Abstract
Nitric oxide plays an important role in immune regulation. We have shown that although high concentrations of NO generally were immune-suppressive, low concentrations of NO selectively enhanced the differentiation of T helper (Th)1 cells but not Th2 cells. This finding provided an explanation for the crucial role of NO in defense against intracellular pathogens. However, the mechanism for the selective induction of Th1 cells was unknown. We report here that at low concentrations, NO activates soluble guanylyl cyclase, leading to the up-regulation of cGMP, which selectively induces the expression of IL-12 receptor β2 but has no effect on IL-4 receptor. Because IL-12 and IL-4 are the key cytokines for induction of Th1 and Th2 cells, respectively, these results, therefore, provide the mechanism for the selective action of NO on T cell subset differentiation. Furthermore, this selectivity also applies to CD8+ cytotoxic and human T cells and, thus, demonstrates the general implication of this observation in immune regulation. Our results also provide an example of the regulation of cytokine receptor expression by NO. The selectivity of such action via cGMP suggests that it is amenable to therapeutic intervention.
Nitric oxide is associated with some of the most important immunopathologies, including rheumatoid arthritis, diabetes, systemic lupus erythematosus, and septic shock (1–6). Conversely, NO also is a key effector molecule for the defense against intracellular pathogens, including virus, bacteria, and parasites (7–9). A common feature among these diseases is the prominent role of type 1 (both CD4+ and CD8+) T cells (10–13). Type 1 T cells, exemplified by CD4+ T helper 1 (Th1) cells, characteristically produce IFN-γ, which can strongly activate macrophage to produce high concentrations of NO via inducible NO synthase. In contrast, Th2 cells produce IL-4 and IL-5, which can inhibit the inducible NO synthase induction by IFN-γ (14, 15). Th1 cells are associated with inflammatory diseases and elimination of intracellular pathogens, whereas Th2 cells are closely involved in allergy and expulsion of extracellular parasites (16). This dichotomy of Th1 and Th2 cells is crucial to the balance of immune response and forms the basis of the current concept of immune therapy. Both type 1 and 2 cells are derived from the same precursor and are differentiated into the two distinct lineages principally under the influence of cytokines in the microenvironment. IL-12 drives the differentiation of type 1 cells during specific antigenic activation of precursor T cells (Tp), whereas IL-4 is the main driving cytokine for the differentiation of type 2 cells (17, 18). Given the close relationship between Th1 cells and NO in disease, it is likely that there exists a reciprocal regulatory mechanism between them.
We have shown (19) that whereas high concentrations of NO were generally cytotoxic, low concentrations of NO had a selective enhancing effect on the induction and differentiation of Th1 but not Th2 cells. NO acted directly on T cells but in synergy with IL-12 produced by antigen-presenting cells (APCs). This biphasic function of NO in immune regulation could contribute importantly to immune homeostasis. We now provide direct evidence that low concentrations of NO preferentially activate Th1 cells by up-regulating cGMP, which selectively induces the expression of IL-12 receptor β2 (IL-12Rβ2) but not IL-4R in T cells. These data, therefore, provide a mechanistic explanation for the selective potentiation of Th1 but not Th2 cell differentiation, a central question of immune regulation. Our results represent an example for the effect of NO on cytokine receptor expression. The selectivity and the participation of cGMP in this process would open a venue of investigation into the role of NO in immune modulation. This finding could have a general implication because it also applies to CD8+ T cells and human T cells.
Methods
Mice.
BALB/c mice were obtained from Harlan Olac (Bichester, U.K.). Ovalbumin (OVA)–T cell receptor-αβ (TCRαβ) CD4 transgenic mice (D011.10) of the BALB/c background were provided originally by Ken Murphy (Washington University, St. Louis) (20). All mice were kept in conventional facilities according to the U.K. Home Office guidelines. Mice, both male and female, were used at 6–10 weeks of age.
Cell Culture.
CD4+ and CD8+ T cells were purified from the spleen and lymph nodes of mice by negative selection, using magnetic beads (MACS; Miltenyi Biotec, Auburn, CA), as described (21). The purity of the cell preparations was determined by FACS analysis with phycoerythrin-conjugated anti-CD4 or anti-CD8 antibodies (PharMingen). Routinely, the purity of the cell preparations was >90%. The cells were cultured in 96- or 24-well plates with OVA peptide (0.3 μM, OVA323–339) and mitomycin-C-treated BALB/c spleen cells (for D011.10 cells) or with plate-bound anti-CD3 antibody (2 μg/ml, for polyclonally activated T cells; PharMingen) for 4–5 days. For the induction of Th1 (CD4+) or cytotoxic T cell 1 (Tc1) (CD8+) cells, IL-12 (10 ng/ml; kindly provided by Genetics Institute, Cambridge, MA) and anti-IL-4 antibody (1 μg/ml; R & D Systems) were added at the start of the culture. Conversely, for the induction of Th2 or Tc2 cells, IL-4 (10 ng/ml) plus anti-IL-12 antibody (1 μg/ml) and anti-IFN-γ antibody (1 μg/ml) (all from R & D Systems) were added into the cultures. For experiments on human T cells, CD4+ T cells were purified from human cord blood by negative selection by using MACS. The blood samples were obtained from informed, consented mothers in the Yorkhill Hospital for Sick Children, Glasgow. Purified cells then were cultured with phytohemagglutinin (1 mg/ml) in full culture medium containing human IL-12 and anti-hIL-4 and anti-hIFN-γ antibody as described (22). In some cultures, IL-2 (10 ng/ml; Genzyme) was added on day 2 of the culture. To examine the effect of NO on the differentiation of T cells, graded concentrations of an NO donor, 2,2′-(hydroxynitrosohydrazino)bis-ethamine (NOC-18, also known as DETA-NO; Alexis, Nottingham, U.K.) were added at the beginning of the culture. In some experiments, graded concentrations of the specific competitive inhibitor of cGMP activation, 1-H-oxodiazolo-(1,2,4)-(4,3-a) guinoxaline-1-one (ODQ; Alexis), or the cGMP analogue, 8-Br-cGMP (Sigma), also were added. At the end of the culture period, supernatants were collected and assayed for IFN-γ or IL-5 concentration by ELISA. T cell proliferation was estimated by uptake of [3H]thymidine.
Measurement of Intracellular cGMP Levels.
Purified CD4+ T cells (1 × 106 cells per ml, 99% pure) from BALB/c mice were cultured in 24-well plates for 30 min with plate-bound anti-CD3, IL-12, and anti-IL-4 as described above (for driving polyclonal Th1 cells) in the presence of graded concentrations of NOC-18. The nonspecific phosphodiesterase inhibitor 3-isobutyl-1-methylxantine (500 μM; Alexis) was added at the beginning of culture. cGMP was isolated and the levels were determined by using a cGMP [3H]assay system (Amersham Pharmacia) according to the manufacturer's instruction and expressed as the ratio of Co (cpm bound in the absence of unlabeled cGMP) and Cx (cpm bound in the presence of samples). Data are pooled from two experiments.
Cytokine Assays.
IFN-γ and IL-5 were assayed by ELISA by using paired antibodies (PharMingen) according to the manufacturer's instructions. Lower limits of detections were: IFN-γ, 80 pg/ml; IL-5, 40 pg/ml.
Quantitative PCR.
Total RNA was prepared by using RNAzol B (Biogenesis, Bournemouth, U.K.), treated with DNase 1 (Ambion, Austin, TX) and reverse-transcribed to cDNA by using Superscript II RNase H− reverse transcriptase (Life Technologies, Paisley, U.K.). Negative control samples (no first-strand synthesis) were prepared by performing reverse transcription reactions in the absence of reverse transcriptase. cDNA levels of IL-12Rβ2, IL-4R, and IL-18Rα and hypoxanthine phosphoribosyltransferase were quantitated by real-time PCR by using an ABI prism 7700 sequence detector (Perkin–Elmer). cDNA levels during the linear phase of amplification were normalized against hypoxanthine phosphoribosyltransferase controls. Determination was carried out in triplicate and expressed as mean ± SD. Primers (f, forward; r, reverse) and 5′-6-carboxy-fluorescein-labeled/3′-6-carboxy-tetramethyl rhodamine-labeled probes (p) used to detect expression of the corresponding murine genes were as follows: IL-12Rβ2 (f, 5′-AATTCTTCTTCACTTCCGCATACG-3′; r, 5′-GCTCCCAGAAGCATTTAGAAAGT-3′; p, 5′-TCCCTCTTCCTCCGTGGGACATCAG-3′); IL-4R (f, 5′-TGTTCTGAGTCTCTGAAAACCT-3′; r, 5′-TATTCATTTCCATGTGGCACACA-3′; p, CATCCCGAGGAACAGTGCCAGCA); IL-18Rα (f, 5′-TCGAGGAGATCCATTCACTGATAG-3′; r, 5′-CCTGGCTCCGTTAGTGAGGTAA-3′; p, 5′-AAAAGCCGGAGGCTAATCATCGTTCTCA-3′).
Flow Cytometry Analysis.
The induction of Th1 cells also were analyzed by intracellular staining of IFN-γ as described (23). Briefly, Th1 cells at the end of 2 days of culture were harvested and stimulated with phorbol 12-myristate 13-acetate (50 ng/ml; Calbiochem)/ionomycin (500 ng/ml; Calbiochem) for 4 h. Brefeldin A (10 ng/ml; Sigma) was added during the last 2 h. The cells then were fixed with paraformaldehyde (2%) and, after washing, permeabilized at room temperature with 0.5% saponin (Sigma) in PBS and FCS (5%) and stained with FITC-conjugated antimurine IFN-γ antibody (IgG2a; PharMingen) or isotype control antibody. Samples were analyzed with a FACScan flow cytometer.
Detection of Apoptosis.
Apoptotic cells were detected by flow cytometry after staining with FITC-conjugated annexin V and propidium iodide by using a commercially available kit (annexin V apoptosis kit; PharMingen). Cells were considered apoptotic when they were annexin V-positive and propidium iodide-negative. Staining of cells by propidium iodide was an indicator of the loss of plasma membrane integrity.
Statistics.
These were performed by using MINITAB software for Macintosh. The analyses were performed by using Student's t test: *, P < 0.05; **, P < 0.01.
Results
Low Concentrations of NO Enhance Differentiation of Tc1 and Human Th1 Cells.
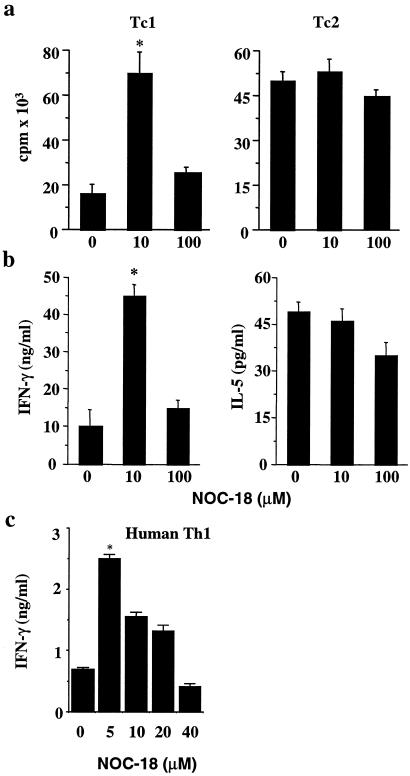
We had demonstrated that whereas high doses of NO were cytotoxic, low doses of NO selectively enhanced the differentiation of murine Th1 but not Th2 cells. We have since investigated whether this phenomenon also is applicable to subsets of CD8+ cells (Tc1 and Tc2) and human CD4+ T cells. Purified BALB/c CD8+ cells were cultured with immobilized anti-CD3 antibody and anti-CD28 in the presence of IL-12 + anti-IL-4 (Tc1 cells) or IL-4 + anti-IL-12 and anti-IFN-γ (Tc2 cells). Graded concentrations of the NO donor, NOC-18, were added at the beginning of culture, and cellular proliferation and IFN-γ and IL-5 production in the supernatant were determined by ELISA 4 days into the culture. At 10 μM, NOC-18 markedly enhanced the differentiation of Tc1 cells but not Tc2 cells. This enhancing effect was absent at 100 μM NOC-18 (Fig. 1a). The cellular proliferation was mirrored by the production of cytokines. Tc1 cells differentiated in the presence of 10 μM NOC-18 produced significantly more IFN-γ compared with Tc1 treated with 100 μM NOC-18 or medium alone (Fig. 1b). There was no evidence of increased IL-5 synthesis during Tc2 differentiation at all of the doses of NOC-18 tested. The increased IFN-γ concentration in the culture supernatant is likely caused by an increased number of type 1 T cells rather than a larger amount of the cytokine produced by individual cells. Flow cytometric analysis of intracellular IFN-γ revealed that there was no evidence of increased staining intensity of Th1/Tc1 cells in the cultures treated with 10 μM NOC-18 compared with the medium control (data not shown). We then investigated whether this enhancing effect of low concentrations of NO also applied to human T cells. CD4+ T cells were purified from human cord blood and driven to the Th1 cell lineage by culturing with phytohemagglutinin in the presence of human IL-12 and anti-IL-4 as described (22, 24). Graded concentrations of NOC-18 were added at the beginning of the culture, which was terminated on day 5. The differentiation of human Th1 cells was enhanced markedly by the presence of 5 μM NOC-18, and the development clearly was suppressed by higher concentrations (>40 μM) of NOC-18 compared with medium control (Fig. 1c). These data therefore extend our earlier finding on the selective enhancing effect of low doses of NO on murine Th1 cells to Tc1 cells and also to human T cells. Furthermore, it appears that human CD4+ T cells are even more sensitive to the varying concentrations of NO than murine T cells.
Fig 1.
Low concentrations of NO enhance differentiation of Tc1 cells and human Th1 cells. (a and b) CD8+ T cells were purified from pooled BALB/c spleen and lymph node cells and cultured with immobilized anti-CD3 antibodies and IL-12 + anti-IL-4 (Tc1), or IL-4 + anti-IL-12/anti-IFN-γ (Tc2), in the presence of graded concentrations of the NO donor (NOC-18) for 4 days. T cell proliferation was determined by [3H]thymidine incorporation and cytokine production by ELISA. (c) CD4+ T cells were purified from human cord blood and cultured with phytohemagglutinin and recombinant human IL-12 and anti-human IL-4 antibodies. IFN-γ concentrations in the culture supernatant were determined on day 4. Results, representative of three to four experiments, are mean ± SD, n = 4–5. *, P < 0.05 compared with culture without NO.
The Effect Is Associated with cGMP Activity and Not Apoptosis.
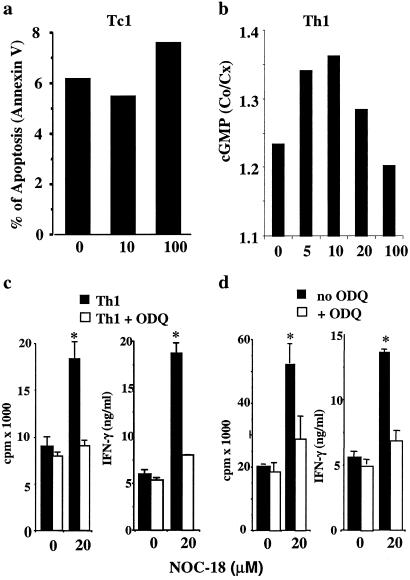
We next investigated the mechanism involved in the enhancing effect of low concentrations of NO on type 1 cell differentiation. Because NO is known to influence apoptosis, the most straightforward explanation for our finding would be that low doses of NO inhibited T cell apoptosis and thereby contribute to the increased viability of the treated cells. In repeated experiments, there was no evidence that low doses of NO (5–25 μM NOC-18) significantly influenced the apoptosis of T cells, although, at higher concentrations (>100 μM, NOC-18), NO consistently increased the level of apoptosis (Fig. 2a). We then examined the possibility that NO may exert its influence via the activation of soluble guanylyl cyclase (sGC), thus elevating cGMP, a well-established NO-effector pathway. We measured the levels of intracellular cGMP in CD4+ T cells cultured for 30 min under the Th1 driving condition in the presence of graded concentrations of NOC-18. cGMP concentration was elevated significantly by 5 and 10 μM NOC-18. This amount declined to the control level at 100 μM NOC-18 (Fig. 1b). The pattern of cGMP elevation closely correlated with the enhanced Th1 cell activation by NO (see Fig. 1 and ref. 19). We then tested the effect of ODQ, a competitive inhibitor of the activation of cGMP. ODQ oxidizes the ferrous iron of sGC to ferric iron and so prevents the effective binding of NO to sGC; thus, it is a unique and potent inhibitor of the NO/cGMP pathway. CD4+ T cells were purified from D011.10 OVA-TCRαβ transgenic mice (BALB/c background) and cultured with the OVA peptide and mitomycin C-treated BALB/c spleen cells (APC) for 4 days in the presence of IL-12 plus anti-IL-4 antibody (Th1) or IL-4 plus anti-IL-12 and anti-IFN-γ antibodies (Th2) and graded concentrations of NOC-18. ODQ (10–40 μM) was added at the beginning of the culture. ODQ abrogated the enhanced Th1 differentiation induced by 20 μM NOC-18 (Fig. 2c). In contrast, ODQ had no effect on the induction of Th2 cells across a range of NOC-18 concentrations (data not shown). In additional experiments, polyclonally activated (anti-CD3 stimulated) Th1 cells also were investigated. The enhanced Th1 cell development in the presence of 20 μM NOC-18 and in the absence of APC was also blocked completely by ODQ (Fig. 2d). These results therefore strongly indicate that the enhancing effect of low concentrations of NO is mediated by cGMP. Furthermore, this effect is exerted directly and selectively on Th1 cells and not via APCs.
Fig 2.
The enhancing effect of low concentrations of NO on type 1 cell differentiation results not from affecting apoptosis but via cGMP. (a) Tc1 cells were purified from BALB/c mice and cultured in the presence of graded concentrations of NO as described in the legend for Fig. 1. Apoptosis (annexin V+ and positive propidium iodide) were determined on day 4. Results are representative of three experiments. Similar results also were obtained with Th1 cells (not shown). (b) Negatively purified CD4+ T cells from BALB/c mice were polyclonally activated for 30 min with anti-CD3 under Th1 conditions in the presence of graded concentrations of NOC-18 and 3-isobutyl-1-methylxantine. Intracellular cGMP was extracted and assayed. (c) CD4+ T cells were negatively purified from pooled spleen and lymph nodes of OVA-TCRαβ transgenic mice (D0.11) and cultured with APC and OVA peptide under Th1 or Th2 conditions as described in Methods. NO had no effect on Th2 cells whose activation also was not affected by the presence of ODQ (data not shown). (d) CD4+ T cells from BALB/c mice were polyclonally activated with plate-bound anti-CD3 antibodies under Th1 conditions. NOC-18 and ODQ were added at the beginning of culture, and cellular proliferation and cytokine production were determined on day 4 of culture. For clarity, in c and d, only results for 20 μM NOC-18 and 10 μM ODQ were shown. Data, representative of four experiments, are mean ± SD, n = 4–5, *, P < 0.05, compared with culture without NO.
8-Br-cGMP Selectively Enhances Th1 Cell Differentiation.
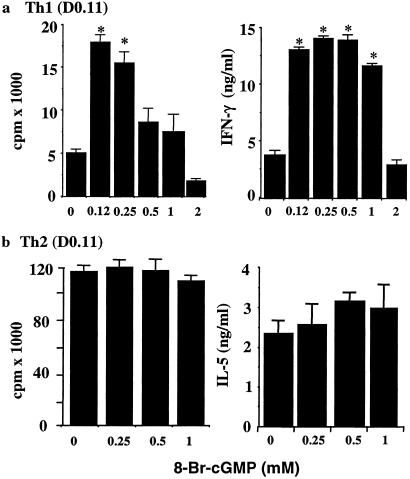
To confirm that cGMP plays a key role in enhanced Th1 cell differentiation by NO, 8-Br-cGMP, the membrane-permeable form of cGMP, was added directly into the Th1- and Th2-inducing cultures as described above in the absence of exogenously added NO. 8-Br-cGMP enhanced Th1 development in a dose-dependent manner (Fig. 3a). In contrast, it had little or no effect on the induction of Th2 cells (Fig. 3b). Similar results were obtained with human Th1 cell differentiation (data not shown). It should be noted that higher concentrations of 8-Br-cGMP (500 and 1,000 μM) did not enhance Th1 cell proliferation at the end of the 4-day culture but did lead to higher concentrations of IFN-γ in the culture supernatant (Fig. 3a). It is likely that these higher doses of 8-Br-cGMP induced an early accumulation of high concentrations of IFN-γ, which subsequently inhibited the proliferation of Th1 cells (detected on day 4 of culture) in a negative feedback manner. However, at very high concentrations (>2 mM), 8-Br-cGMP inhibited both the proliferation and cytokine production by T cells. These data, therefore, clearly demonstrate that low concentrations of NO enhanced Th1 cell differentiation via cGMP.
Fig 3.
8-Br-cGMP selectively enhanced type 1 cell differentiation. CD4+ T cells from D0.11 mice were negatively purified and cultured under Th1 or Th2 conditions as in Fig. 2 b and c. Graded concentrations of 8-Br-cGMP were added at the beginning of cultures, which were harvested on day 4. Results, representative of four similar experiments, are mean ± SD, n = 4, *, P < 0.05, compared with culture without 8-Br-cGMP. Similar results were obtained with polyclonally activated (anti-CD3 antibody-treated BALB/c CD4+ T cells) Th1 and Th2 cells and Tc1 and Tc2 cells (data not shown).
NO Selectively Enhances the Expression of IL-12Rβ2.
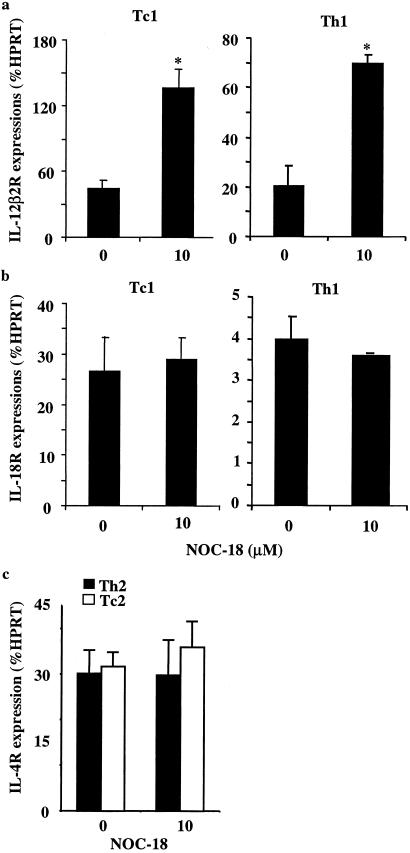
We next investigated the mechanism by which cGMP selectively enhances Th1 cell differentiation. Because NO only increased Th1 cell differentiation and had no effect on established Th1 clones or lines and because it acted on the T cell directly and in synergism with IL-12 (19), we reasoned that NO might enhance expression of the inducible IL-12Rβ2 via cGMP. To test this possibility, CD4+ or CD8+ T cells from BALB/c mice were driven to polyclonal Th1/Tc1 or Th2/Tc2 lineages by using immobilized anti-CD3 antibodies in the presence of the appropriate cytokines. NOC-18 (10 μM) was added at the beginning of the cultures, which were harvested at 16 h. mRNA was extracted and the levels of IL-12Rβ2, IL-18Rα, and IL-4R were quantified by real-time PCR (TaqMan, AB1 Prism 7000 Sequence Detector, Perkin–Elmer). Th1 and Tc1 cells treated with NOC-18 showed significantly higher levels of IL-12Rβ2 message compared with those not treated with NO (Fig. 4 a and b). In contrast, the levels of IL-4R message in the Th2/Tc2 cells were not affected by the treatment with NO (Fig. 4c). Interestingly, the levels of IL-18Rα, characteristic of Th1/Tc1 cells (24), also were not affected by the treatment with low doses of NO. These data, therefore, strongly indicate that NO selectively enhanced the expression of IL-12Rβ2 during type 1 cell differentiation and that this may account for the preferential induction of type 1 cell development by low doses of NO.
Fig 4.
Low concentrations of NO selectively enhanced IL-12Rβ2 induction. Purified CD4+ and CD8+ T cells from BALB/c mice were cultured under type 1 or type 2 conditions with immobilized anti-CD3 antibody for 16 h. Cells were harvested, mRNA was extracted, and the expression of IL-12Rβ2, IL-18Rα, and IL-4R was determined by quantitative PCR as described in Methods. Results, representative of three experiments, are mean ± SD, n = 3, *, P < 0.05, compared with culture without NO.
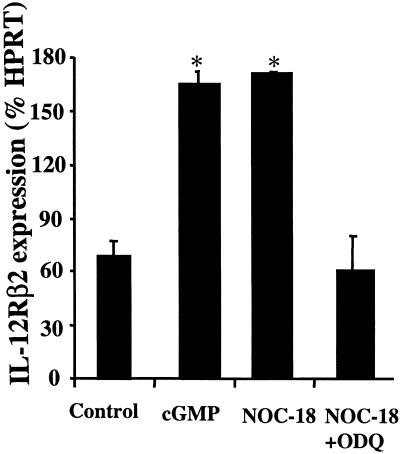
ODQ Inhibits but 8-Br-cGMP Enhances IL-12Rβ2 Expression.
We next sought direct evidence for the induction of IL-12Rβ2 by NO via cGMP. CD4+ T cells from BALB/c mice were cultured under Th1 conditions with immobilized anti-CD3 plus IL-12 and anti-IL-4 in the presence of NOC-18 (10 μM), NOC-18 (10 μM) + ODQ (10 μM), or 8-Br-cGMP (120 μM) for 16 h, and the expression of IL-12Rβ2 was determined by quantitative PCR. 8-Br-cGMP significantly enhanced IL-12Rβ2 expression compared with untreated cells. As expected, NOC-18 enhanced IL-12Rβ2 expression, which was suppressed completely by ODQ (Fig. 5). Together, these data demonstrate directly that low concentrations of NO preferentially enhance type 1 cell differentiation by selectively up-regulating IL-12Rβ2 via cGMP.
Fig 5.
cGMP enhanced and ODQ inhibited IL-12Rβ2 expression during type 1 cell differentiation. Purified CD4+ T cells from BALB/c mice were cultured under Th1 driving conditions in the presence of 8-Br-cGMP (120 μM), NOC-18 (10 μM), or NOC-18 (10 μM) + ODQ (10 μM). Cells were harvested at 16 h, and mRNA was extracted for quantitative PCR analysis for IL-12Rβ2 expression. Results, representative of two experiments, are mean ± SD, n = 5, *, P < 0.05.
Discussion
A strong, immune regulatory role for NO has long been recognized (25, 26). Earlier studies showed that mice deficient in NO developed enhanced Th1 response after infections and antigenic stimulation, producing more IFN-γ and less IL-4 compared with similarly treated, intact mice (27–29). These observations indicated that NO selectively inhibits the expansion of Th1 cells by a negative-feedback mechanism. This is achieved, at least in part, by the selective inhibition of IL-12 synthesis by activated macrophages (30). This mechanism potentially would be highly beneficial during inflammatory diseases predominantly mediated by type 1 cells. In contrast, a strong type 1 T cell response is highly desirable for an effective host defense against intracellular pathogens. This could be enhanced and accelerated by low concentrations of NO provoked during the early stage of infection (19). Thus, immunologically, NO may have evolved to fine-tune the immune homeostasis in health and disease.
Data presented here significantly advance this concept by providing a mechanistic explanation for the selective enhancing effect of low doses of NO on type 1 T cell differentiation. In the present study, we used NOC-18 as an NO donor, which, at 5–20 μM, constantly released 10–40 nM NO and a half-life of 20 h (31, 32). At these concentrations, NO has no detectable effect on the apoptosis of T cells. Because apoptosis would not be expected to be type 1 T cell-specific, this is consistent with our finding that NO, at these concentrations, has little or no effect on the differentiation of Th2 cells. Instead, our results clearly demonstrate that low doses of NO preferentially promoted type 1 T cell differentiation by selective induction of IL-12Rβ2 via cGMP. The effect of NO on T cell activation is likely to be immediate. Elevated levels of cGMP, corresponding to enhanced Th1 cell activation, were detected in CD4+ T cells 30 min after the cells were exposed to NO (Fig. 1b). Furthermore, CD4+ T cells stimulated with NO for 3.5 h, and washed, produced enhanced Th1 response when subsequently cultured with antigen on APCs and IL-12 (19).
Reports of cyclic nucleotide effects on T cell activation indicate that cAMP is inhibitory, whereas effects of cGMP have not been elucidated conclusively. Our results demonstrate an enhancing effect of cGMP on T cell activation. Furthermore, we provide here the mechanism by which cGMP selectively promotes type 1 T cell response. It should be noted that the effect of cGMP again is dose-dependent and is influenced by the signal by which the cells were induced to differentiate. For Th1 cells induced with specific antigen (OVA peptide) and APCs, low doses (125–250 μM) of 8-Br-cGMP enhanced cellular proliferation, whereas a high dose (2,000 μM) clearly was inhibitory. The inhibitory effect of cGMP is in agreement with previous reports, which showed that high levels of cGMP, induced by high concentrations of NO, inhibited T cell proliferation (33) by blocking IL-2 synthesis (34). Furthermore, high doses of NO (>100 μM) could inhibit cytokine production by T cells via cGMP-dependent as well as cGMP-independent pathways, presumably involving apoptosis (35).
Among the several factors reported to be important for inducing type 1 T cell differentiation (36–38), IL-12 clearly plays the dominant role (17, 18). IL-12R is composed of two subunits, β1 and β2, which are both required for high-affinity IL-12 binding and signaling (39–41). IL-12Rβ1 is widely expressed in cells of hematopoietic origin, whereas IL-12Rβ2 is detected selectively in cells responsive to IL-12 such as T cells. Naïve T cells express IL-12R upon TCR engagement. The expression of IL-12R appears to be transient and is down-regulated in committed memory Th1 cells (42), which can be maintained and expanded by other factors including IL-15 (43–45). However, the initial expression of IL-12R is sustained by the presence of the innately induced cytokine IL-18 (24). This has been used to explain the often-observed, synergistic effect of IL-18 and IL-12 in the induction of type 1 T cells (46, 47). Our results here demonstrate that a low concentration of NO is an additional factor for enhancing IL-12R expression and, thus, promoting type 1 cell differentiation. These data also explain the earlier observation that NO acted at the inductive phase of Th1 cell development and had no effect on established memory T cells (19).
The mechanism by which cGMP selectively induces IL-12Rβ2, rather than IL-4R or IL-18R, is unclear at present. In endothelial cells (48) and macrophages (49), low concentrations of NO were shown to up-regulate and high doses of NO to down-regulate NFκB activity. Alternatively, cGMP may act through the P-Raf/MEK/ERK pathway. The precise mechanism(s) of the action of low concentrations of NO in the type 1 T cell differentiation system currently is being explored.
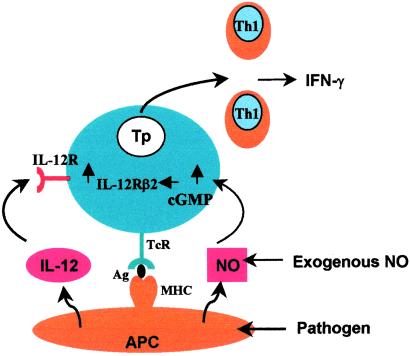
Our results, therefore, establish the following sequence of events, which lead to the selective induction of type 1 T cell differentiation by low concentrations of NO (Fig. 6). NO, produced by activated APCs or provided exogenously in the microenvironment, activates sGC and leads to increased cGMP production. This, then, leads to the induction of increased IL-12Rβ2 expression, which facilitates the differentiation of type 1 T cells by IL-12 and TCR engagement. IL-12 is produced by the APC or present exogenously in the microenvironment. These findings, therefore, provide an explanation for the hitherto puzzling but important observation that low concentrations of NO enhance T cell proliferation and that this enhancement is selective for the induction of Th1 cells, but not Th2 cells, or established memory Th1 cells. We, therefore, have revealed a pathway in which NO represents an additional signal for the induction of T cell subset response, thus contributing to the understanding of the increasingly complex network of immune regulation essential for health and disease.
Fig 6.
Schematic representation of the mechanism by which low concentrations of NO selectively enhance type 1 T cell differentiation. Type 1 cells differentiate from Tp cells through TCR activation by antigen (peptide) presented by the MHC of the APC (e.g., dendritic cells and macrophages) in the presence of IL-12. NO produced by the APC in response to infection or present exogenously in the microenvironment can up-regulate the levels of cGMP in the Tp cells by activating sGC. cGMP, in turn, selectively enhances the expression of IL-12Rβ2. This then increases the response of Tp cells to IL-12 and, hence, preferentially promotes the differentiation of type 1 T cells.
Acknowledgments
This work received financial support from the Wellcome Trust, the Medical Research Council, and the Chief Scientist's Office, Scotland. W.N. also was supported by the Committee of Scientific Research of Poland (Grant 4PO5B 01319).
Abbreviations
APC, antigen-presenting cell
IL-12R, IL-12 receptor
NOC-18, 2,2′-(hydroxynitrosohydrazino)bis-ethamine
ODQ, 1-H-oxodiazolo-(1,2,4)-(4,3-a) guinoxaline-1-one
OVA, ovalbumin
sGC, soluble guanylyl cyclase
TCR, T cell receptor
Th, T helper
Tc, cytotoxic T cell
Tp, precursor T cell
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Moncada S., Palmer, R. M. & Higgs, E. A. (1991) Pharmacol. Rev. 43, 109-142. [PubMed] [Google Scholar]
- 2.Nathan C. & Xie, Q. W. (1994) Cell 78, 915-918. [DOI] [PubMed] [Google Scholar]
- 3.Vladutiu A. O. (1995) Clin. Immunol. Immunopathol. 76, 1-11. [DOI] [PubMed] [Google Scholar]
- 4.Liew F. Y. (1995) Curr. Opin. Immunol. 7, 396-399. [DOI] [PubMed] [Google Scholar]
- 5.Kolb H. & Kolb-Bachofen, V. (1998) Immunol. Today 19, 556-561. [DOI] [PubMed] [Google Scholar]
- 6.Van't Hof R. J. & Ralston, S. H. (2001) Immunology 103, 255-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathan C. F. & Hibbs, J. B., Jr. (1991) Curr. Opin. Immunol. 3, 65-70. [DOI] [PubMed] [Google Scholar]
- 8.Liew F. Y. & Cox, F. E. (1991) Immunol. Today 12, A17-A21. [DOI] [PubMed] [Google Scholar]
- 9.Akaike T. & Maeda, H. (2000) Immunology 101, 300-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason D. & Fowell, D. (1992) Curr. Opin. Immunol. 4, 728-732. [DOI] [PubMed] [Google Scholar]
- 11.Sher A. & Coffman, R. L. (1992) Annu. Rev. Immunol. 10, 385-409. [DOI] [PubMed] [Google Scholar]
- 12.Bogdan C., Rollinghoff, M. & Diefenbach, A. (2000) Curr. Opin. Immunol. 12, 64-76. [DOI] [PubMed] [Google Scholar]
- 13.Liew F. Y. (2002) Nat. Rev. Immunol. 2, 55-60. [DOI] [PubMed] [Google Scholar]
- 14.Liew F. Y., Li, Y., Moss, D., Parkinson, C., Rogers, M. V. & Moncada, S. (1991) Eur. J. Immunol. 21, 3009-3014. [DOI] [PubMed] [Google Scholar]
- 15.Bogdan C., Vodovotz, Y., Paik, J., Xie, Q. W. & Nathan, C. (1994) J. Leukocyte Biol. 55, 227-233. [DOI] [PubMed] [Google Scholar]
- 16.Mosmann T. R. & Coffman, R. L. (1989) Annu. Rev. Immunol. 7, 145-173. [DOI] [PubMed] [Google Scholar]
- 17.Seder R. A. & Paul, W. E. (1994) Annu. Rev. Immunol. 12, 635-673. [DOI] [PubMed] [Google Scholar]
- 18.Abbas A. K., Murphy, K. M. & Sher, A. (1996) Nature 383, 787-793. [DOI] [PubMed] [Google Scholar]
- 19.Niedbala W., Wei, X. Q., Piedrafita, D., Xu, D. & Liew, F. Y. (1999) Eur. J. Immunol. 29, 2498-2505. [DOI] [PubMed] [Google Scholar]
- 20.Murphy K. M., Heimberger, A. B. & Loh, D. Y. (1990) Science 250, 1720-1723. [DOI] [PubMed] [Google Scholar]
- 21.Openshaw P., Murphy, E. E., Hosken, N. A., Maino, V., Davis, K., Murphy, K. & O'Garra, A. (1995) J. Exp. Med. 182, 1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sallusto F., Lenig, D., Mackay, C. R. & Lanzavecchia, A. (1998) J. Exp. Med. 187, 875-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu D., Chan, W. L., Leung, B. P., Huang, F. P., Wheeler, R., Piedrafita, D., Robinson, J. H. & Liew, F. Y. (1998) J. Exp. Med. 187, 787-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu D., Chan, W. L., Leung, B. P., Hunter, D., Schulz, K., Carter, R. W., McInnes, I. B., Robinson, J. H. & Liew, F. Y. (1998) J. Exp. Med. 188, 1485-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor-Robinson A. W., Phillips, R. S., Severn, A., Moncada, S. & Liew, F. Y. (1993) Science 260, 1931-1934. [DOI] [PubMed] [Google Scholar]
- 26.Bogdan C. (2001) Nat. Immunol. 2, 907-916. [DOI] [PubMed] [Google Scholar]
- 27.Wei X. Q., Charles, I. G., Smith, A., Ure, J., Feng, G. J., Huang, F. P., Xu, D., Muller, W., Moncada, S. & Liew, F. Y. (1995) Nature 375, 408-411. [DOI] [PubMed] [Google Scholar]
- 28.McInnes I. B., Leung, B., Wei, X. Q., Gemmell, C. C. & Liew, F. Y. (1998) J. Immunol. 160, 308-315. [PubMed] [Google Scholar]
- 29.MacLean A., Wei, X. Q., Huang, F. P., Al-Alem, U. A., Chan, W. L. & Liew, F. Y. (1998) J. Gen. Virol. 79, 825-830. [DOI] [PubMed] [Google Scholar]
- 30.Huang F. P., Niedbala, W., Wei, X. Q., Xu, D., Feng, G. J., Robinson, J. H., Lam, C. & Liew, F. Y. (1998) Eur. J. Immunol. 28, 4062-4070. [DOI] [PubMed] [Google Scholar]
- 31.Keefer L. K., Nims, R. W., Davies, K. M. & Wink, D. A. (1996) Methods Enzymol. 268, 281-293. [DOI] [PubMed] [Google Scholar]
- 32.Beltran B., Mathur, A., Duchen, M. R., Erusalimsky, J. D. & Moncada, S. (2000) Proc. Natl. Acad. Sci. USA 97, 14602-14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bauer H., Jung, T., Tsikas, D., Stichgenoth, D. O., Frolich, J. C. & Neumann, C. (1997) Immunology 90, 205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fischer T. A., Palmetshofer, A., Gambaryan, S., Butt, E., Jassoy, C., Walter, U., Sopper, S. & Lohmann, S. M. (2001) J. Biol. Chem. 276, 5967-5974. [DOI] [PubMed] [Google Scholar]
- 35.Roozendaal R., Vellenga, E., Postma, D. S., De Monchy, J. G. & Kauffman, H. F. (1999) Immunology 98, 393-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Constant S., Pfeiffer, C., Woodard, A., Pasqualini, T. & Bottomly, K. (1995) J. Exp. Med. 182, 1591-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuchroo V. K., Das, M. P., Brown, J. A., Ranger, A. M., Zamvil, S. S., Sobel, R. A., Weiner, H. L., Nabavi, N. & Glimcher, L. H. (1995) Cell 80, 707-718. [DOI] [PubMed] [Google Scholar]
- 38.Coyle A. J., Lehar, S., Lloyd, C., Tian, J., Delaney, T., Manning, S., Nguyen, T., Burwell, T., Schneider, H., Gonzalo, J. A., et al. (2000) Immunity 13, 95-105. [DOI] [PubMed] [Google Scholar]
- 39.Chizzonite R., Truitt, T., Desai, B. B., Nunes, P., Podlaski, F. J., Stern, A. S. & Gately, M. K. (1992) J. Immunol. 148, 3117-3124. [PubMed] [Google Scholar]
- 40.Chua A. O., Chizzonite, R., Desai, B. B., Truitt, T. P., Nunes, P., Minetti, L. J., Warrier, R. R., Presky, D. H., Levine, J. F., Gately, M. K., et al. (1994) J. Immunol. 153, 128-136. [PubMed] [Google Scholar]
- 41.Presky D. H., Yang, H., Minetti, L. J., Chua, A. O., Nabavi, N., Wu, C. Y., Gately, M. K. & Gubler, U. (1996) Proc. Natl. Acad. Sci. USA 93, 14002-14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murphy K. M., Ouyang, W., Farrar, J. D., Yang, J., Ranganath, S., Asnagli, H., Afkarian, M. & Murphy, T. L. (2000) Annu. Rev. Immunol. 18, 451-494. [DOI] [PubMed] [Google Scholar]
- 43.Zheng X., Sun, S., Hwang, I., Tough, D. F. & Sprent, J. (1988) Immunity 8, 591-599. [DOI] [PubMed] [Google Scholar]
- 44.Geginat J., Sallusto, F. & Lanzavecchia, A. (2001) J. Exp. Med. 194, 1711-1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niedbala W., Wei, X. & Liew, F. Y. (2002) Eur. J. Immunol. 32, 341-347. [DOI] [PubMed] [Google Scholar]
- 46.Kohno K., Kataoka, J., Ohtsuki, T., Suemoto, Y., Okamoto, I., Usui, M., Ikeda, M. & Kurimoto, M. (1997) J. Immunol. 158, 1541-1550. [PubMed] [Google Scholar]
- 47.Robinson D., Shibuya, K., Mui, A., Zonin, F., Murphy, E., Sana, T., Hartley, S. B., Menon, S., Kastelein, R., Bazan, F., et al. (1997) Immunity 7, 571-581. [DOI] [PubMed] [Google Scholar]
- 48.Umansky V., Hehner, S. P., Dumont, A., Hofmann, T. G., Schirrmacher, V., Droge, W. & Schmitz, M. L. (1998) Eur. J. Immunol. 28, 2276-2282. [DOI] [PubMed] [Google Scholar]
- 49.Connelly L., Palacios-Callender, M., Ameixa, C., Moncada, S. & Hobbs, A. J. (2001) J. Immunol. 166, 3873-3881. [DOI] [PubMed] [Google Scholar]