Abstract
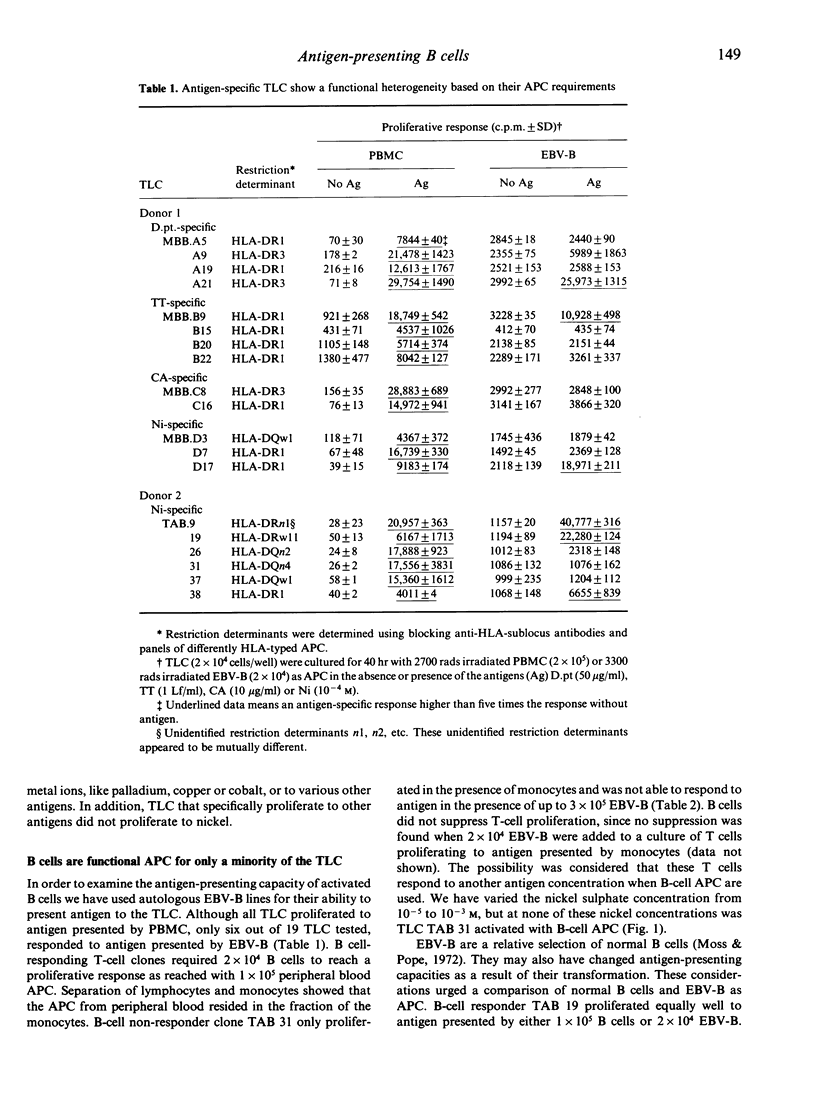
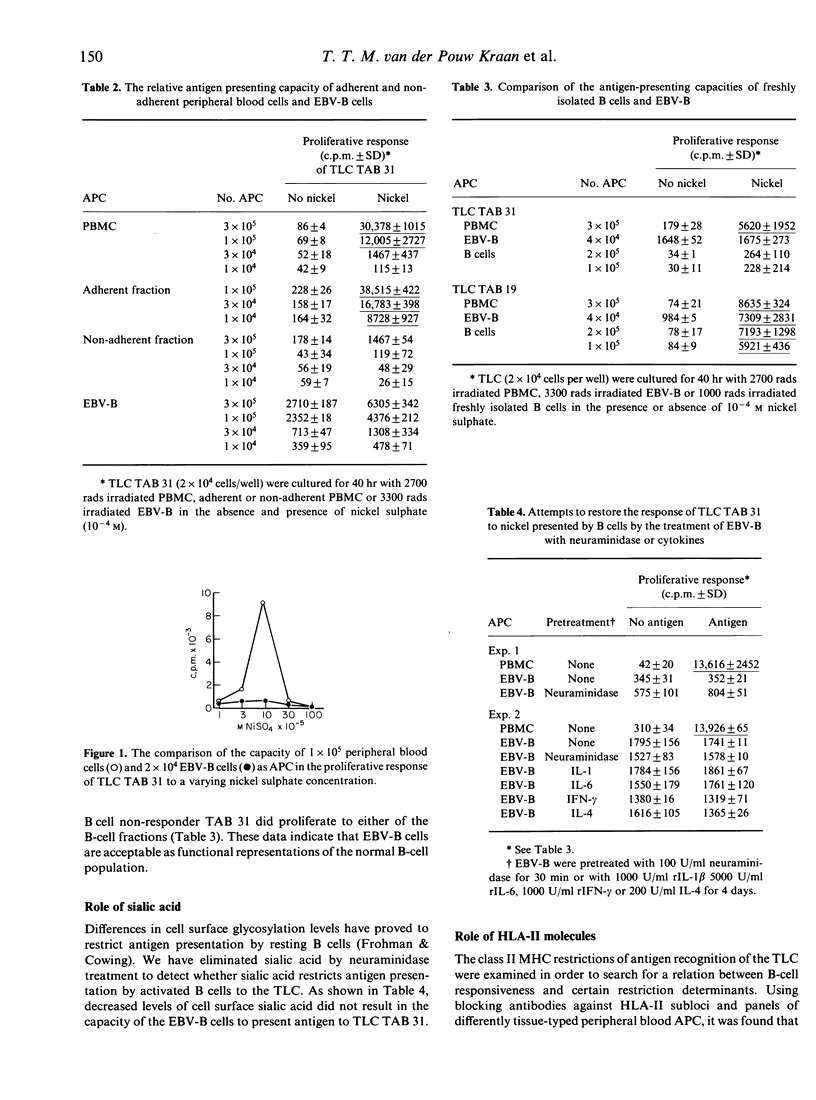
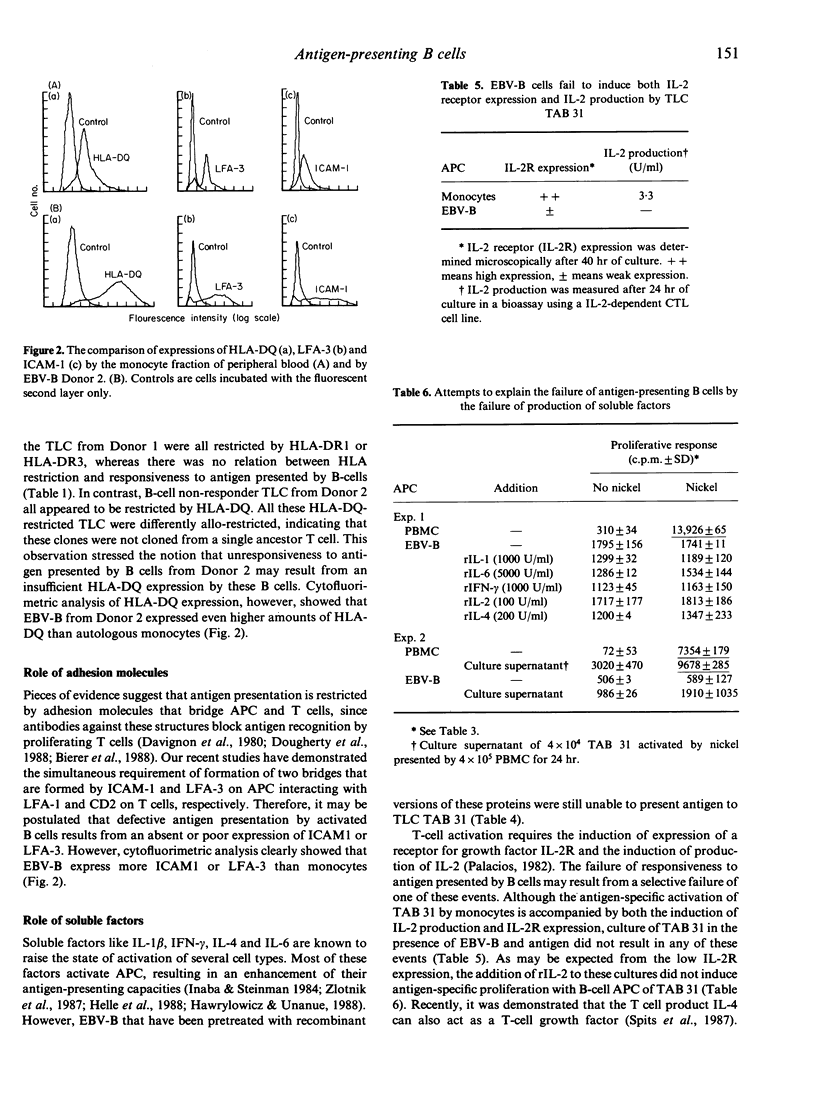
We examined the role of antigen-presenting B lymphocytes using panels of antigen-specific CD4+8-T-lymphocyte clones (TLC). All 19 TLC showed a class II major histocompatibility complex-encoded (HLA-II) restricted proliferation to antigen presented by antigen-presenting cells (APC) from the monocyte fraction of peripheral blood. Only six TLC were effectively activated by antigen presented by autologous B lymphocytes activated by EBV transformation. This failure of B lymphocytes was not due to: (i) a high degree of cell surface sialic acid; (ii) a low expression of the cell surface proteins HLA-II, ICAM-1 or LFA-3 that restrict antigen presentation; (iii) lack of secretion of the cytokine IL-1 or other soluble factors that may be required as secondary signals; or (iv) induction of incomplete T-cell activation resulting in the production of growth factor interleukin-2 (IL-2) or the expression of receptors for IL-2 only. These data suggest the involvement of another cell surface interaction in antigen presentation acting besides the interactions known so far.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashwell J. D., DeFranco A. L., Paul W. E., Schwartz R. H. Antigen presentation by resting B cells. Radiosensitivity of the antigen-presentation function and two distinct pathways of T cell activation. J Exp Med. 1984 Mar 1;159(3):881–905. doi: 10.1084/jem.159.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Bergholtz B. O., Thorsby E. Macrophage-dependent response of immune human T lymphocytes to PPD in vitro. Influence of HLA-D histocompatibility. Scand J Immunol. 1977;6(8):779–786. doi: 10.1111/j.1365-3083.1977.tb02151.x. [DOI] [PubMed] [Google Scholar]
- Bierer B. E., Barbosa J., Herrmann S., Burakoff S. J. Interaction of CD2 with its ligand, LFA-3, in human T cell proliferation. J Immunol. 1988 May 15;140(10):3358–3363. [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Studies on the capacity of B cells to serve as antigen-presenting cells. J Immunol. 1981 Mar;126(3):1075–1079. [PubMed] [Google Scholar]
- Clement L. T., Shevach E. M. Characterization of major histocompatibility antigens on trinitrophenyl-modified cells. Mol Immunol. 1979 Jan;16(1):67–76. doi: 10.1016/0161-5890(79)90029-4. [DOI] [PubMed] [Google Scholar]
- Cowing C., Chapdelaine J. M. T cells discriminate between Ia antigens expressed on allogeneic accessory cells and B cells: a potential function for carbohydrate side chains on Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6000–6004. doi: 10.1073/pnas.80.19.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon D., Martz E., Reynolds T., Kürzinger K., Springer T. A. Monoclonal antibody to a novel lymphocyte function-associated antigen (LFA-1): mechanism of blockade of T lymphocyte-mediated killing and effects on other T and B lymphocyte functions. J Immunol. 1981 Aug;127(2):590–595. [PubMed] [Google Scholar]
- Dekruyff R. H., Cantor H., Dorf M. E. Activation requirements of cloned inducer T cells. II. The failure of some clones to respond to antigen presented by activated B cells. J Immunol. 1986 Jan;136(2):446–451. [PubMed] [Google Scholar]
- Dougherty G. J., Murdoch S., Hogg N. The function of human intercellular adhesion molecule-1 (ICAM-1) in the generation of an immune response. Eur J Immunol. 1988 Jan;18(1):35–39. doi: 10.1002/eji.1830180107. [DOI] [PubMed] [Google Scholar]
- Fleischer B. A novel pathway of human T cell activation via a 103 kD T cell activation antigen. J Immunol. 1987 Mar 1;138(5):1346–1350. [PubMed] [Google Scholar]
- Forman J., Vitetta E. S., Hart D. A., Klein J. Relationship between trinitrophenyl and H-2 antigens on trinitrophenyl-modified spleen cells. I. H-2 antigens on cells treated with trinitrobenzene sulfonic acid are derivatized. J Immunol. 1977 Mar;118(3):797–802. [PubMed] [Google Scholar]
- Frohman M., Cowing C. Presentation of antigen by B cells: functional dependence on radiation dose, interleukins, cellular activation, and differential glycosylation. J Immunol. 1985 Apr;134(4):2269–2275. [PubMed] [Google Scholar]
- Geppert T. D., Lipsky P. E. Antigen presentation by interferon-gamma-treated endothelial cells and fibroblasts: differential ability to function as antigen-presenting cells despite comparable Ia expression. J Immunol. 1985 Dec;135(6):3750–3762. [PubMed] [Google Scholar]
- Glimcher L. H., Kim K. J., Green I., Paul W. E. Ia antigen-bearing B cell tumor lines can present protein antigen and alloantigen in a major histocompatibility complex-restricted fashion to antigen-reactive T cells. J Exp Med. 1982 Feb 1;155(2):445–459. doi: 10.1084/jem.155.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke J. H., Cook R. G., Lopez L. A., Rich R. R. Differential antigen-presenting cell requirements of hapten-specific T cell lines restricted to class II determinants. J Immunol. 1987 Apr 15;138(8):2384–2391. [PubMed] [Google Scholar]
- Hara T., Fu S. M., Hansen J. A. Human T cell activation. II. A new activation pathway used by a major T cell population via a disulfide-bonded dimer of a 44 kilodalton polypeptide (9.3 antigen). J Exp Med. 1985 Jun 1;161(6):1513–1524. doi: 10.1084/jem.161.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylowicz C. M., Unanue E. R. Regulation of antigen-presentation-I. IFN-gamma induces antigen-presenting properties on B cells. J Immunol. 1988 Dec 15;141(12):4083–4088. [PubMed] [Google Scholar]
- Helle M., Brakenhoff J. P., De Groot E. R., Aarden L. A. Interleukin 6 is involved in interleukin 1-induced activities. Eur J Immunol. 1988 Jun;18(6):957–959. doi: 10.1002/eji.1830180619. [DOI] [PubMed] [Google Scholar]
- Hirschberg H., Braathen L. R., Thorsby E. Antigen presentation by vascular endothelial cells and epidermal Langerhans cells: the role of HLA-DR. Immunol Rev. 1982;66:57–77. doi: 10.1111/j.1600-065x.1982.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. P., Wank R. Identification of two cis-encoded HLA-DQ molecules that carry distinct alloantigenic specificities. J Exp Med. 1984 Nov 1;160(5):1350–1359. doi: 10.1084/jem.160.5.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapsenberg M. L., Van der Pouw-Kraan T., Stiekema F. E., Schootemeijer A., Bos J. D. Direct and indirect nickel-specific stimulation of T lymphocytes from patients with allergic contact dermatitis to nickel. Eur J Immunol. 1988 Jul;18(7):977–982. doi: 10.1002/eji.1830180702. [DOI] [PubMed] [Google Scholar]
- Karr R. W., Alber C., Goyert S. M., Silver J., Duquesnoy R. J. The complexity of HLA-DS molecules. A homozygous cell line expresses multiple HLA-DS alpha chains. J Exp Med. 1984 May 1;159(5):1512–1531. doi: 10.1084/jem.159.5.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Liano D., HayGlass K. A., Benacerraf B., Sy M. S., Abbas A. K. The role of antigen-presenting B cells in T cell priming in vivo. Studies of B cell-deficient mice. J Immunol. 1988 Jun 1;140(11):3773–3778. [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985 Apr 11;314(6011):537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Matsui M., Ariga H., Kakiuchi T., Nariuchi H. Dissociation between the expression of IL 2 receptor and IL 2 receptor mRNA in the antigen-specific T cell clone stimulated by the specific antigen with B cell APC. J Immunol. 1987 Jul 15;139(2):373–379. [PubMed] [Google Scholar]
- Metlay J. P., Puré E., Steinman R. M. Distinct features of dendritic cells and anti-Ig activated B cells as stimulators of the primary mixed leukocyte reaction. J Exp Med. 1989 Jan 1;169(1):239–254. doi: 10.1084/jem.169.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Pope J. H. Assay of the infectivity of Epstein-Barr virus by transformation of human leucocytes in vitro. J Gen Virol. 1972 Nov;17(2):233–236. doi: 10.1099/0022-1317-17-2-233. [DOI] [PubMed] [Google Scholar]
- Palacios R. Mechanism of T cell activation: role and functional relationship of HLA-DR antigens and interleukins. Immunol Rev. 1982;63:73–110. doi: 10.1111/j.1600-065x.1982.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Res P., Kapsenberg M. L., Bos J. D., Stiekema F. The crucial role of human dendritic antigen-presenting cell subsets in nickel-specific T-cell proliferation. J Invest Dermatol. 1987 May;88(5):550–554. doi: 10.1111/1523-1747.ep12470142. [DOI] [PubMed] [Google Scholar]
- Ron Y., Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987 May 1;138(9):2848–2856. [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala G., Oppenheim J. J. Antigen presentation by human monocytes: evidence for stimulant processing and requirement for interleukin 1. J Immunol. 1983 Sep;131(3):1160–1166. [PubMed] [Google Scholar]
- Shimonkevitz R., Kappler J., Marrack P., Grey H. Antigen recognition by H-2-restricted T cells. I. Cell-free antigen processing. J Exp Med. 1983 Aug 1;158(2):303–316. doi: 10.1084/jem.158.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Yssel H., Takebe Y., Arai N., Yokota T., Lee F., Arai K., Banchereau J., de Vries J. E. Recombinant interleukin 4 promotes the growth of human T cells. J Immunol. 1987 Aug 15;139(4):1142–1147. [PubMed] [Google Scholar]
- Steinman R. M., Nussenzweig M. C. Dendritic cells: features and functions. Immunol Rev. 1980;53:127–147. doi: 10.1111/j.1600-065x.1980.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Zlotnik A., Fischer M., Roehm N., Zipori D. Evidence for effects of interleukin 4 (B cell stimulatory factor 1) on macrophages: enhancement of antigen presenting ability of bone marrow-derived macrophages. J Immunol. 1987 Jun 15;138(12):4275–4279. [PubMed] [Google Scholar]
- Zlotnik A., Shimonkevitz R. P., Gefter M. L., Kappler J., Marrack P. Characterization of the gamma-interferon-mediated induction of antigen-presenting ability in P388D1 cells. J Immunol. 1983 Dec;131(6):2814–2820. [PubMed] [Google Scholar]


