Abstract
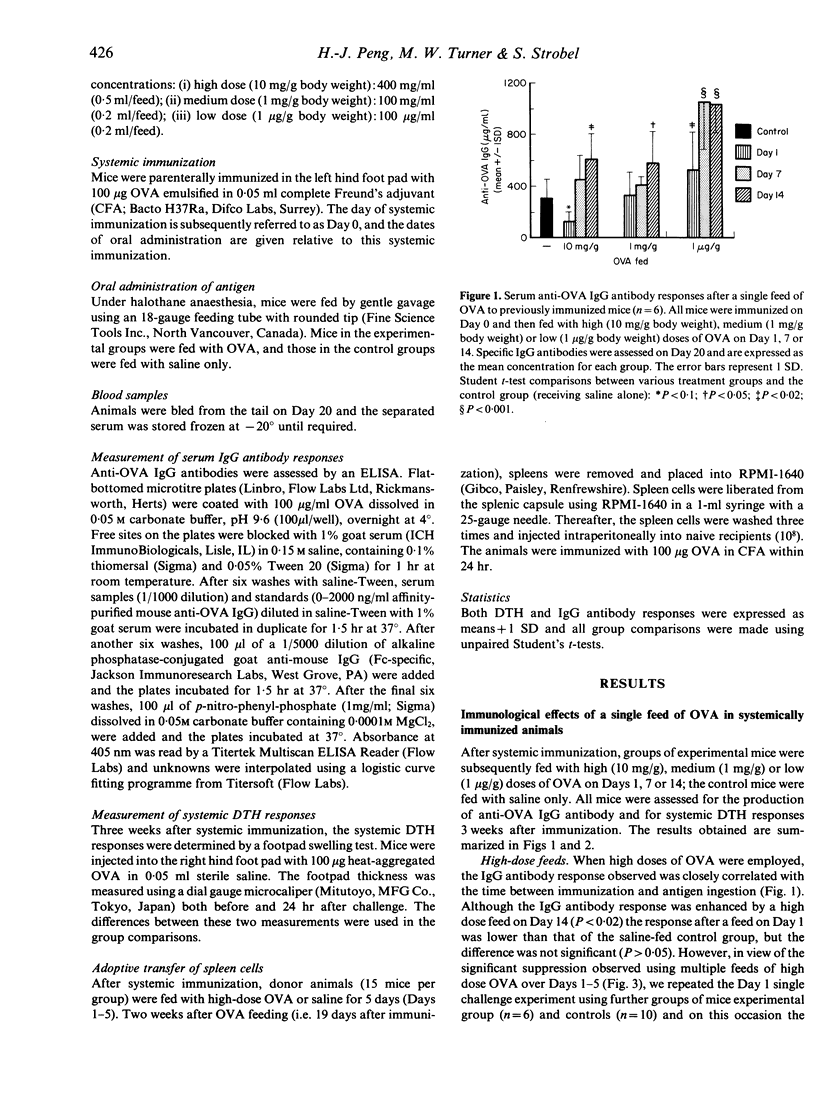
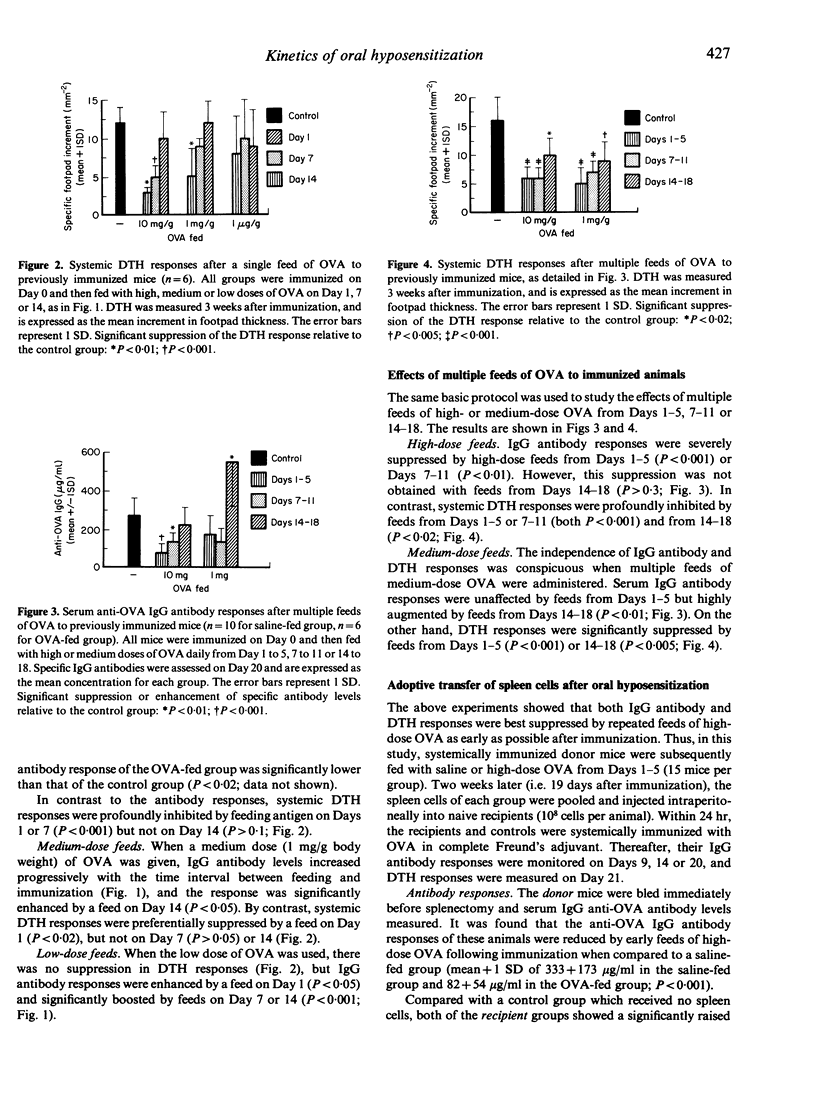
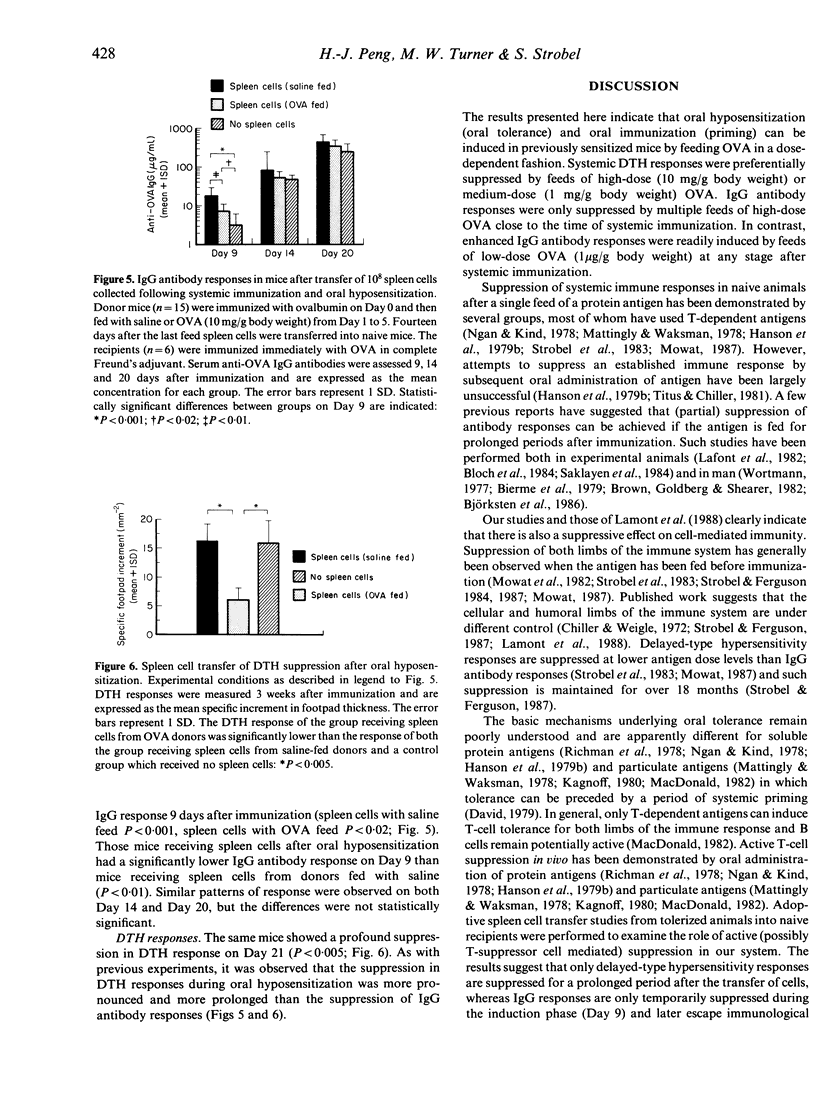
We have investigated the immunological consequences of feeding a protein antigen to previously immunized animals. BALB/c mice were systemically primed with ovalbumin (OVA) in complete Freund's adjuvant (CFA) and fed with high (10 mg/g body weight), medium (1 mg/g body weight) or low (1 microgram/g body weight) doses of OVA once (Day 1, 7 or 14) or sequentially for 5 days (Days 1-5, 7-11, 14-18). The specific IgG antibody response was suppressed only by early feeds of high-dose OVA (Days 1-5). Medium-dose OVA fed on Day 14 or low-dose OVA fed at any stage after immunization enhanced the IgG antibody response. In contradistinction, systemic delayed-type hypersensitivity responses (DTH) were usually suppressed by early feeds of high or medium doses of OVA but never after feeding low-dose OVA. The results suggest that systemic DTH and IgG antibody responses to oral antigen are subject to different control mechanisms in previously primed animals. Such responses depend on the immune status of the animal and are controlled by antigen dose, time and frequency of feeding. The immunological effects observed are also demonstrable following adoptive transfer of spleen cells collected 14 days after multiple feeds of high-dose OVA to immunized mice. Our findings suggest that oral hyposensitization after systemic immunization is regulated by (suppressor) spleen cells which are activated by gut-processed antigen.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bazin H., Platteau B. Production of circulating reaginic (IgE) antibodies by oral administration of ovalbumin to rats. Immunology. 1976 May;30(5):679–684. [PMC free article] [PubMed] [Google Scholar]
- Bierme S. J., Blanc M., Abbal M., Fournie A. Oral Rh treatment for severely immunised mothers. Lancet. 1979 Mar 17;1(8116):604–605. doi: 10.1016/s0140-6736(79)91024-9. [DOI] [PubMed] [Google Scholar]
- Björkstén B., Möller C., Broberger U., Ahlstedt S., Dreborg S., Johansson S. G., Juto P., Lanner A. Clinical and immunological effects of oral immunotherapy with a standardized birch pollen extract. Allergy. 1986 May;41(4):290–295. doi: 10.1111/j.1398-9995.1986.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Brown L. A., Goldberg N. D., Shearer W. T. Long-term ticarcillin desensitization by the continuous oral administration of penicillin. J Allergy Clin Immunol. 1982 Jan;69(1 Pt 1):51–54. doi: 10.1016/0091-6749(82)90087-2. [DOI] [PubMed] [Google Scholar]
- David M. F. Induction of hyporesponsiveness to particulate antigen by feeding: the sequence of immunologic response to fed antigen. J Allergy Clin Immunol. 1979 Sep;64(3):164–172. doi: 10.1016/0091-6749(79)90091-5. [DOI] [PubMed] [Google Scholar]
- Golbert T. M. A review of controversial diagnostic and therapeutic techniques employed in allergy. J Allergy Clin Immunol. 1975 Sep;56(3):170–190. doi: 10.1016/0091-6749(75)90089-5. [DOI] [PubMed] [Google Scholar]
- Hanson D. G., Vaz N. M., Maia L. C., Lynch J. M. Inhibition of specific immune responses by feeding protein antigens. III. Evidence against maintenance of tolerance to ovalbumin by orally induced antibodies. J Immunol. 1979 Nov;123(5):2337–2343. [PubMed] [Google Scholar]
- Hanson D. G., Vaz N. M., Rawlings L. A., Lynch J. M. Inhibition of specific immune responses by feeding protein antigens. II. Effects of prior passive and active immunization. J Immunol. 1979 Jun;122(6):2261–2266. [PubMed] [Google Scholar]
- Jarrett E. E., Haig D. M., McDougall W., McNulty E. Rat IgE production. II. Primary and booster reaginic antibody responses following intradermal or oral immunization. Immunology. 1976 May;30(5):671–677. [PMC free article] [PubMed] [Google Scholar]
- Lafont S., André C., André F., Gillon J., Fargier M. C. Abrogation by subsequent feeding of antibody response including IgE, in parenterally immunized mice. J Exp Med. 1982 May 1;155(5):1573–1578. doi: 10.1084/jem.155.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont A. G., Bruce M. G., Watret K. C., Ferguson A. Suppression of an established DTH response to ovalbumin in mice by feeding antigen after immunization. Immunology. 1988 May;64(1):135–139. [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T. Immunosuppression caused by antigen feeding. I. Evidence for the activation of a feedback suppressor pathway in the spleens of antigen-fed mice. Eur J Immunol. 1982 Sep;12(9):767–773. doi: 10.1002/eji.1830120912. [DOI] [PubMed] [Google Scholar]
- Mattingly J. A., Waksman B. H. Immunologic suppression after oral administration of antigen. I. Specific suppressor cells formed in rat Peyer's patches after oral administration of sheep erythrocytes and their systemic migration. J Immunol. 1978 Nov;121(5):1878–1883. [PubMed] [Google Scholar]
- Mowat A. M., Strobel S., Drummond H. E., Ferguson A. Immunological responses to fed protein antigens in mice. I. Reversal of oral tolerance to ovalbumin by cyclophosphamide. Immunology. 1982 Jan;45(1):105–113. [PMC free article] [PubMed] [Google Scholar]
- Ngan J., Kind L. S. Suppressor T cells for IgE and IgG in Peyer's patches of mice made tolerant by the oral administration of ovalbumin. J Immunol. 1978 Mar;120(3):861–865. [PubMed] [Google Scholar]
- Reisman R. E. Presidential address. Standards of practice--our responsibility. J Allergy Clin Immunol. 1981 Jul;68(1):1–4. doi: 10.1016/0091-6749(81)90115-9. [DOI] [PubMed] [Google Scholar]
- Richman L. K., Chiller J. M., Brown W. R., Hanson D. G., Vaz N. M. Enterically induced immunologic tolerance. I. Induction of suppressor T lymphoyctes by intragastric administration of soluble proteins. J Immunol. 1978 Dec;121(6):2429–2434. [PubMed] [Google Scholar]
- Saklayen M. G., Pesce A. J., Pollak V. E., Michael J. G. Kinetics of oral tolerance: study of variables affecting tolerance induced by oral administration of antigen. Int Arch Allergy Appl Immunol. 1984;73(1):5–9. doi: 10.1159/000233428. [DOI] [PubMed] [Google Scholar]
- Strobel S., Ferguson A. Immune responses to fed protein antigens in mice. 3. Systemic tolerance or priming is related to age at which antigen is first encountered. Pediatr Res. 1984 Jul;18(7):588–594. doi: 10.1203/00006450-198407000-00004. [DOI] [PubMed] [Google Scholar]
- Strobel S., Ferguson A. Persistence of oral tolerance in mice fed ovalbumin is different for humoral and cell-mediated immune responses. Immunology. 1987 Feb;60(2):317–318. [PMC free article] [PubMed] [Google Scholar]
- Strobel S., Mowat A. M., Drummond H. E., Pickering M. G., Ferguson A. Immunological responses to fed protein antigens in mice. II. Oral tolerance for CMI is due to activation of cyclophosphamide-sensitive cells by gut-processed antigen. Immunology. 1983 Jul;49(3):451–456. [PMC free article] [PubMed] [Google Scholar]
- Titus R. G., Chiller J. M. Orally induced tolerance. Definition at the cellular level. Int Arch Allergy Appl Immunol. 1981;65(3):323–338. [PubMed] [Google Scholar]
- Wortmann F. Oral hyposensitization of children with pollinosis or house-dust asthma. Allergol Immunopathol (Madr) 1977 Jan-Feb;5(1):15–26. [PubMed] [Google Scholar]


