Abstract
Our recent study revealed that soluble factors derived from accessory cells (AC; monocytes) and physical interaction with T cells of the accessory cells are both required for the induction of the proliferation of human peripheral blood T cells by anti-CD3 antibody coupled on latex beads. The accessory cell-derived soluble factor could be replaced by IL-1 and IL-6, and the role of live macrophages for physical interaction with T cells was found to be replaceable with paraformaldehyde(PFA)-fixed macrophages, provided the macrophages were pretreated with interferon-gamma (IFN-gamma) before fixation. Quantitative analysis in the present study revealed that IL-1 and IL-6 act synergistically to induce T-cell proliferation in the above system but either one of the factors alone reveals only a marginal or weak activity. Furthermore, it was shown that the potentiating activity of the culture supernatants of monocytes was substantially inhibited by anti-IL-6 antibody. Taken together with our previous results that anti-IL-1 serum strongly inhibited the potentiating activity of the culture supernatant, these results indicate that the main responsible molecules in the culture supernatant are IL-1 and IL-6, although a presence of other effective factors is not excluded. The anti-CD3-induced thymidine uptake by T cells in the presence of IL-1 and IL-6 was significantly inhibited by anti-Tac antibody, suggesting that the proliferation of T cells in this system is mostly mediated by a IL-2-dependent pathway. Our study further showed that accessory cells seem to acquire cell surface properties necessary for the effective interaction with T cells during 6-24 hr of culture with IFN-gamma. Presumably, a certain molecule(s) required for the interaction is induced on the cell surface of the AC by IFN-gamma.
Full text
PDF


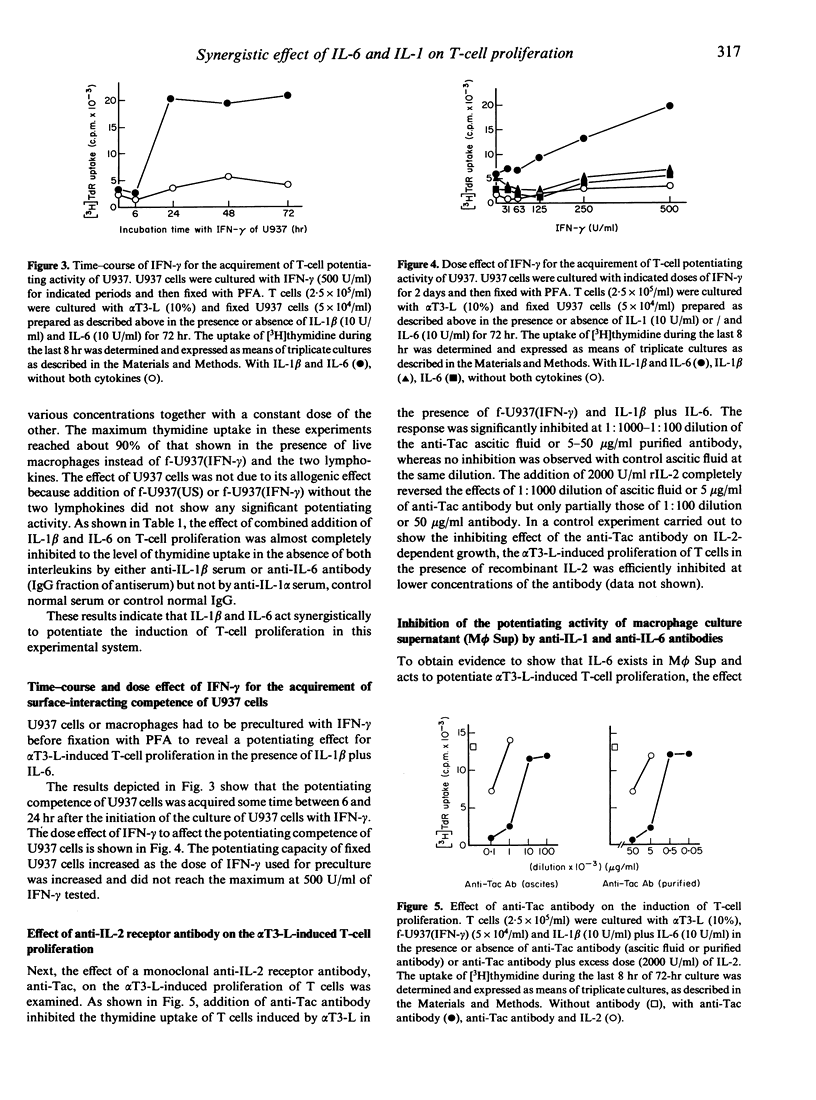
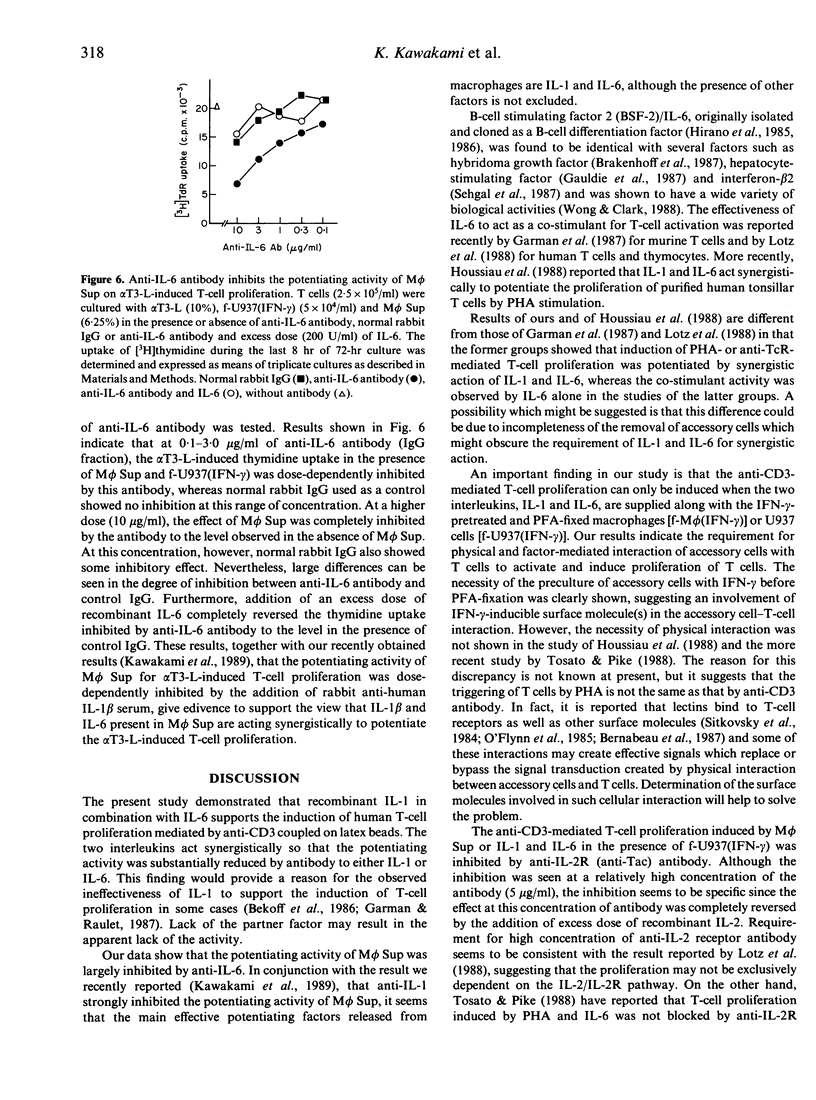


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Bekoff M., Kubo R., Grey H. M. Activation requirements for normal T cells: accessory cell-dependent and -independent stimulation by anti-receptor antibodies. J Immunol. 1986 Sep 1;137(5):1411–1419. [PubMed] [Google Scholar]
- Bernabeu C., Carrera A. C., De Landázuri M. O., Sánchez-Madrid F. Interaction between the CD45 antigen and phytohemagglutinin. Inhibitory effect on the lectin-induced T cell proliferation by anti-CD45 monoclonal antibody. Eur J Immunol. 1987 Oct;17(10):1461–1466. doi: 10.1002/eji.1830171012. [DOI] [PubMed] [Google Scholar]
- Brakenhoff J. P., de Groot E. R., Evers R. F., Pannekoek H., Aarden L. A. Molecular cloning and expression of hybridoma growth factor in Escherichia coli. J Immunol. 1987 Dec 15;139(12):4116–4121. [PubMed] [Google Scholar]
- Fujimoto K., Yamamoto Y., Ohmura T., Kawakami K., Onoue K. Dissection of the role of macrophages in triggering T lymphocytes for interleukin 2 production by monoclonal antibody OKT3. Microbiol Immunol. 1986;30(6):561–572. doi: 10.1111/j.1348-0421.1986.tb02982.x. [DOI] [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garman R. D., Raulet D. H. Characterization of a novel murine T cell-activating factor. J Immunol. 1987 Feb 15;138(4):1121–1129. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Hosoi K., Okano A., Matsui H., Kishimoto T. Absence of antiviral activity in recombinant B cell stimulatory factor 2 (BSF-2). Immunol Lett. 1988 Jan;17(1):41–45. doi: 10.1016/0165-2478(88)90099-5. [DOI] [PubMed] [Google Scholar]
- Hirano T., Taga T., Nakano N., Yasukawa K., Kashiwamura S., Shimizu K., Nakajima K., Pyun K. H., Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2). Proc Natl Acad Sci U S A. 1985 Aug;82(16):5490–5494. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssiau F. A., Coulie P. G., Olive D., Van Snick J. Synergistic activation of human T cells by interleukin 1 and interleukin 6. Eur J Immunol. 1988 Apr;18(4):653–656. doi: 10.1002/eji.1830180427. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Yamamoto Y., Kakimoto K., Onoue K. Requirement for delivery of signals by physical interaction and soluble factors from accessory cells in the induction of receptor-mediated T cell proliferation. Effectiveness of IFN-gamma modulation of accessory cells for physical interaction with T cells. J Immunol. 1989 Mar 15;142(6):1818–1825. [PubMed] [Google Scholar]
- Kaye J., Gillis S., Mizel S. B., Shevach E. M., Malek T. R., Dinarello C. A., Lachman L. B., Janeway C. A., Jr Growth of a cloned helper T cell line induced by a monoclonal antibody specific for the antigen receptor: interleukin 1 is required for the expression of receptors for interleukin 2. J Immunol. 1984 Sep;133(3):1339–1345. [PubMed] [Google Scholar]
- Koide S. L., Inaba K., Steinman R. M. Interleukin 1 enhances T-dependent immune responses by amplifying the function of dendritic cells. J Exp Med. 1987 Feb 1;165(2):515–530. doi: 10.1084/jem.165.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing T. J., Weiss A. Evidence for IL-2 independent proliferation in human T cells. J Immunol. 1988 Feb 15;140(4):1056–1062. [PubMed] [Google Scholar]
- Lotz M., Jirik F., Kabouridis P., Tsoukas C., Hirano T., Kishimoto T., Carson D. A. B cell stimulating factor 2/interleukin 6 is a costimulant for human thymocytes and T lymphocytes. J Exp Med. 1988 Mar 1;167(3):1253–1258. doi: 10.1084/jem.167.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malek T. R., Schmidt J. A., Shevach E. M. The murine IL 2 receptor. III. Cellular requirements for the induction of IL 2 receptor expression on T cell subpopulations. J Immunol. 1985 Apr;134(4):2405–2413. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Cantrell D. A., Hodgdon J. C., Schlossman S. F., Smith K. A., Reinherz E. L. Triggering of the T3-Ti antigen-receptor complex results in clonal T-cell proliferation through an interleukin 2-dependent autocrine pathway. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1509–1513. doi: 10.1073/pnas.81.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Meyer zum Büschenfelde K. H. T cell receptor triggering induces responsiveness to interleukin 1 and interleukin 2 but does not lead to T cell proliferation. J Immunol. 1986 Jun 1;136(11):4106–4112. [PubMed] [Google Scholar]
- Moldwin R. L., Lancki D. W., Herold K. C., Fitch F. W. An antigen receptor-driven, interleukin 2-independent pathway for proliferation of murine cytolytic T lymphocyte clones. J Exp Med. 1986 Jun 1;163(6):1566–1582. doi: 10.1084/jem.163.6.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flynn K., Krensky A. M., Beverley P. C., Burakoff S. J., Linch D. C. Phytohaemagglutinin activation of T cells through the sheep red blood cell receptor. Nature. 1985 Feb 21;313(6004):686–687. doi: 10.1038/313686a0. [DOI] [PubMed] [Google Scholar]
- Roska A. K., Lipsky P. E. Dissection of the functions of antigen-presenting cells in the induction of T cell activation. J Immunol. 1985 Nov;135(5):2953–2961. [PubMed] [Google Scholar]
- Scala G., Oppenheim J. J. Antigen presentation by human monocytes: evidence for stimulant processing and requirement for interleukin 1. J Immunol. 1983 Sep;131(3):1160–1166. [PubMed] [Google Scholar]
- Sehgal P. B., May L. T., Tamm I., Vilcek J. Human beta 2 interferon and B-cell differentiation factor BSF-2 are identical. Science. 1987 Feb 13;235(4790):731–732. doi: 10.1126/science.3492764. [DOI] [PubMed] [Google Scholar]
- Sitkovsky M. V., Pasternack M. S., Lugo J. P., Klein J. R., Eisen H. N. Isolation and partial characterization of concanavalin A receptors on cloned cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1519–1523. doi: 10.1073/pnas.81.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Teranishi T., Hirano T., Arima N., Onoue K. Human helper T cell factor(s) (ThF). II. Induction of IgG production in B lymphoblastoid cell lines and identification of T cell-replacing factor- (TRF) like factor(s). J Immunol. 1982 Apr;128(4):1903–1908. [PubMed] [Google Scholar]
- Thiele D. L., Kurosaka M., Lipsky P. E. Phenotype of the accessory cell necessary for mitogen-stimulated T and B cell responses in human peripheral blood: delineation by its sensitivity to the lysosomotropic agent, L-leucine methyl ester. J Immunol. 1983 Nov;131(5):2282–2290. [PubMed] [Google Scholar]
- Thiele D. L., Lipsky P. E. The immunosuppressive activity of L-leucyl-L-leucine methyl ester: selective ablation of cytotoxic lymphocytes and monocytes. J Immunol. 1986 Feb 1;136(3):1038–1048. [PubMed] [Google Scholar]
- Tosato G., Pike S. E. Interferon-beta 2/interleukin 6 is a co-stimulant for human T lymphocytes. J Immunol. 1988 Sep 1;141(5):1556–1562. [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Williams J. M., Deloria D., Hansen J. A., Dinarello C. A., Loertscher R., Shapiro H. M., Strom T. B. The events of primary T cell activation can be staged by use of Sepharose-bound anti-T3 (64.1) monoclonal antibody and purified interleukin 1. J Immunol. 1985 Oct;135(4):2249–2255. [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]


