Abstract
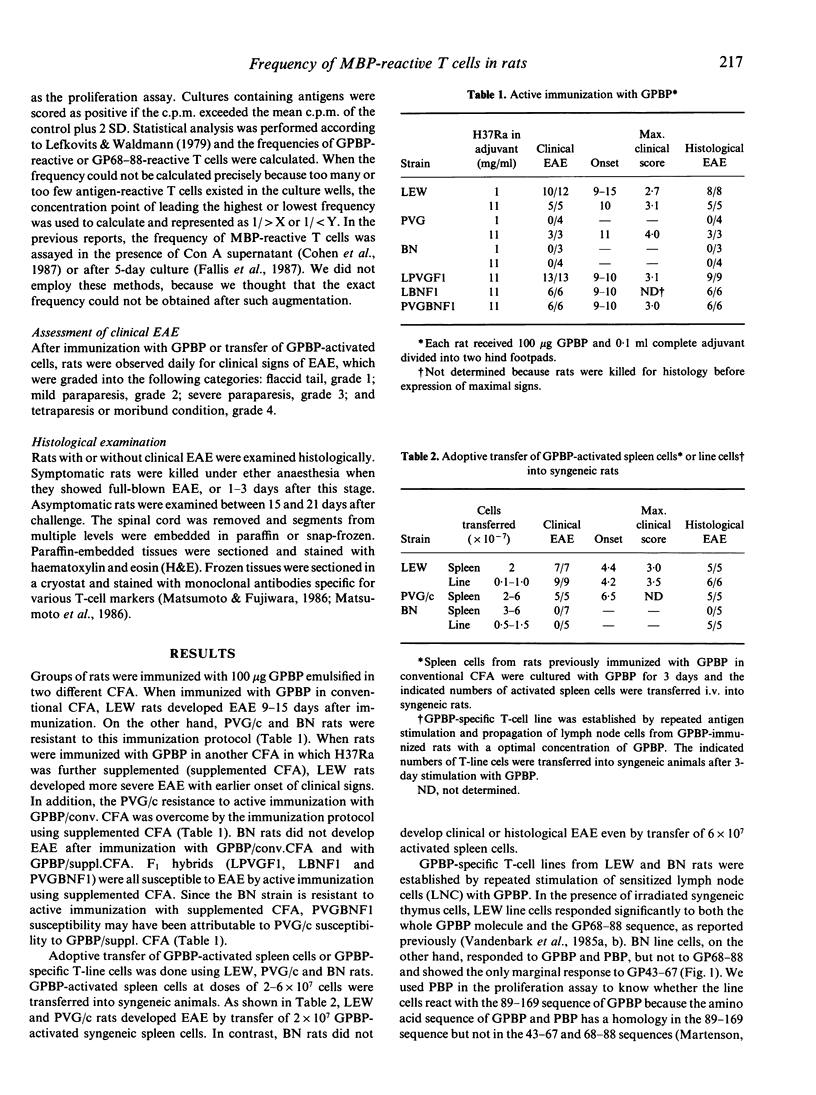
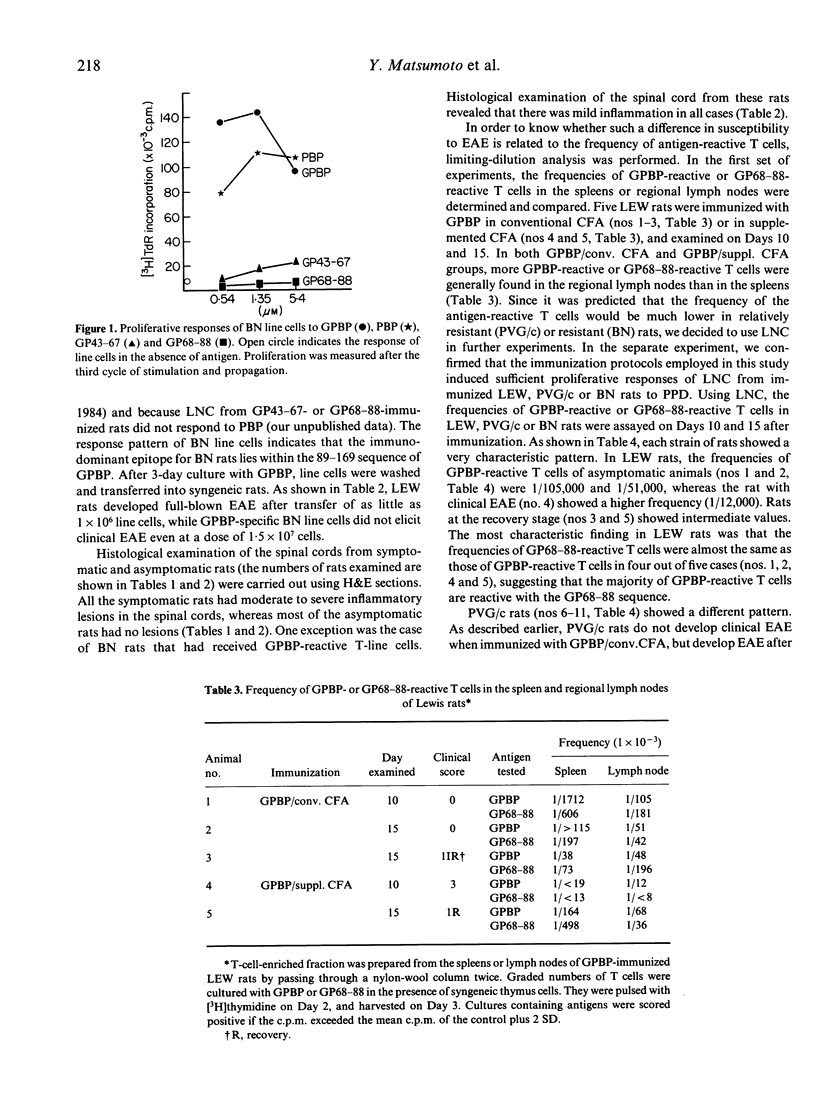
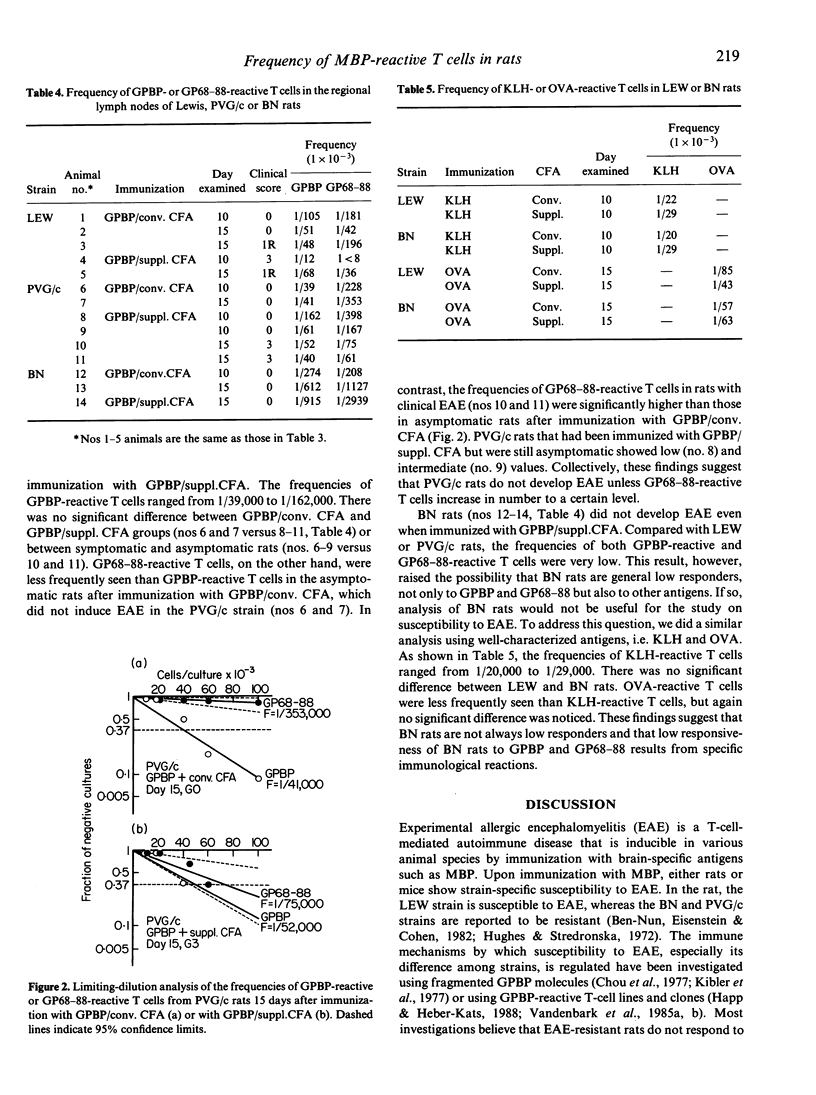
Susceptibility to experimental autoimmune encephalomyelitis (EAE), which is an autoimmune disease inducible by immunization with a brain-specific antigen in complete Freund's adjuvant (CFA), is different among strains. In an attempt to resolve the immune mechanisms by which the difference in susceptibility to EAE is regulated, we re-estimated susceptibility of several strains of rats, and the frequency of antigen-reactive T cells in each strain was determined by limiting-dilution analysis. EAE was induced in Lewis (LEW), PVG/c and BN rats using four different methods: (i) active immunization with guinea-pig myelin basic protein (GPBP) in CFA; (ii) immunization with GPBP in CFA that had been further supplemented with Mycobacterium tuberculosis H37Ra (supplemented CFA); (iii) adoptive transfer of GPBP-activated spleen cells into syngeneic rats; and (iv) transfer of a GPBP-specific T-cell line. The LEW strain was susceptible to all four methods. The PVG/c strain was resistant to immunization with GPBP in conventional CFA (GPBP/conv. CFA), but was susceptible to immunization with GPBP in supplemented CFA (GPBP/suppl. CFA) and to transfer of activated spleen cells. The BN strain was resistant to all methods. Limiting-dilution analysis using T cells from LEW, PVG/c or BN rats has revealed that each strain of rat displays a different pattern of frequencies of GPBP-reactive or the 68-88 sequence (GP68-88)-reactive T cells. LEW rats showed relatively high frequencies of GPBP-reactive and GP68-88-reactive T cells after immunization with either GPBP/conv. CFA or GPBP/suppl. CFA, symptomatic rats showing higher values than asymptomatic rats. In asymptomatic PVG/c rats, the frequency of GP68-88-reactive T cells was lower than that of GPBP-reactive T cells. In PVG/c rats with clinical EAE, however, GP68-88-reactive T cells increased in frequency and were almost the same as GPBP-reactive T cells. BN rats, on the other hand, responded very poorly not only to the GP68-88 sequence but also to the whole GPBP molecule, even after immunization with GPBP/suppl. CFA. These findings, obtained by limiting-dilution analysis, strongly suggest that the development of EAE in LEW, PVG/c and BN rats is closely related to the frequency of GPBP-reactive T cells. Furthermore, it is shown that resistance to EAE found in PVG/c and BN rats may be generated by different immune mechanisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Nun A., Eisenstein S., Cohen I. R. Experimental autoimmune encephalomyelitis (EAE) in genetically resistant rats: PVG rats resist active induction of EAE but are susceptible to and can generate EAE effector T cell lines. J Immunol. 1982 Sep;129(3):918–919. [PubMed] [Google Scholar]
- Beraud E., Reshef T., Vandenbark A. A., Offner H., Friz R., Chou C. H., Bernard D., Cohen I. R. Experimental autoimmune encephalomyelitis mediated by T lymphocyte lines: genotype of antigen-presenting cells influences immunodominant epitope of basic protein. J Immunol. 1986 Jan;136(2):511–515. [PubMed] [Google Scholar]
- Chou C. H., Chou F. C., Kowalski T. J., Shapira R., Kibler R. F. The major site of guinea-pig myelin basic protein encephalitogenic in Lewis rats. J Neurochem. 1977 Jan;28(1):115–119. doi: 10.1111/j.1471-4159.1977.tb07716.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. A., Essayan D. M., Zweiman B., Lisak R. P. Limiting dilution analysis of the frequency of antigen-reactive lymphocytes isolated from the central nervous system of Lewis rats with experimental allergic encephalomyelitis. Cell Immunol. 1987 Aug;108(1):203–213. doi: 10.1016/0008-8749(87)90204-8. [DOI] [PubMed] [Google Scholar]
- Deibler G. E., Martenson R. E., Kies M. W. Large scale preparation of myelin basic protein from central nervous tissue of several mammalian species. Prep Biochem. 1972;2(2):139–165. doi: 10.1080/00327487208061467. [DOI] [PubMed] [Google Scholar]
- Fallis R. J., Powers M. L., Sy M. S., Weiner H. L. Adoptive transfer of murine chronic-relapsing autoimmune encephalomyelitis. Analysis of basic protein-reactive cells in lymphoid organs and nervous system of donor and recipient animals. J Neuroimmunol. 1987 Mar;14(2):205–219. doi: 10.1016/0165-5728(87)90055-5. [DOI] [PubMed] [Google Scholar]
- Gasser D. L., Hickey W. F., Gonatas N. K. The genes for nonsusceptibility to EAE in the Le-R and BH rat strains are not linked to RT1. Immunogenetics. 1983;17(4):441–444. doi: 10.1007/BF00372462. [DOI] [PubMed] [Google Scholar]
- Gasser D. L., Newlin C. M., Palm J., Gonatas N. K. Genetic control of susceptibility to experimental allergic encephalomyelitis in rats. Science. 1973 Aug 31;181(4102):872–873. doi: 10.1126/science.181.4102.872. [DOI] [PubMed] [Google Scholar]
- Gasser D. L., Palm J., Gonatas N. K. Genetic control of susceptibility to experimental allergic encephalomyelitis and the Ag-B locus of rats. J Immunol. 1975 Aug;115(2):431–433. [PubMed] [Google Scholar]
- Günther E., Odenthal H., Wechsler W. Association between susceptibility to experimental allergic encephalomyelitis and the major histocompatibility system in congenic rat strains. Clin Exp Immunol. 1978 Jun;32(3):429–434. [PMC free article] [PubMed] [Google Scholar]
- Happ M. P., Heber-Katz E. Differences in the repertoire of the Lewis rat T cell response to self and non-self myelin basic proteins. J Exp Med. 1988 Feb 1;167(2):502–513. doi: 10.1084/jem.167.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happ M. P., Wettstein P., Dietzschold B., Heber-Katz E. Genetic control of the development of experimental allergic encephalomyelitis in rats. Separation of MHC and non-MHC gene effects. J Immunol. 1988 Sep 1;141(5):1489–1494. [PubMed] [Google Scholar]
- Hughes R. A., Stedronska J. The susceptibility of rat strains to experimental allergic encephalomyelitis. Immunology. 1973 May;24(5):879–884. [PMC free article] [PubMed] [Google Scholar]
- Kibler R. F., Fritz R. B., Chou F., Jen Chou C-H, Peacocke N. Y., Brown N. M., McFarlin D. E. Immune response of Lewis rats to peptide C1 (residues 68-88) of guinea pig and rat myelin basic proteins. J Exp Med. 1977 Nov 1;146(5):1323–1331. doi: 10.1084/jem.146.5.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE S., WENK E. J. Studies on the mechanism of altered susceptibility to experimental allergic encephalomyelitis. Am J Pathol. 1961 Oct;39:419–441. [PMC free article] [PubMed] [Google Scholar]
- Levine S., Sowinski R. Allergic encephalomyelitis in the reputedly resistant Brown Norway strain of rats. J Immunol. 1975 Feb;114(2 Pt 1):597–601. [PubMed] [Google Scholar]
- Matsumoto Y., Fujiwara M. In situ detection of class I and II major histocompatibility complex antigens in the rat central nervous system during experimental allergic encephalomyelitis. An immunohistochemical study. J Neuroimmunol. 1986 Oct;12(4):265–277. doi: 10.1016/0165-5728(86)90033-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Fujiwara M. The immunopathology of adoptively transferred experimental allergic encephalomyelitis (EAE) in Lewis rats. Part 1. Immunohistochemical examination of developing lesions of EAE. J Neurol Sci. 1987 Jan;77(1):35–47. doi: 10.1016/0022-510x(87)90204-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Hara N., Tanaka R., Fujiwara M. Immunohistochemical analysis of the rat central nervous system during experimental allergic encephalomyelitis, with special reference to Ia-positive cells with dendritic morphology. J Immunol. 1986 May 15;136(10):3668–3676. [PubMed] [Google Scholar]
- Matsumoto Y., Kawai K., Fujiwara M. In situ Ia expression on brain cells in the rat: autoimmune encephalomyelitis-resistant strain (BN) and susceptible strain (Lewis) compared. Immunology. 1989 Apr;66(4):621–627. [PMC free article] [PubMed] [Google Scholar]
- Moore M. J., Singer D. E., Williams R. M. Linkage of severity of experimental allergic encephalomyelitis to the rat major histocompatibility locus. J Immunol. 1980 Apr;124(4):1815–1820. [PubMed] [Google Scholar]
- Vandenbark A. A., Gill T., Offner H. A myelin basic protein-specific T lymphocyte line that mediates experimental autoimmune encephalomyelitis. J Immunol. 1985 Jul;135(1):223–228. [PubMed] [Google Scholar]
- Vandenbark A. A., Offner H., Reshef T., Fritz R., Chou C. H., Cohen I. R. Specificity of T lymphocyte lines for peptides of myelin basic protein. J Immunol. 1985 Jul;135(1):229–233. [PubMed] [Google Scholar]
- Watanabe H., Fujiwara M., Nishiyama T., Ito M., Mashiko T., Nisizawa T. Establishment of T-cell clones recognizing difference in H-2K antigen and inducing graft-versus-host disease. Cell Immunol. 1985 Sep;94(2):454–465. doi: 10.1016/0008-8749(85)90270-9. [DOI] [PubMed] [Google Scholar]
- Waxman F. J., Perryman L. E., Hinrichs D. J., Coe J. E. Genetic resistance to the induction of experimental allergic encephalomyelitis in Lewis rats. I. Genetic analysis of an apparent mutant strain with phenotypic resistance to experimental allergic encephalomyelitis. J Exp Med. 1981 Jan 1;153(1):61–74. doi: 10.1084/jem.153.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. M., Moore M. J. Linkage of susceptibility to experimental allergic encephalomyelitis to the major histocompatibility locus in the rat. J Exp Med. 1973 Oct 1;138(4):775–783. doi: 10.1084/jem.138.4.775. [DOI] [PMC free article] [PubMed] [Google Scholar]


