.1016/0161-5890(80)90174-1. [DOI] [PubMed] [Google Scholar]
- Retegui L. A., Masson P. L., Paladini A. C. Specificities of antibodies to human growth hormone (hGH) in patients treated with hGH: longitudinal study and comparison with the specificities of animal antisera. J Clin Endocrinol Metab. 1985 Jan;60(1):184–190. doi: 10.1210/jcem-60-1-184. [DOI] [PubMed] [Google Scholar]
- Retegui L. A., Milne R. W., Cambiaso C. L., Masson P. L. The recognition by monoclonal antibodies of various portions of a major antigenic site of human growth hormone. Mol Immunol. 1982 Jul;19(7):865–875. doi: 10.1016/0161-5890(82)90352-2. [DOI] [PubMed] [Google Scholar]
- Retegui L. A., Paladini A. C. Heteroclitic behaviour of some monoclonal antibodies against bovine growth hormone. Mol Immunol. 1986 Feb;23(2):119–123. doi: 10.1016/0161-5890(86)90032-5. [DOI] [PubMed] [Google Scholar]
- Roth J. Methods for assessing immunologic and biologic properties of iodinated peptide hormones. Methods Enzymol. 1975;37:223–233. doi: 10.1016/s0076-6879(75)37018-3. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Alexander H., Houghten R. A., Olson A. J., Lerner R. A., Hendrickson W. A. The reactivity of anti-peptide antibodies is a function of the atomic mobility of sites in a protein. Nature. 1984 Nov 8;312(5990):127–134. doi: 10.1038/312127a0. [DOI] [PubMed] [Google Scholar]
- Vita N., Etcheverrigaray M., Biscayart P. L., Retegui L. A. Relative distribution of various antigenic determinants on the human growth hormone surface. Mol Immunol. 1986 Jun;23(6):619–624. doi: 10.1016/0161-5890(86)90098-2. [DOI] [PubMed] [Google Scholar]


