Abstract
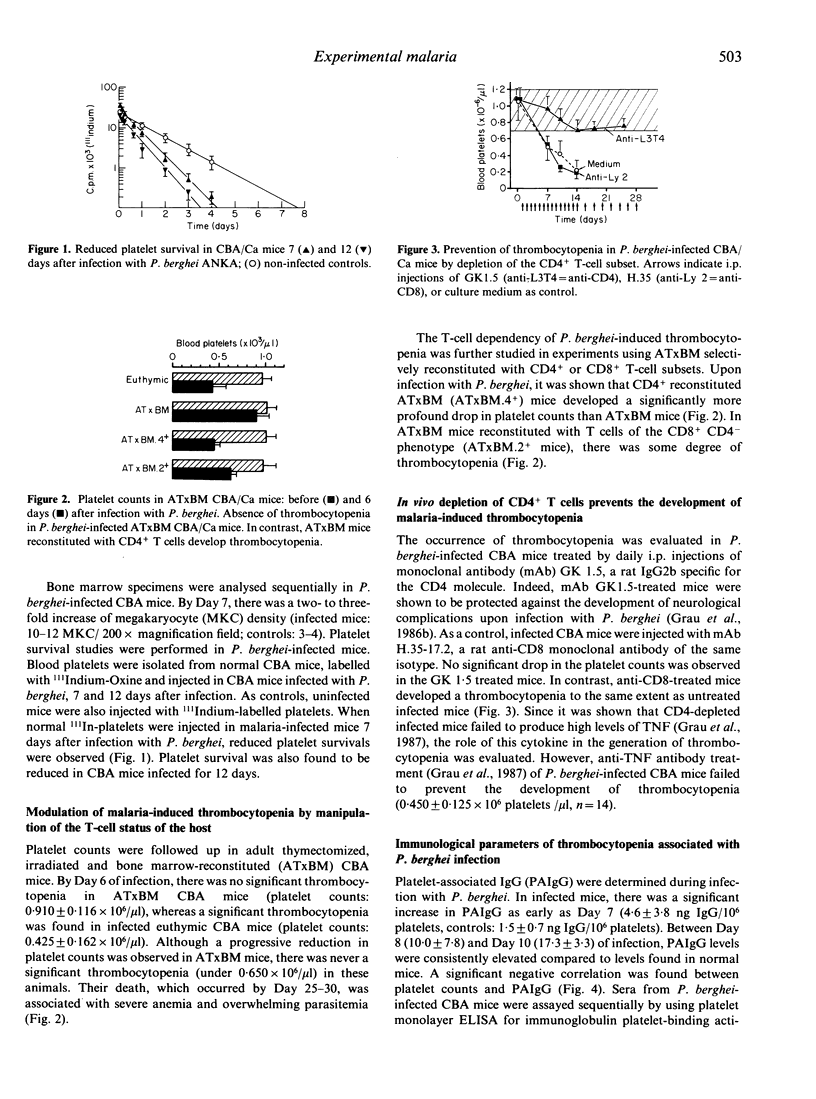
An early thrombocytopenia was observed in CBA mice during acute infection with Plasmodium berghei. This was associated with an increase in bone marrow megakaryocytes and a reduction of normal syngeneic 111Indium-labelled platelet life span. Malaria-induced thrombocytopenia was thus considered to be the result of increased peripheral platelet destruction rather than central hypoproduction. The occurrence of thrombocytopenia was modulated by T-cell depletion. Indeed, thymectomized, irradiated or anti-CD4 monoclonal antibody-treated mice failed to develop thrombocytopenia, although they were infected to the same extent. Conversely, a significant thrombocytopenia was observed in thymectomized mice reconstituted with CD4+ T cells. During the course of infection, a significant inverse correlation was found between platelet counts and platelet-associated IgG. Normal mice passively transferred with serum from syngeneic malaria-infected mice developed thrombocytopenia. The possibility to raise monoclonal anti-platelet antibodies from P. berghei-infected animals further suggested a role for an antibody-mediated platelet destruction during acute murine malaria infection. These results indicate that in murine malaria, thrombocytopenia is mediated by immune mechanisms and that CD4+ T cells might be significantly involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale P. J., Cormack J. D., Oldrey T. B. Thrombocytopenia in malaria with immunoglobulin (IgM) changes. Br Med J. 1972 Feb 5;1(5796):345–349. doi: 10.1136/bmj.1.5796.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borochovitz D., Crosley A. L., Metz J. Disseminated intravascular coagulation with fatal haemorrhage in cerebral malaria. Br Med J. 1970 Jun 20;2(5711):710–710. doi: 10.1136/bmj.2.5711.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler T., Tong M. J., Fletcher J. R., Dostalek R. J., Robbins T. O. Blood coagulation studies in Plasmodium falciparum malaria. Am J Med Sci. 1973 Jan;265(1):63–67. doi: 10.1097/00000441-197301000-00006. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Morrow D., Izui S., Lambert P. H. Pathogenesis of the delayed phase of Rauscher virus-induced thrombocytopenia. J Immunol. 1986 Jan;136(2):686–691. [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Engers H. D., Louis J. A., Vassalli P., Lambert P. H. L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol. 1986 Oct 1;137(7):2348–2354. [PubMed] [Google Scholar]
- Horstmann R. D., Dietrich M., Bienzle U., Rasche H. Malaria-induced thrombocytopenia. Blut. 1981 Mar;42(3):157–164. doi: 10.1007/BF01026385. [DOI] [PubMed] [Google Scholar]
- Izui S., Eisenberg R. A., Dixon F. J. IgM rheumatoid factors in mice injected with bacterial lipopolysaccharides. J Immunol. 1979 May;122(5):2096–2102. [PubMed] [Google Scholar]
- Jayawardena A. N., Targett G. A., Carter R. L., Leuchars E., Davies A. J. The immunological response of CBA mice to P. yoelii. I. General characteristics, the effects of T-cell deprivation and reconstitution with thymus grafts. Immunology. 1977 Jun;32(6):849–859. [PMC free article] [PubMed] [Google Scholar]
- Kelton J. G., Keystone J., Moore J., Denomme G., Tozman E., Glynn M., Neame P. B., Gauldie J., Jensen J. Immune-mediated thrombocytopenia of malaria. J Clin Invest. 1983 Apr;71(4):832–836. doi: 10.1172/JCI110836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelton J. G. The measurement of platelet-bound immunoglobulins: an overview of the methods and the biological relevance of platelet-associated IgG. Prog Hematol. 1983;13:163–199. [PubMed] [Google Scholar]
- Pierres M., Goridis C., Golstein P. Inhibition of murine T cell-mediated cytolysis and T cell proliferation by a rat monoclonal antibody immunoprecipitating two lymphoid cell surface polypeptides of 94 000 and 180 000 molecular weight. Eur J Immunol. 1982 Jan;12(1):60–69. doi: 10.1002/eji.1830120112. [DOI] [PubMed] [Google Scholar]
- Shaw G. M., Axelson J., Maglott J. G., LoBuglio A. F. Quantification of platelet-bound IgG by 125I-Staphylococcal protein A in immune thrombocytopenic purpura and other thrombocytopenic disorders. Blood. 1984 Jan;63(1):154–161. [PubMed] [Google Scholar]
- Wilson J. J., Neame P. B., Kelton J. G. Infection-induced thrombocytopenia. Semin Thromb Hemost. 1982 Jul;8(3):217–233. doi: 10.1055/s-2007-1005053. [DOI] [PubMed] [Google Scholar]


