Abstract
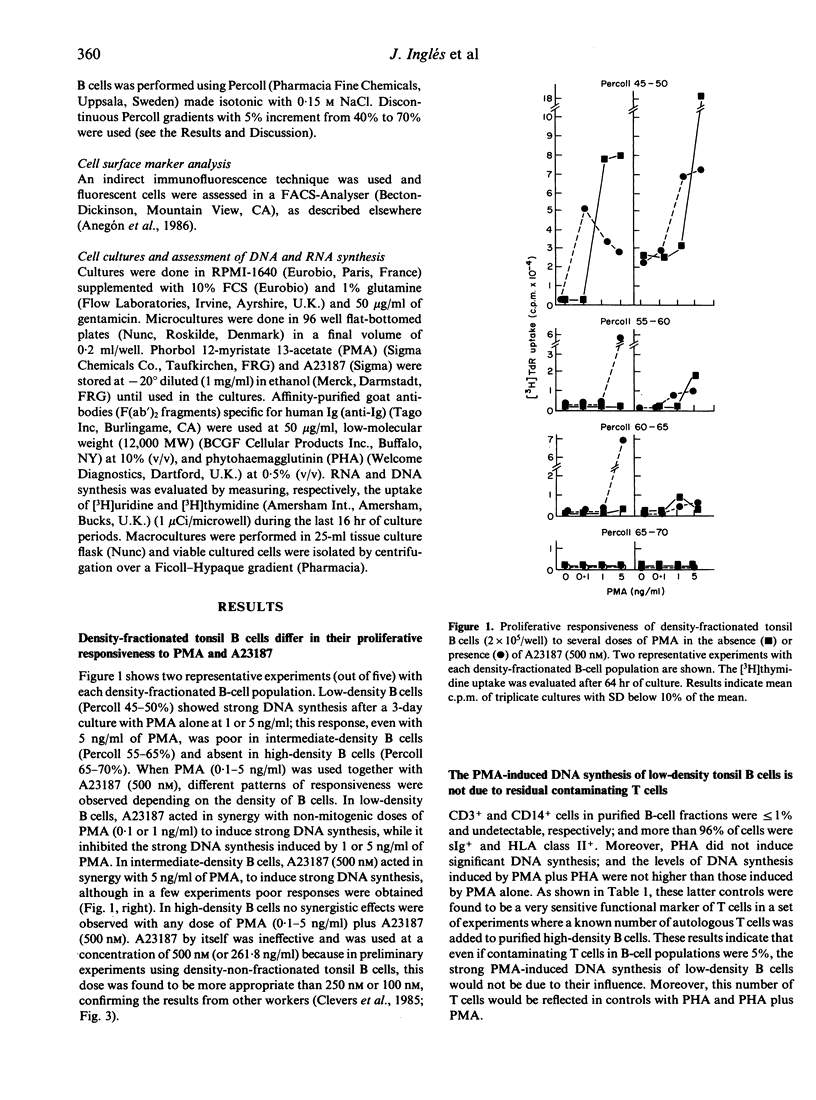
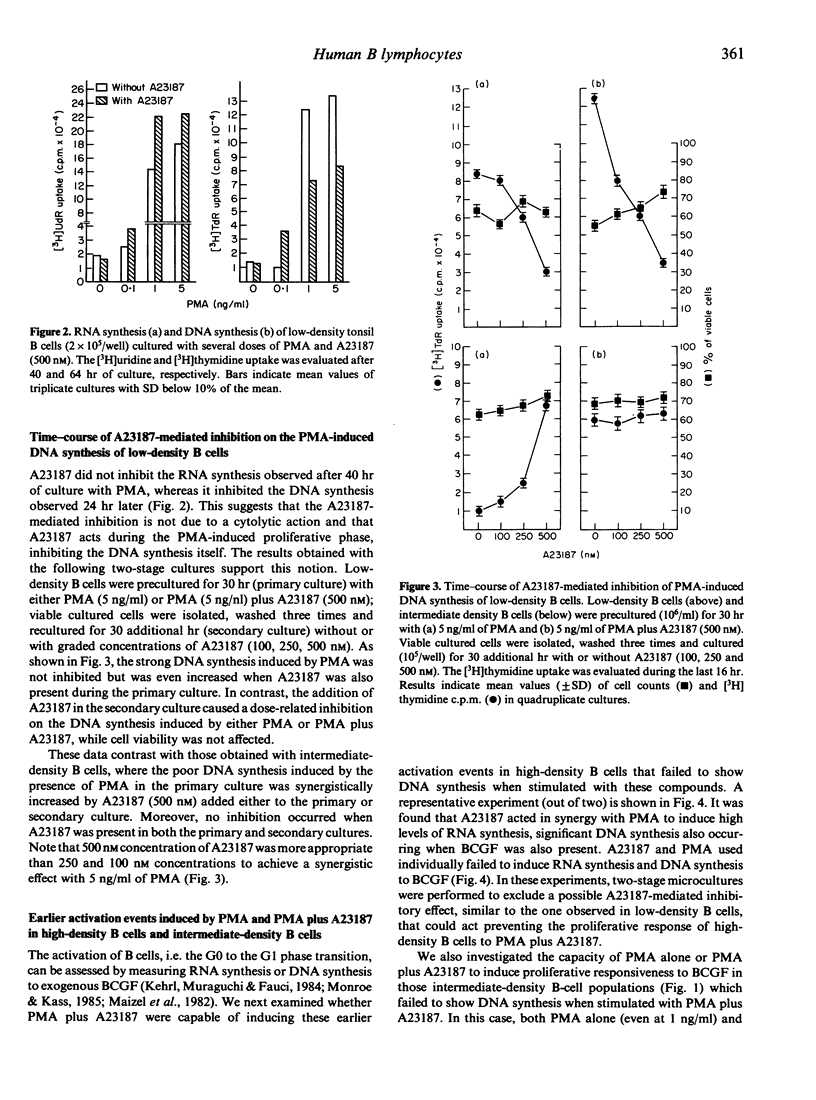
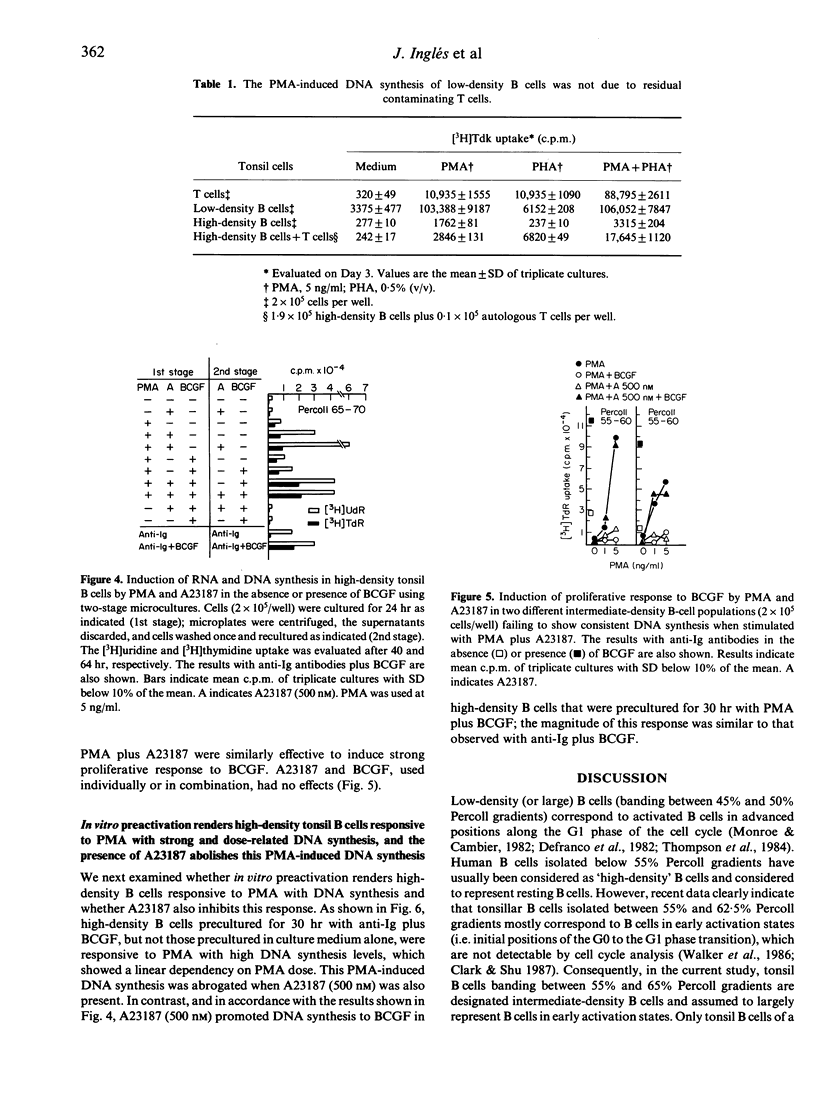
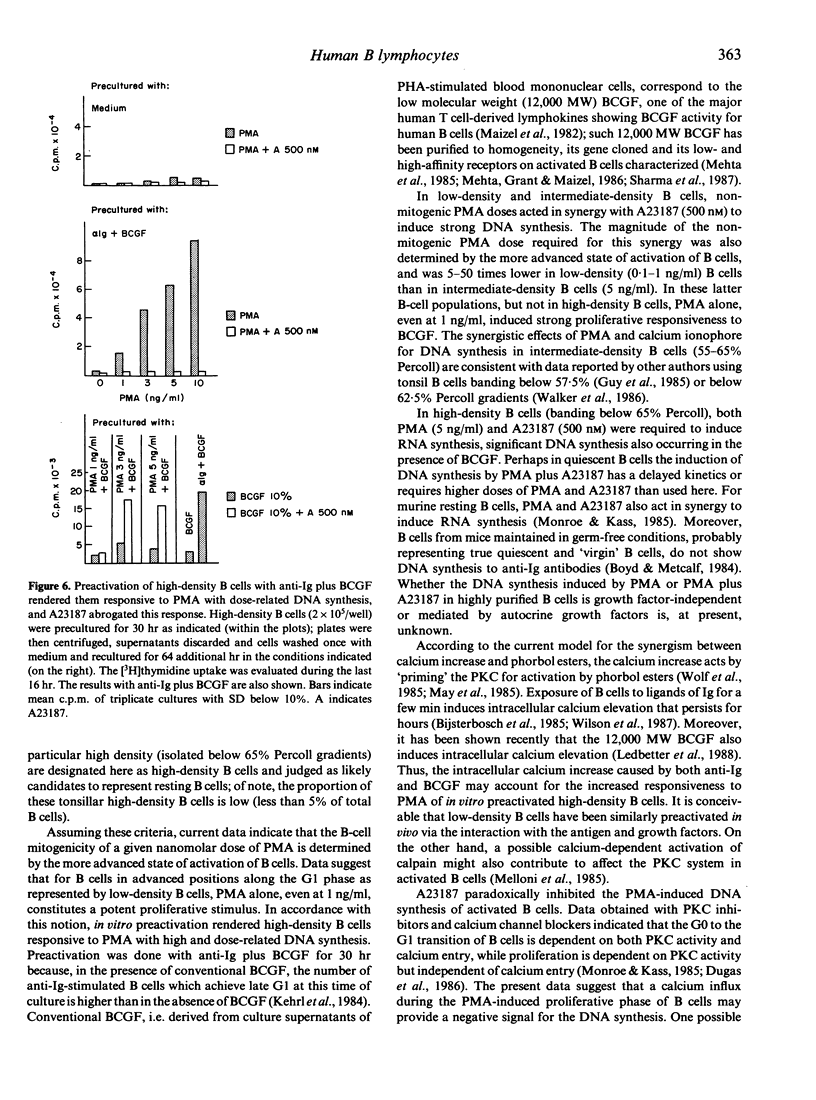
For tonsil B cells of a particular high density (below 65% Percoll), both phorbol myristate acetate (PMA) (5 ng/ml) and calcium ionophore A23187 (500 nM) were required to induce RNA synthesis, significant DNA synthesis also occurring in the presence of 12,000 MW B-cell growth factor (BCGF). In contrast, PMA alone, even at 1 ng/ml, was a sufficient stimulus to induce strong DNA synthesis in low-density B cells (45-50% Percoll) and strong proliferative responsiveness to BCGF in intermediate-density B cells (55-65% Percoll). In these latter B-cell populations, A23187 (500 nM), acted synergistically with non-mitogenic PMA doses to induce strong DNA synthesis, the PMA dose required being 5-50 times lower in low-density B cells (0.1-1 ng/ml) than in intermediate-density B cells (5 ng/ml). Preactivation for 30 hr with anti-Ig antibodies plus BCGF, known to drive B cells into late G1, rendered high-density B cells responsive to PMA (1-10 ng/ml) with high, dose-related DNA synthesis. These data indicate that the B-cell mitogenicity of a given nanomolar dose of PMA depends on the more advanced state of activation of B cells. It was also found that the above optimal dose of A23187 (500 nM) paradoxically inhibited the PMA-induced DNA synthesis of low-density B cells and in vitro preactivated high-density B cells. Data obtained with low-density B cells suggest that a calcium influx during the PMA-induced proliferative phase of B cells may provide a negative signal for the DNA synthesis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bijsterbosch M. K., Meade C. J., Turner G. A., Klaus G. G. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985 Jul;41(3):999–1006. doi: 10.1016/s0092-8674(85)80080-5. [DOI] [PubMed] [Google Scholar]
- Boyd A. W., Metcalf D. Activation of human and murine B cells by anti-immunoglobulin antibody: dependence of the response on the preexisting level of B-cell activation. Cell Immunol. 1984 Nov;89(1):75–83. doi: 10.1016/0008-8749(84)90199-0. [DOI] [PubMed] [Google Scholar]
- Chen Z. Z., Coggeshall K. M., Cambier J. C. Translocation of protein kinase C during membrane immunoglobulin-mediated transmembrane signaling in B lymphocytes. J Immunol. 1986 Mar 15;136(6):2300–2304. [PubMed] [Google Scholar]
- Clark E. A., Shu G. Activation of human B cell proliferation through surface Bp35 (CD20) polypeptides or immunoglobulin receptors. J Immunol. 1987 Feb 1;138(3):720–725. [PubMed] [Google Scholar]
- Defranco A. L., Raveche E. S., Asofsky R., Paul W. E. Frequency of B lymphocytes responsive to anti-immunoglobulin. J Exp Med. 1982 May 1;155(5):1523–1536. doi: 10.1084/jem.155.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy G. R., Bunce C. M., Gordon J., Michell R. H., Brown G. A combination of calcium ionophore and 12-O-tetradecanoyl-phorbol-13-acetate (TPA) stimulates the growth of purified resting B cells. Scand J Immunol. 1985 Nov;22(5):591–596. doi: 10.1111/j.1365-3083.1985.tb01919.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Stall A. M., Herzenberg L. A., Herzenberg L. A. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986 Oct;16(10):1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- Kehrl J. H., Muraguchi A., Fauci A. S. Human B cell activation and cell cycle progression: stimulation with anti-mu and Staphylococcus aureus Cowan strain I. Eur J Immunol. 1984 Feb;14(2):115–121. doi: 10.1002/eji.1830140203. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., O'Garra A., Bijsterbosch M. K., Holman M. Activation and proliferation signals in mouse B cells. VIII. Induction of DNA synthesis in B cells by a combination of calcium ionophores and phorbol myristate acetate. Eur J Immunol. 1986 Jan;16(1):92–97. doi: 10.1002/eji.1830160118. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Rabinovitch P. S., June C. H., Song C. W., Clark E. A., Uckun F. M. Antigen-independent regulation of cytoplasmic calcium in B cells with a 12-kDa B-cell growth factor and anti-CD19. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1897–1901. doi: 10.1073/pnas.85.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maizel A., Sahasrabuddhe C., Mehta S., Morgan J., Lachman L., Ford R. Biochemical separation of a human B cell mitogenic factor. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5998–6002. doi: 10.1073/pnas.79.19.5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May W. S., Jr, Sahyoun N., Wolf M., Cuatrecasas P. Role of intracellular calcium mobilization in the regulation of protein kinase C-mediated membrane processes. Nature. 1985 Oct 10;317(6037):549–551. doi: 10.1038/317549a0. [DOI] [PubMed] [Google Scholar]
- Mehta S. R., Conrad D., Sandler R., Morgan J., Montagna R., Maizel A. L. Purification of human B cell growth factor. J Immunol. 1985 Nov;135(5):3298–3302. [PubMed] [Google Scholar]
- Mehta S. R., Grant S. R., Maizel A. L. Characterization of the cell surface receptors for human B cell growth factor of 12,000 molecular weight. J Immunol. 1986 Oct 1;137(7):2210–2214. [PubMed] [Google Scholar]
- Mold C., Cooper N. R., Nemerow G. R. Incorporation of the purified Epstein Barr virus/C3d receptor (CR2) into liposomes and demonstration of its dual ligand binding functions. J Immunol. 1986 Jun 1;136(11):4140–4145. [PubMed] [Google Scholar]
- Monroe J. G., Cambier J. C. Cell cycle dependence for expression of membrane associated IgD, IgM and Ia antigen on mitogen-stimulated murine B-lymphocytes. Ann N Y Acad Sci. 1982;399:238–254. doi: 10.1111/j.1749-6632.1982.tb25677.x. [DOI] [PubMed] [Google Scholar]
- Monroe J. G., Kass M. J. Molecular events in B cell activation. I. Signals required to stimulate G0 to G1 transition of resting B lymphocytes. J Immunol. 1985 Sep;135(3):1674–1682. [PubMed] [Google Scholar]
- Muraguchi A., Butler J. L., Kehrl J. H., Fauci A. S. Differential sensitivity of human B cell subsets to activation signals delivered by anti-mu antibody and proliferative signals delivered by a monoclonal B cell growth factor. J Exp Med. 1983 Feb 1;157(2):530–546. doi: 10.1084/jem.157.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roifman C. M., Benedict S. H., Cheung R. K., Gelfand E. W. Induction of human B cell proliferation and differentiation by the combination of phorbol ester and ionomycin. Eur J Immunol. 1987 May;17(5):701–706. doi: 10.1002/eji.1830170519. [DOI] [PubMed] [Google Scholar]
- Sharma S., Mehta S., Morgan J., Maizel A. Molecular cloning and expression of a human B-cell growth factor gene in Escherichia coli. Science. 1987 Mar 20;235(4795):1489–1492. doi: 10.1126/science.3547651. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Scher I., Schaefer M. E., Lindsten T., Finkelman F. D., Mond J. J. Size-dependent B lymphocyte subpopulations: relationship of cell volume to surface phenotype, cell cycle, proliferative response, and requirements for antibody production to TNP-Ficoll and TNP-BA. J Immunol. 1984 Nov;133(5):2333–2342. [PubMed] [Google Scholar]
- Walker L., Guy G., Brown G., Rowe M., Milner A. E., Gordon J. Control of human B-lymphocyte replication. I. Characterization of novel activation states that precede the entry of G0 B cells into cycle. Immunology. 1986 Aug;58(4):583–589. [PMC free article] [PubMed] [Google Scholar]
- Wilson H. A., Greenblatt D., Taylor C. W., Putney J. W., Tsien R. Y., Finkelman F. D., Chused T. M. The B lymphocyte calcium response to anti-Ig is diminished by membrane immunoglobulin cross-linkage to the Fc gamma receptor. J Immunol. 1987 Mar 15;138(6):1712–1718. [PubMed] [Google Scholar]
- Wolf M., LeVine H., 3rd, May W. S., Jr, Cuatrecasas P., Sahyoun N. A model for intracellular translocation of protein kinase C involving synergism between Ca2+ and phorbol esters. Nature. 1985 Oct 10;317(6037):546–549. doi: 10.1038/317546a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]


