Abstract
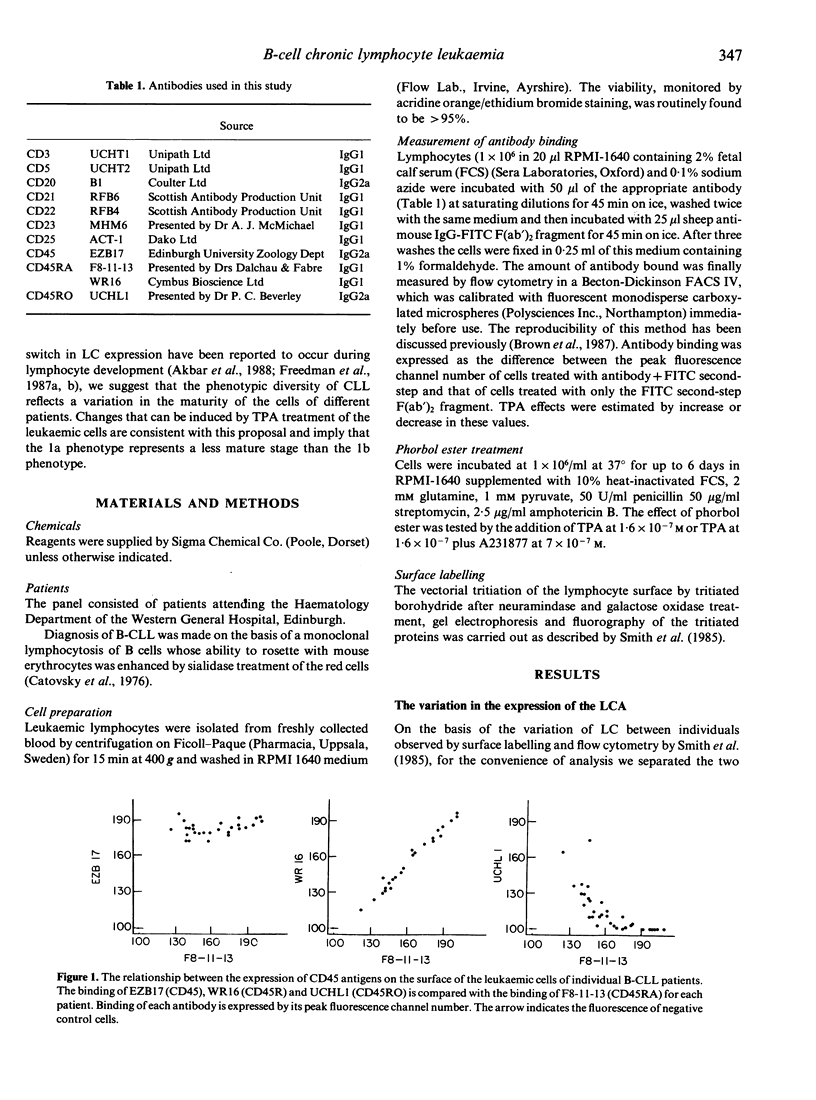
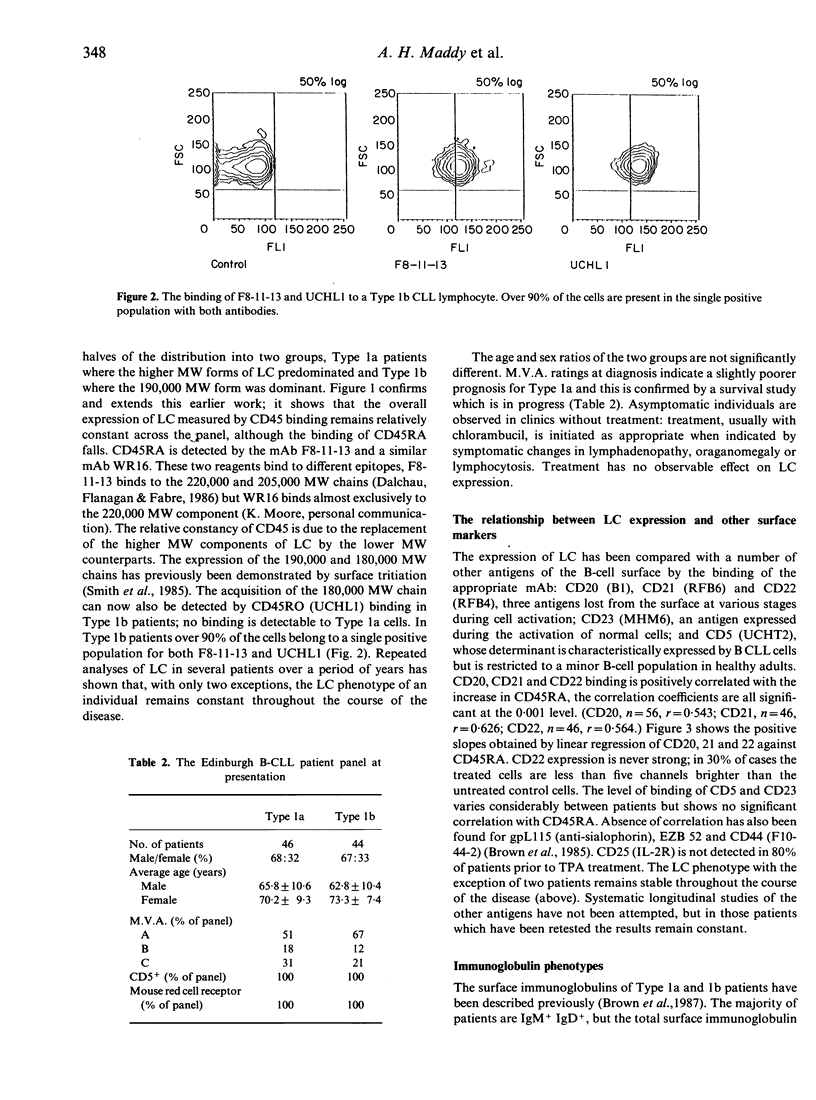
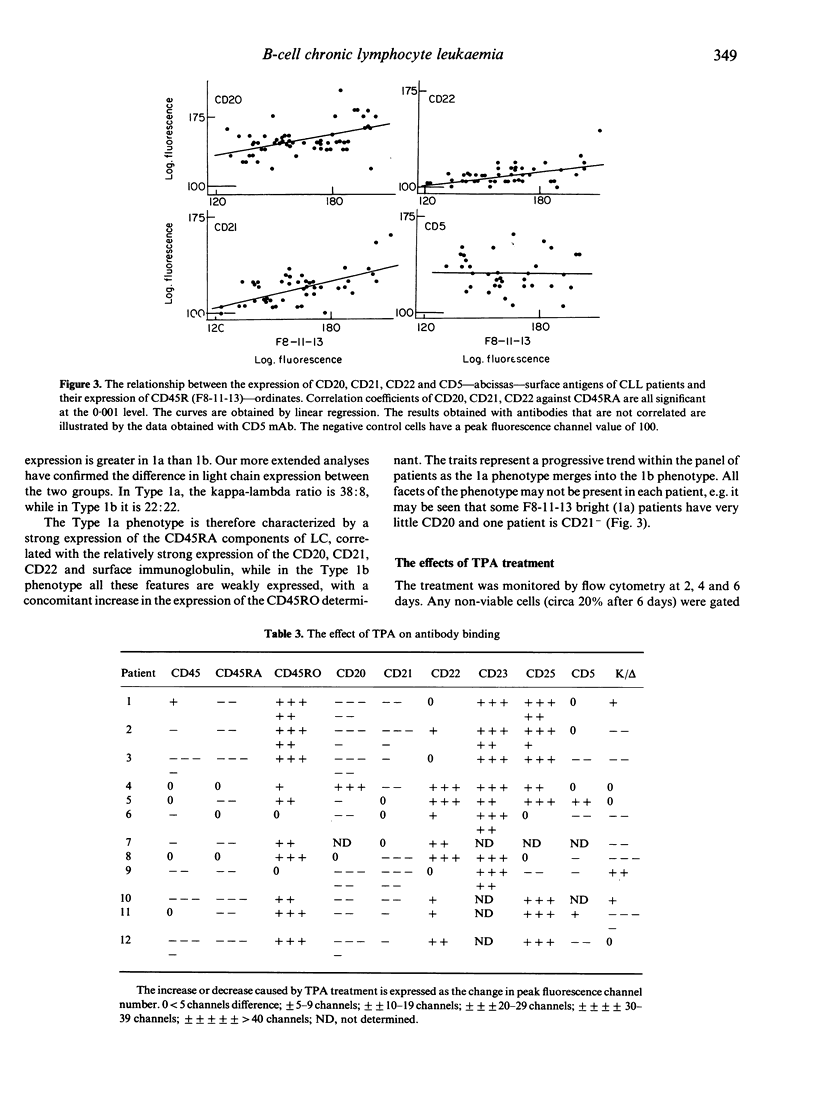
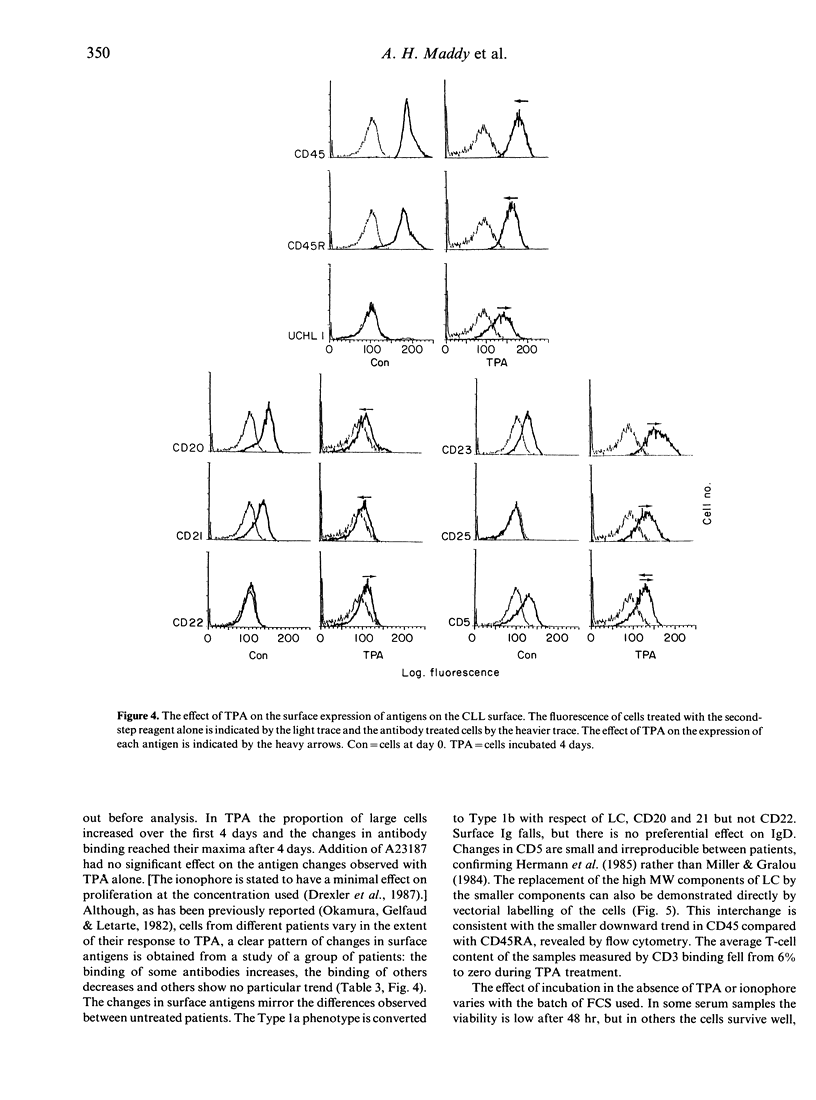
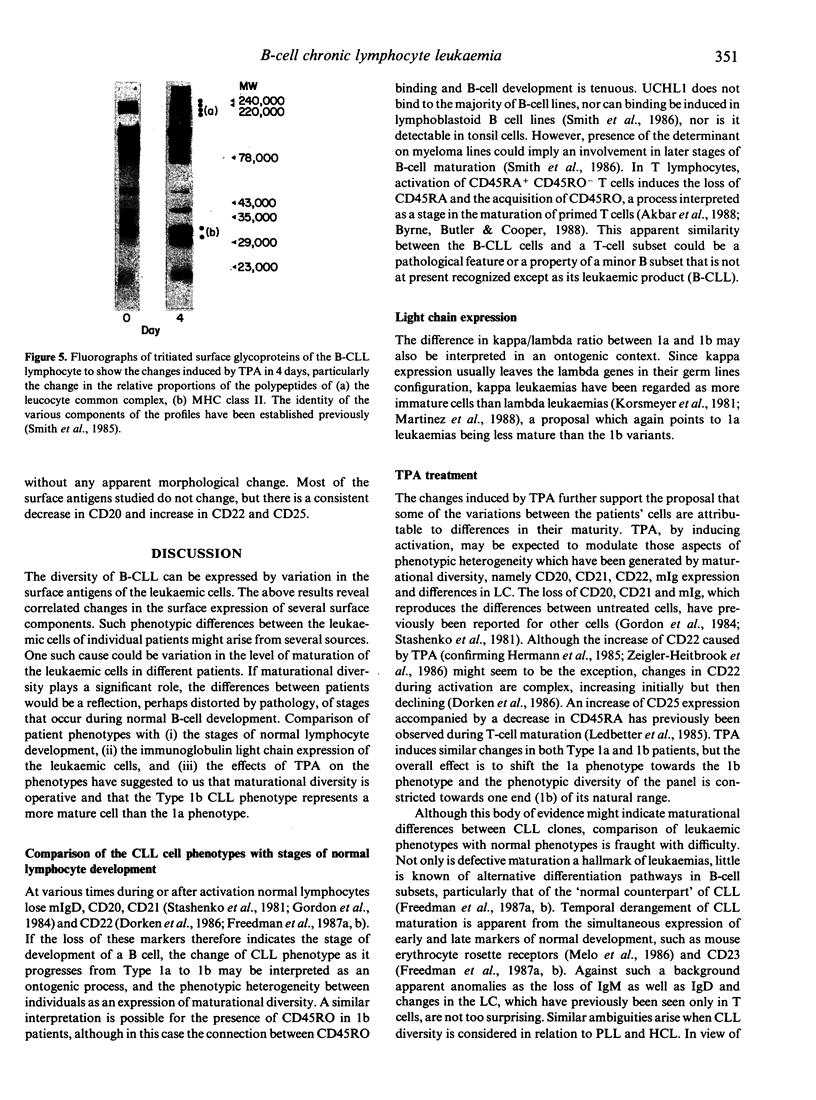
B-chronic lymphocytic leukaemia (B-CLL) patients can be ranked along a progression of phenotypes characterized by a decreasing surface expression of CD20, CD21, CD22 and membrane immunoglobulin and a gradual replacement of the high molecular weight (MW) glycoproteins of the leucocyte-common antigen (LC) CD45RA by the lower MW components, including the CD45RO determinant. As CD20, CD21, CD22 and membrane immunoglobulin change during or after B-cell activation, and the CD45RA/CD45RO inversion is implicated in T-cell maturation, the possibility that the phenotypic differences are generated by a maturational diversity of the CLL clones has been investigated by testing the effects of TPA treatment of the leukaemic cells. TPA reduces the level of expression of CD20, CD21, mIg and CD45RA and increases CD45RO binding, thereby minimizing the phenotypic heterogeneity of the CLL clones and causing them to converge towards one end of the natural range. We propose that the phenotypic diversity in CLL is, at least in part, a consequence of maturational diversity where lymphocyte development is disrupted at different stages in different patients.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Terry L., Timms A., Beverley P. C., Janossy G. Loss of CD45R and gain of UCHL1 reactivity is a feature of primed T cells. J Immunol. 1988 Apr 1;140(7):2171–2178. [PubMed] [Google Scholar]
- Brown V. A., Smith S. K., Dewar A. E., Stockdill G., Maddy A. H. Surface glycoproteins as markers of the cellular status of B chronic lymphocytic leukaemia lymphocytes. Clin Exp Immunol. 1985 Oct;62(1):95–103. [PMC free article] [PubMed] [Google Scholar]
- Brown V., Smith S., Dewar E., Maddy A. The correlation between surface immunoglobulin expression and the leucocyte-common antigen in B-cell chronic lymphocytic leukaemia. Leuk Res. 1987;11(10):903–910. doi: 10.1016/0145-2126(87)90136-6. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Butler J. L., Cooper M. D. Differential activation requirements for virgin and memory T cells. J Immunol. 1988 Nov 15;141(10):3249–3257. [PubMed] [Google Scholar]
- Caligaris-Cappio F., Bergui L., Rege-Cambrin G., Tesio L., Migone N., Malavasi F. Phenotypic, cytogenetic and molecular characterization of a new B-chronic lymphocytic leukaemia (B-CLL) cell line. Leuk Res. 1987;11(7):579–588. doi: 10.1016/0145-2126(87)90029-4. [DOI] [PubMed] [Google Scholar]
- Catovsky D., Cherchi M., Okos A., Hegde U., Galton D. A. Mouse red-cell rosettes in B-lymphoproliferative disorders. Br J Haematol. 1976 Jun;33(2):173–177. doi: 10.1111/j.1365-2141.1976.tb03528.x. [DOI] [PubMed] [Google Scholar]
- Dalchau R., Flanagan B. F., Fabre J. W. Structural implications of the location and stability to proteolytic enzymes of immunodominant determinants of the human leukocyte common molecule. Eur J Immunol. 1986 Aug;16(8):993–999. doi: 10.1002/eji.1830160820. [DOI] [PubMed] [Google Scholar]
- Drexler H. G., Brenner M. K., Coustan-Smith E., Wickremasinghe R. G., Hoffbrand A. V. Synergistic action of calcium ionophore A23187 and phorbol ester TPA on B-chronic lymphocytic leukemia cells. Blood. 1987 Nov;70(5):1536–1542. [PubMed] [Google Scholar]
- Dörken B., Moldenhauer G., Pezzutto A., Schwartz R., Feller A., Kiesel S., Nadler L. M. HD39 (B3), a B lineage-restricted antigen whose cell surface expression is limited to resting and activated human B lymphocytes. J Immunol. 1986 Jun 15;136(12):4470–4479. [PubMed] [Google Scholar]
- Freedman A. S., Boyd A. W., Bieber F. R., Daley J., Rosen K., Horowitz J. C., Levy D. N., Nadler L. M. Normal cellular counterparts of B cell chronic lymphocytic leukemia. Blood. 1987 Aug;70(2):418–427. [PubMed] [Google Scholar]
- Gale R. P., Foon K. A. Biology of chronic lymphocytic leukemia. Semin Hematol. 1987 Oct;24(4):209–229. [PubMed] [Google Scholar]
- Gordon J., Mellstedt H., Aman P., Biberfeld P., Klein G. Phenotypic modulation of chronic lymphocytic leukemia cells by phorbol ester: induction of IgM secretion and changes in the expression of B cell-associated surface antigens. J Immunol. 1984 Jan;132(1):541–547. [PubMed] [Google Scholar]
- Herrmann F., Dörken B., Ludwig W. D., Schwarting R. A comparison of membrane marker phenotypes in hairy-cell leukemia and phorbol-ester induced B-cll cells using monoclonal antibodies. Leuk Res. 1985;9(5):529–536. doi: 10.1016/0145-2126(85)90132-8. [DOI] [PubMed] [Google Scholar]
- Korsmeyer S. J., Hieter P. A., Ravetch J. V., Poplack D. G., Waldmann T. A., Leder P. Developmental hierarchy of immunoglobulin gene rearrangements in human leukemic pre-B-cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7096–7100. doi: 10.1073/pnas.78.11.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Rose L. M., Spooner C. E., Beatty P. G., Martin P. J., Clark E. A. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL 2 receptor expression. J Immunol. 1985 Sep;135(3):1819–1825. [PubMed] [Google Scholar]
- Martinez G., Ferreira R., Hernandez A., Hernandez P., Cruz C., Fernandez O., Lagarde M., Felicetti L., Colombo B. The molecular heterogeneity of chronic lymphocytic leukemia: further data. Haematologica. 1988 May-Jun;73(3):169–172. [PubMed] [Google Scholar]
- Melo J. V., Wardle J., Chetty M., England J., Lewis S. M., Galton D. A., Catovsky D. The relationship between chronic lymphocytic leukaemia and prolymphocytic leukaemia. III. Evaluation of cell size by morphology and volume measurements. Br J Haematol. 1986 Nov;64(3):469–478. doi: 10.1111/j.1365-2141.1986.tb02202.x. [DOI] [PubMed] [Google Scholar]
- Merson A., Brochier J. Phenotypic heterogeneity of B cell chronic lymphocytic leukaemia. Immunol Lett. 1988 Dec;19(4):269–271. doi: 10.1016/0165-2478(88)90153-8. [DOI] [PubMed] [Google Scholar]
- Miller R. A., Gralow J. The induction of Leu-1 antigen expression in human malignant and normal B cells by phorbol myristic acetate (PMA). J Immunol. 1984 Dec;133(6):3408–3414. [PubMed] [Google Scholar]
- Okamura J., Gelfand E. W., Letarte M. Heterogeneity of the response of chronic lymphocytic leukemia cells to phorbol ester. Blood. 1982 Nov;60(5):1082–1088. [PubMed] [Google Scholar]
- Roxburgh A. E., Cooper I. A. Expression of leucocyte-common antigen and large sialoglycoprotein on leukemic cells in B-cell chronic lymphocytic leukemia and non-Hodgkin's lymphoma. Leuk Res. 1987;11(10):891–901. doi: 10.1016/0145-2126(87)90135-4. [DOI] [PubMed] [Google Scholar]
- Smith S. K., Brown V. A., Dewar A. E., Stockdill G., Cohen B., Maddy A. H. Abnormalities in the expression of the leucocyte-common antigen in chronic lymphocytic leukaemia. Clin Exp Immunol. 1985 Jan;59(1):55–63. [PMC free article] [PubMed] [Google Scholar]
- Stashenko P., Nadler L. M., Hardy R., Schlossman S. F. Expression of cell surface markers after human B lymphocyte activation. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3848–3852. doi: 10.1073/pnas.78.6.3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
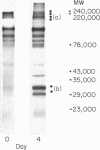
- Terry L. A., Brown M. H., Beverley P. C. The monoclonal antibody, UCHL1, recognizes a 180,000 MW component of the human leucocyte-common antigen, CD45. Immunology. 1988 Jun;64(2):331–336. [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Munker R., Dörken B., Gaedicke G., Thiel E. Induction of features characteristic of hairy cell leukemia in chronic lymphocytic leukemia and prolymphocytic leukemia cells. Cancer Res. 1986 Apr;46(4 Pt 2):2172–2178. [PubMed] [Google Scholar]