Abstract
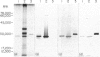
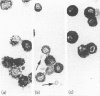
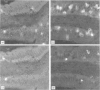
In order to define further mast cell heterogeneity in the mouse, affinity-purified antibodies against a 28,000 MW serine proteinase from mouse intestinal mast cells (IMCP) and against rat mast cell proteinase I (RMCPI) were used to characterize mast cell cytoplasmic granules immunohistochemically. On Western blot, anti-IMCP cross-reacted with RMCPI and with a 25,000 MW antigen from isolated mouse serosal mast cells (SMC). Anti-RMCPI did not react with IMCP, although it identified the same 25,000 MW antigen from SMC. Isolated SMC (85-90% pure) lacked the 28,000 MW IMCP on Western blot, even though, immunohistochemically, the cells were stained with both anti-RMCPI and anti-IMCP. Anti-IMCP stained the granules of more than 85% of all mast cells detected with toluidine blue in the tongue or gastrointestinal mucosa. The specificity of anti-RMCPI which, in the rat, detects very few mucosal mast cells was almost identical to that of anti-IMCP for murine tongue and gastric and large intestinal mucosae, but a significant proportion of cells in distal jejunal, ileal and caecal mucosae were not stained with this antibody. The immunohistochemistry of the large numbers of mast cells recruited to jejunum following infection 10 days previously with 300 Trichinella spiralis muscle larvae was similar to that of uninfected control mice. The results show that considerable mast cell heterogeneity exists within the gastrointestinal mucosa of the mouse and indicate that there are both similarities and differences between mouse and rat in the distribution of mast cells and of their granule proteinases.
Full text
PDF
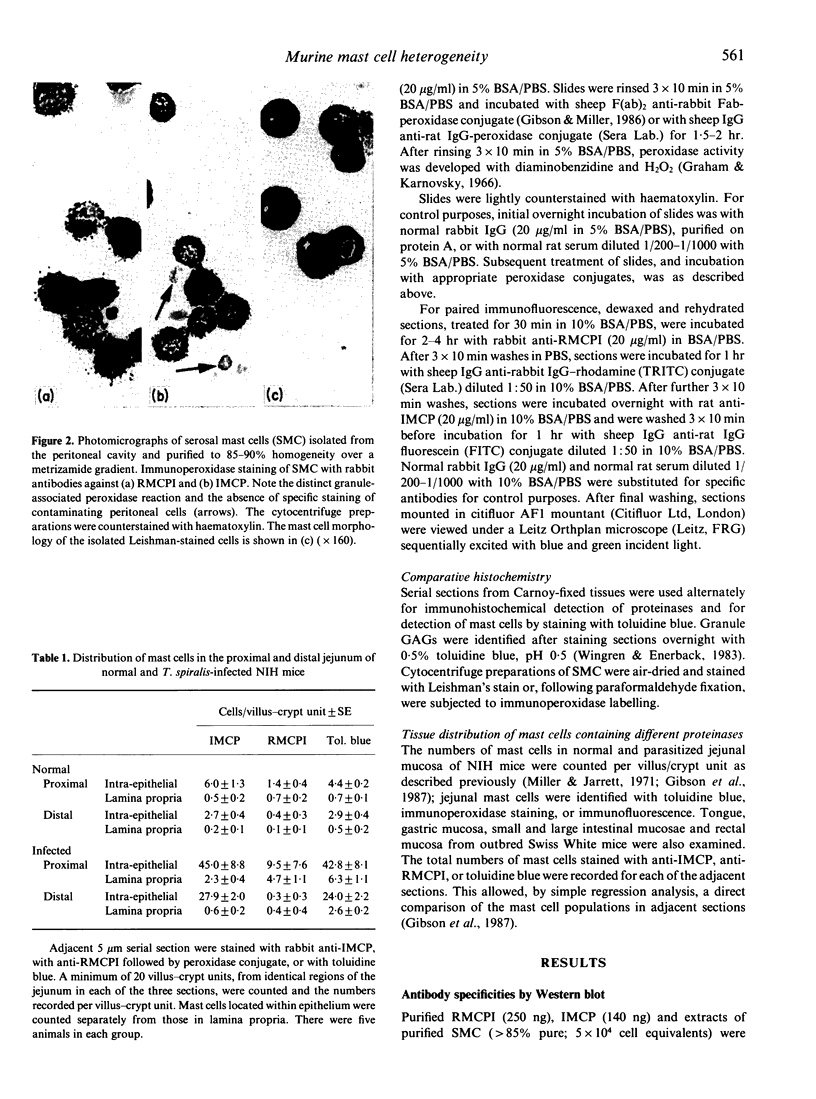


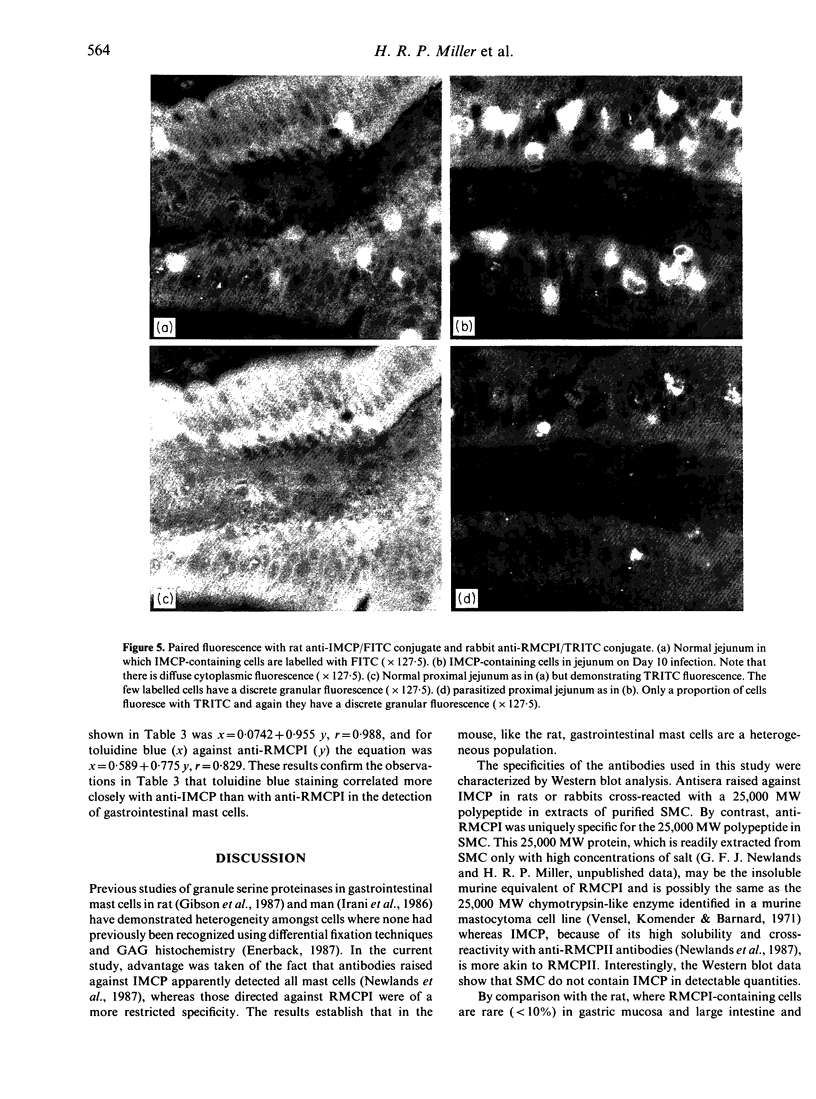
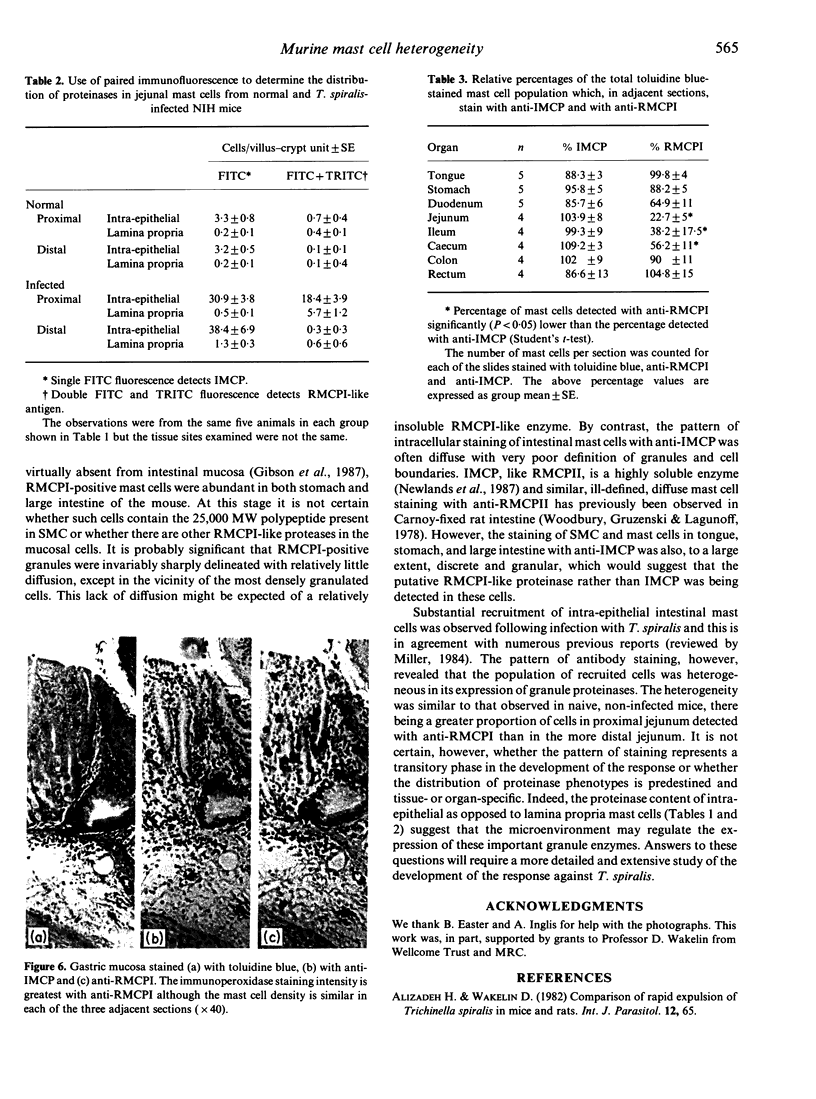

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizadeh H., Wakelin D. Comparison of rapid expulsion of Trichinella spiralis in mice and rats. Int J Parasitol. 1982 Feb;12(1):65–73. doi: 10.1016/0020-7519(82)90097-2. [DOI] [PubMed] [Google Scholar]
- Befus A. D., Pearce F. L., Gauldie J., Horsewood P., Bienenstock J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J Immunol. 1982 Jun;128(6):2475–2480. [PubMed] [Google Scholar]
- Crowle P. K., Phillips D. E. Characteristics of mast cells in Chediak-Higashi mice: light and electron microscopic studies of connective tissue and mucosal mast cells. Exp Cell Biol. 1983;51(3):130–139. doi: 10.1159/000163183. [DOI] [PubMed] [Google Scholar]
- Davidson S., Mansour A., Gallily R., Smolarski M., Rofolovitch M., Ginsburg H. Mast cell differentiation depends on T cells and granule synthesis on fibroblasts. Immunology. 1983 Mar;48(3):439–452. [PMC free article] [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. I. Effects of fixation. Acta Pathol Microbiol Scand. 1966;66(3):289–302. doi: 10.1111/apm.1966.66.3.289. [DOI] [PubMed] [Google Scholar]
- Enerbäck L. Mucosal mast cells in the rat and in man. Int Arch Allergy Appl Immunol. 1987;82(3-4):249–255. doi: 10.1159/000234199. [DOI] [PubMed] [Google Scholar]
- Gibson S., Mackeller A., Newlands G. F., Miller H. R. Phenotypic expression of mast cell granule proteinases. Distribution of mast cell proteinases I and II in the rat digestive system. Immunology. 1987 Dec;62(4):621–627. [PMC free article] [PubMed] [Google Scholar]
- Gibson S., Miller H. R. Mast cell subsets in the rat distinguished immunohistochemically by their content of serine proteinases. Immunology. 1986 May;58(1):101–104. [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hamaguchi Y., Kanakura Y., Fujita J., Takeda S., Nakano T., Tarui S., Honjo T., Kitamura Y. Interleukin 4 as an essential factor for in vitro clonal growth of murine connective tissue-type mast cells. J Exp Med. 1987 Jan 1;165(1):268–273. doi: 10.1084/jem.165.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyderman E., Neville A. M. A shorter immunoperoxidase technique for the demonstration of carcinoembryonic antigen and other cell products. J Clin Pathol. 1977 Feb;30(2):138–140. doi: 10.1136/jcp.30.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani A. A., Schechter N. M., Craig S. S., DeBlois G., Schwartz L. B. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4464–4468. doi: 10.1073/pnas.83.12.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y., Kanakura Y., Sonoda S., Asai H., Nakano T. Mutual phenotypic changes between connective tissue type and mucosal mast cells. Int Arch Allergy Appl Immunol. 1987;82(3-4):244–248. doi: 10.1159/000234198. [DOI] [PubMed] [Google Scholar]
- Lee T. D., Swieter M., Bienenstock J., Befus A. D. Heterogeneity in mast cell populations. Clin Immunol Rev. 1985;4(2):143–199. [PubMed] [Google Scholar]
- Levi-Schaffer F., Dayton E. T., Austen K. F., Hein A., Caulfield J. P., Gravallese P. M., Liu F. T., Stevens R. L. Mouse bone marrow-derived mast cells cocultured with fibroblasts. Morphology and stimulation-induced release of histamine, leukotriene B4, leukotriene C4, and prostaglandin D2. J Immunol. 1987 Nov 15;139(10):3431–3441. [PubMed] [Google Scholar]
- Miller H. R., Jarrett W. F. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology. 1971 Mar;20(3):277–288. [PMC free article] [PubMed] [Google Scholar]
- Miller H. R. The protective mucosal response against gastrointestinal nematodes in ruminants and laboratory animals. Vet Immunol Immunopathol. 1984 May;6(1-2):167–259. doi: 10.1016/0165-2427(84)90051-5. [DOI] [PubMed] [Google Scholar]
- Newlands G. F., Gibson S., Knox D. P., Grencis R., Wakelin D., Miller H. R. Characterization and mast cell origin of a chymotrypsin-like proteinase isolated from intestines of mice infected with Trichinella spiralis. Immunology. 1987 Dec;62(4):629–634. [PMC free article] [PubMed] [Google Scholar]
- Newlands G. F., Huntley J. F., Miller H. R. Concomitant detection of mucosal mast cells and eosinophils in the intestines of normal and Nippostrongylus-immune rats. A re-evaluation of histochemical and immunocytochemical techniques. Histochemistry. 1984;81(6):585–589. doi: 10.1007/BF00489539. [DOI] [PubMed] [Google Scholar]
- Sredni B., Friedman M. M., Bland C. E., Metcalfe D. D. Ultrastructural, biochemical, and functional characteristics of histamine-containing cells cloned from mouse bone marrow: tentative identification as mucosal mast cells. J Immunol. 1983 Aug;131(2):915–922. [PubMed] [Google Scholar]
- Vensel W. H., Komender J., Barnard E. A. Non-pancreatic proteases of the chymotrypsin family. II. Two proteases from a mouse mast cell tumor. Biochim Biophys Acta. 1971 Nov 13;250(2):395–407. doi: 10.1016/0005-2744(71)90196-3. [DOI] [PubMed] [Google Scholar]
- Wingren U., Enerbäck L. Mucosal mast cells of the rat intestine: a re-evaluation of fixation and staining properties, with special reference to protein blocking and solubility of the granular glycosaminoglycan. Histochem J. 1983 Jun;15(6):571–582. doi: 10.1007/BF01954148. [DOI] [PubMed] [Google Scholar]
- Woodbury R. G., Gruzenski G. M., Lagunoff D. Immunofluorescent localization of a serine protease in rat small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2785–2789. doi: 10.1073/pnas.75.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]