Abstract
Leucocytes and vascular cells interact closely in inflammation and immunity and cytokines are important mediators of this interaction. The present study was designed to define the capacity of human endothelial cells (HEC) to produce a monocyte-derived neutrophil chemotactic factor (provisionally termed IL-8). IL-8 is a polypeptide chemotactic for neutrophils originally identified in the culture supernatant of lipopolysaccharide (LPS)-stimulated monocytes. IL-1 induced high levels of production of neutrophil chemotactic activity in culture supernatants of HEC. Optimal stimulation of activity was observed when HEC were cultured with 10-100 ng/ml IL-1 beta for 16 hr. Anti-IL-8 antibody blocked the chemotactic activity for neutrophils of IL-1-activated HEC supernatants. IL-1-treated HEC expressed high levels of IL-8 mRNA transcripts, as assessed by Northern blot analysis. Tumour necrosis factor (TNF) and LPS, unlike the inflammatory monokine IL-6, also induced IL-8 expression. Nuclear run-off experiments revealed that IL-1 activated transcription of the IL-8 gene. The production of IL-8 may represent a mechanism whereby endothelial cells, exposed to inflammatory signals, participate in the regulation of neutrophil extravasation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anisowicz A., Bardwell L., Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averbook B. J., Yamamoto R. S., Ulich T. R., Jeffes E. W., Masunaka I., Granger G. A. Purified native and recombinant human alpha lymphotoxin [tumor necrosis factor (TNF)-beta] induces inflammatory reactions in normal skin. J Clin Immunol. 1987 Jul;7(4):333–340. doi: 10.1007/BF00915556. [DOI] [PubMed] [Google Scholar]
- Barone A. D., Ghrayeb J., Hammerling U., Zucker M. B., Thorbecke G. J. The expression in Escherichia coli of recombinant human platelet factor 4, a protein with immunoregulatory activity. J Biol Chem. 1988 Jun 25;263(18):8710–8715. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Colotta F., Lampugnani M. G., Polentarutti N., Dejana E., Mantovani A. Interleukin-1 induces c-fos protooncogene expression in cultured human endothelial cells. Biochem Biophys Res Commun. 1988 May 16;152(3):1104–1110. doi: 10.1016/s0006-291x(88)80398-x. [DOI] [PubMed] [Google Scholar]
- Cybulsky M. I., Movat H. Z., Dinarello C. A. Role of interleukin-1 and tumour necrosis factor-alpha in acute inflammation. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):505–512. doi: 10.1016/s0769-2625(87)80068-5. [DOI] [PubMed] [Google Scholar]
- Dejana E., Breviario F., Balconi G., Rossi V., Remuzzi G., de Gaetano G., Mantovani A. Stimulation of prostacyclin synthesis in vascular cells by mononuclear cell products. Blood. 1984 Dec;64(6):1280–1283. [PubMed] [Google Scholar]
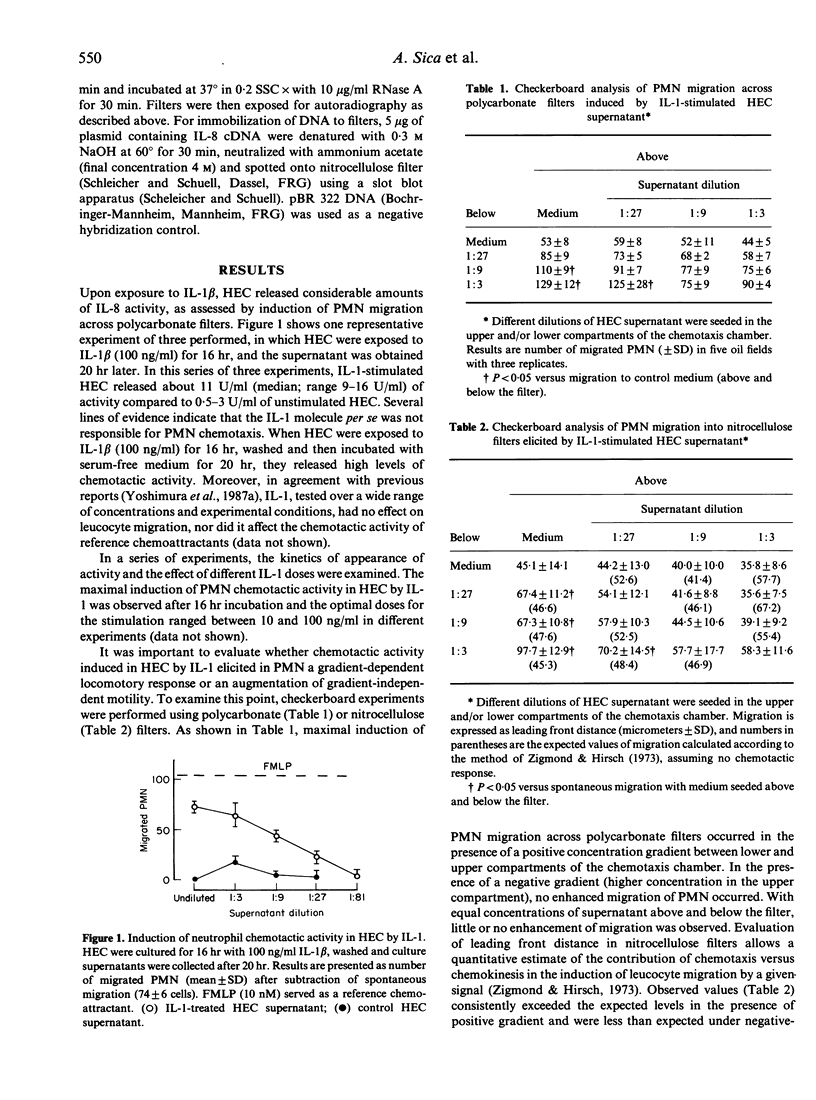
- Falk W., Goodwin R. H., Jr, Leonard E. J. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33(3):239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- Figari I. S., Mori N. A., Palladino M. A., Jr Regulation of neutrophil migration and superoxide production by recombinant tumor necrosis factors-alpha and -beta: comparison to recombinant interferon-gamma and interleukin-1 alpha. Blood. 1987 Oct;70(4):979–984. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Holt J. C., Harris M. E., Holt A. M., Lange E., Henschen A., Niewiarowski S. Characterization of human platelet basic protein, a precursor form of low-affinity platelet factor 4 and beta-thromboglobulin. Biochemistry. 1986 Apr 22;25(8):1988–1996. doi: 10.1021/bi00356a023. [DOI] [PubMed] [Google Scholar]
- Luster A. D., Unkeless J. C., Ravetch J. V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985 Jun 20;315(6021):672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- Maestrelli P., Tsai J. J., Cromwell O., Kay A. B. The identification and partial characterization of a human mononuclear cell-derived neutrophil chemotactic factor apparently distinct from IL-1, IL-2, GM-CSF, TNF and IFN-gamma. Immunology. 1988 Jun;64(2):219–225. [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989 Nov;10(11):370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
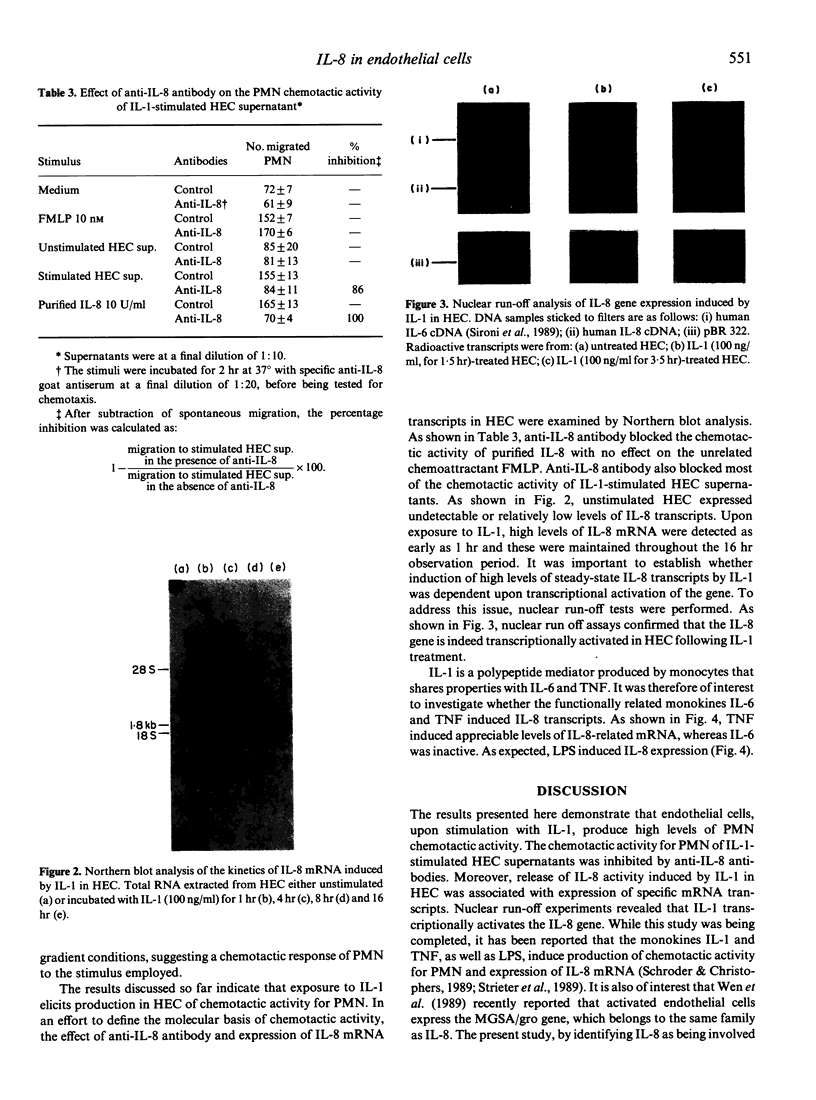
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming W. J., Bersani L., Mantovani A. Tumor necrosis factor is chemotactic for monocytes and polymorphonuclear leukocytes. J Immunol. 1987 Mar 1;138(5):1469–1474. [PubMed] [Google Scholar]
- Movat H. Z., Burrowes C. E., Cybulsky M. I., Dinarello C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987 Dec;129(3):463–476. [PMC free article] [PubMed] [Google Scholar]
- Peveri P., Walz A., Dewald B., Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988 May 1;167(5):1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A., Balentien E., Thomas H. G., Flaggs G., Barton D. E., Spiess J., Bordoni R., Francke U., Derynck R. Molecular characterization and chromosomal mapping of melanoma growth stimulatory activity, a growth factor structurally related to beta-thromboglobulin. EMBO J. 1988 Jul;7(7):2025–2033. doi: 10.1002/j.1460-2075.1988.tb03042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi V., Breviario F., Ghezzi P., Dejana E., Mantovani A. Prostacyclin synthesis induced in vascular cells by interleukin-1. Science. 1985 Jul 12;229(4709):174–176. doi: 10.1126/science.2409598. [DOI] [PubMed] [Google Scholar]
- Sayers T. J., Wiltrout T. A., Bull C. A., Denn A. C., 3rd, Pilaro A. M., Lokesh B. Effect of cytokines on polymorphonuclear neutrophil infiltration in the mouse. Prostaglandin- and leukotriene-independent induction of infiltration by IL-1 and tumor necrosis factor. J Immunol. 1988 Sep 1;141(5):1670–1677. [PubMed] [Google Scholar]
- Schmid J., Weissmann C. Induction of mRNA for a serine protease and a beta-thromboglobulin-like protein in mitogen-stimulated human leukocytes. J Immunol. 1987 Jul 1;139(1):250–256. [PubMed] [Google Scholar]
- Schröder J. M., Christophers E. Secretion of novel and homologous neutrophil-activating peptides by LPS-stimulated human endothelial cells. J Immunol. 1989 Jan 1;142(1):244–251. [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Christophers E. Purification and partial biologic characterization of a human lymphocyte-derived peptide with potent neutrophil-stimulating activity. J Immunol. 1988 May 15;140(10):3534–3540. [PubMed] [Google Scholar]
- Sironi M., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989 Jan 15;142(2):549–553. [PubMed] [Google Scholar]
- Strieter R. M., Kunkel S. L., Showell H. J., Remick D. G., Phan S. H., Ward P. A., Marks R. M. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989 Mar 17;243(4897):1467–1469. doi: 10.1126/science.2648570. [DOI] [PubMed] [Google Scholar]
- Van Damme J., Decock B., Conings R., Lenaerts J. P., Opdenakker G., Billiau A. The chemotactic activity for granulocytes produced by virally infected fibroblasts is identical to monocyte-derived interleukin 8. Eur J Immunol. 1989 Jul;19(7):1189–1194. doi: 10.1002/eji.1830190706. [DOI] [PubMed] [Google Scholar]
- Van Damme J., Van Beeumen J., Opdenakker G., Billiau A. A novel, NH2-terminal sequence-characterized human monokine possessing neutrophil chemotactic, skin-reactive, and granulocytosis-promoting activity. J Exp Med. 1988 Apr 1;167(4):1364–1376. doi: 10.1084/jem.167.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. M., Chen Z. G., Cianciolo G. J., Snyderman R., Breviario F., Dejana E., Mantovani A. Production of a retroviral P15E-related chemotaxis inhibitor by IL-1-treated endothelial cells. A possible negative feedback in the regulation of the vascular response to monokines. J Immunol. 1989 Mar 15;142(6):2012–2017. [PubMed] [Google Scholar]
- Wen D. Z., Rowland A., Derynck R. Expression and secretion of gro/MGSA by stimulated human endothelial cells. EMBO J. 1989 Jun;8(6):1761–1766. doi: 10.1002/j.1460-2075.1989.tb03569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. G., Clark S. C. Multiple actions of interleukin 6 within a cytokine network. Immunol Today. 1988 May;9(5):137–139. doi: 10.1016/0167-5699(88)91200-5. [DOI] [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Oppenheim J. J., Leonard E. J. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1). J Immunol. 1987 Aug 1;139(3):788–793. [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]