Abstract
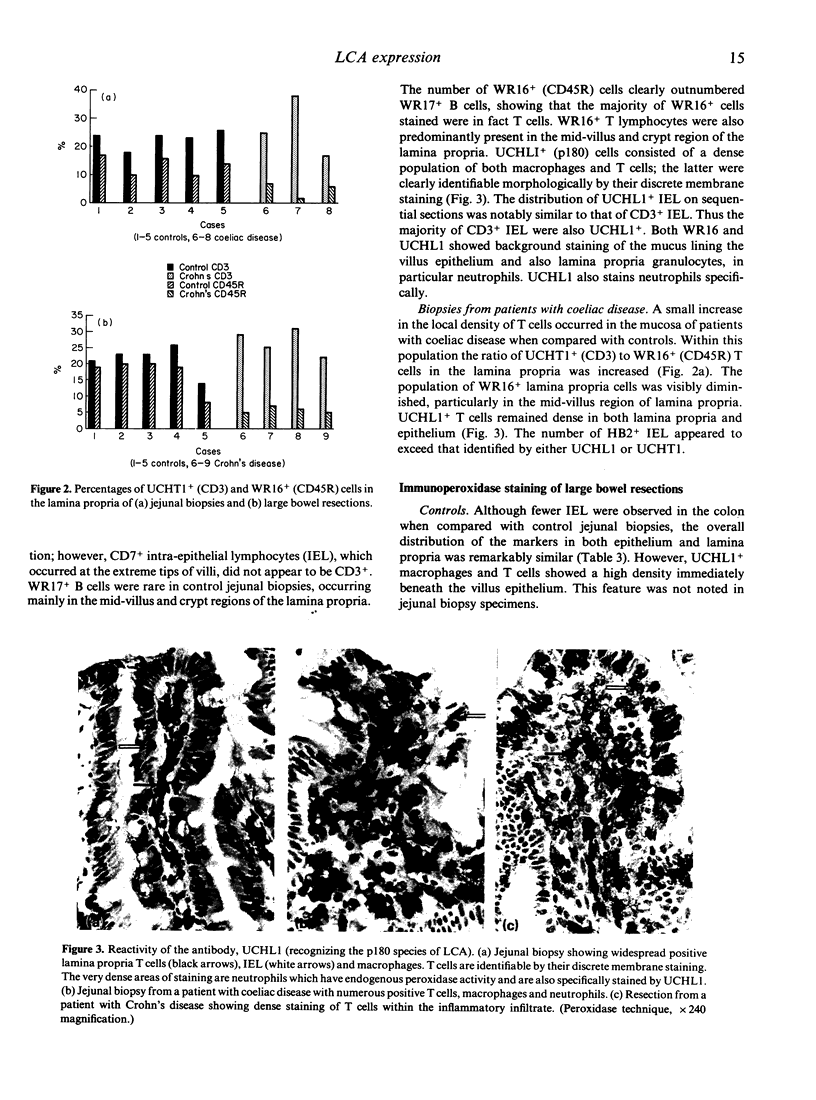
The expression of the 220,000 MW (p220) glycoprotein component of the leucocyte common antigen (LCA) family by intestinal mucosal lymphocytes was studied using the CD45R monoclonal antibody WR16. In normal intestine, a proportion of CD3+ mucosal T cells were WR16+ and this population resided predominantly in the mid-villus and crypt region of lamina propria. In the inflammatory infiltrates of both coeliac disease and Crohn's disease the CD3+, WR16+ population was markedly reduced. The monoclonal antibody UCHL1 identifies the 180,000 MW member of the LCA family and is expressed on T cells and in macrophages. CD3+ lymphocytes expressing this marker were widespread in normal lamina propria and epithelium. In contrast with WR16, UCHL1+ cells remained at a high level in coeliac disease and Crohn's disease. Our results support the view that loss of the p220 molecule occurs upon T-cell activation in inflammation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverley P. C., Callard R. E. Distinctive functional characteristics of human "T" lymphocytes defined by E rosetting or a monoclonal anti-T cell antibody. Eur J Immunol. 1981 Apr;11(4):329–334. doi: 10.1002/eji.1830110412. [DOI] [PubMed] [Google Scholar]
- Beverley P. C. Human T cell subsets. Immunol Lett. 1987 Apr;14(4):263–267. doi: 10.1016/0165-2478(87)90001-0. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N., Schneeberger E. E., Bhan A. K. Immunohistologic and immunoelectron microscopic characterization of the mucosal lymphocytes of human small intestine by the use of monoclonal antibodies. J Immunol. 1983 Jun;130(6):2615–2622. [PubMed] [Google Scholar]
- Dalchau R., Flanagan B. F., Fabre J. W. Structural implications of the location and stability to proteolytic enzymes of immunodominant determinants of the human leukocyte common molecule. Eur J Immunol. 1986 Aug;16(8):993–999. doi: 10.1002/eji.1830160820. [DOI] [PubMed] [Google Scholar]
- Elson C. O., Kagnoff M. F., Fiocchi C., Befus A. D., Targan S. Intestinal immunity and inflammation: recent progress. Gastroenterology. 1986 Sep;91(3):746–768. doi: 10.1016/0016-5085(86)90649-9. [DOI] [PubMed] [Google Scholar]
- Ferguson A., MacDonald T. T., McClure J. P., Holden R. J. Cell-mediated immunity to gliadin within the small-intestinal mucosa in coeliac disease. Lancet. 1975 Apr 19;1(7912):895–897. doi: 10.1016/s0140-6736(75)91689-x. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Eisenbarth G. S., Fauci A. S. Human lymphocyte antigens: production of a monoclonal antibody that defines functional thymus-derived lymphocyte subsets. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5829–5833. doi: 10.1073/pnas.76.11.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S. P., Fiocchi C., Graeff A. S., Strober W. Phenotypic analysis of lamina propria lymphocytes. Predominance of helper-inducer and cytolytic T-cell phenotypes and deficiency of suppressor-inducer phenotypes in Crohn's disease and control patients. Gastroenterology. 1986 Dec;91(6):1483–1489. [PubMed] [Google Scholar]
- Ledbetter J. A., Rose L. M., Spooner C. E., Beatty P. G., Martin P. J., Clark E. A. Antibodies to common leukocyte antigen p220 influence human T cell proliferation by modifying IL 2 receptor expression. J Immunol. 1985 Sep;135(3):1819–1825. [PubMed] [Google Scholar]
- Lefrançois L., Bevan M. J. Functional modifications of cytotoxic T-lymphocyte T200 glycoprotein recognized by monoclonal antibodies. Nature. 1985 Apr 4;314(6010):449–452. doi: 10.1038/314449a0. [DOI] [PubMed] [Google Scholar]
- Leigh R. J., Marsh M. N., Crowe P., Kelly C., Garner V., Gordon D. Studies of intestinal lymphoid tissue. IX. Dose-dependent, gluten-induced lymphoid infiltration of coeliac jejunal epithelium. Scand J Gastroenterol. 1985 Aug;20(6):715–719. doi: 10.3109/00365528509089201. [DOI] [PubMed] [Google Scholar]
- Marsh M. N. Studies of intestinal lymphoid tissue. III. Quantitative analyses of epithelial lymphocytes in the small intestine of human control subjects and of patients with celiac sprue. Gastroenterology. 1980 Sep;79(3):481–492. [PubMed] [Google Scholar]
- Moore K., Cooper S. A., Jones D. B. Use of the monoclonal antibody WR17, identifying the CD37 gp40-45 Kd antigen complex, in the diagnosis of B-lymphoid malignancy. J Pathol. 1987 May;152(1):13–21. doi: 10.1002/path.1711520103. [DOI] [PubMed] [Google Scholar]
- Moore K., Nesbitt A. M. Functional heterogeneity of CD4+ T lymphocytes: two subpopulations with counteracting immunoregulatory functions identified with the monoclonal antibodies WR16 and WR19. Immunology. 1987 Jun;61(2):159–165. [PMC free article] [PubMed] [Google Scholar]
- Moore K., Nesbitt A. M. Identification and isolation of OKT4+ suppressor cells with the monoclonal antibody WR16. Immunology. 1986 Aug;58(4):659–664. [PMC free article] [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Rudd C. E., Hagan M., Takeuchi T., Schlossman S. F. The role of the 2H4 molecule in the generation of suppressor function in Con A-activated T cells. J Immunol. 1986 Nov 15;137(10):3247–3253. [PubMed] [Google Scholar]
- Rudd C. E., Morimoto C., Wong L. L., Schlossman S. F. The subdivision of the T4 (CD4) subset on the basis of the differential expression of L-C/T200 antigens. J Exp Med. 1987 Dec 1;166(6):1758–1773. doi: 10.1084/jem.166.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby W. S., Janossy G., Bofill M., Jewell D. P. Lymphocyte subpopulations in the human small intestine. The findings in normal mucosa and in the mucosa of patients with adult coeliac disease. Clin Exp Immunol. 1983 Apr;52(1):219–228. [PMC free article] [PubMed] [Google Scholar]
- Shanahan F., Deem R., Nayersina R., Leman B., Targan S. Human mucosal T-cell cytotoxicity. Gastroenterology. 1988 Apr;94(4):960–967. doi: 10.1016/0016-5085(88)90554-9. [DOI] [PubMed] [Google Scholar]
- Stein H., Bonk A., Tolksdorf G., Lennert K., Rodt H., Gerdes J. Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas. J Histochem Cytochem. 1980 Aug;28(8):746–760. doi: 10.1177/28.8.7003001. [DOI] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Malizia G., Badr-el-Din S., Smart C. J., Oakes D. J., Southgate J., Howdle P. D., Janossy G., Poulter L. W., Losowsky M. S. T cell and mononuclear phagocyte populations of the human small and large intestine. Adv Exp Med Biol. 1987;216A:465–473. doi: 10.1007/978-1-4684-5344-7_54. [DOI] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]



