Abstract
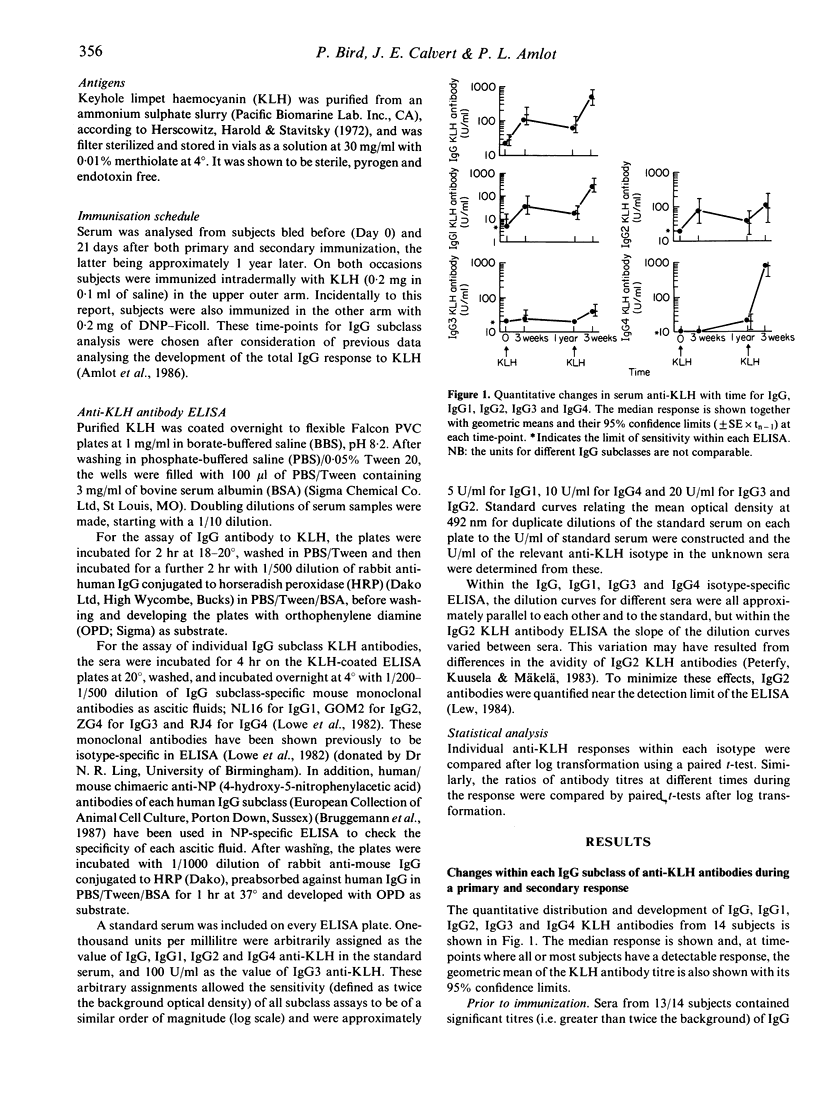
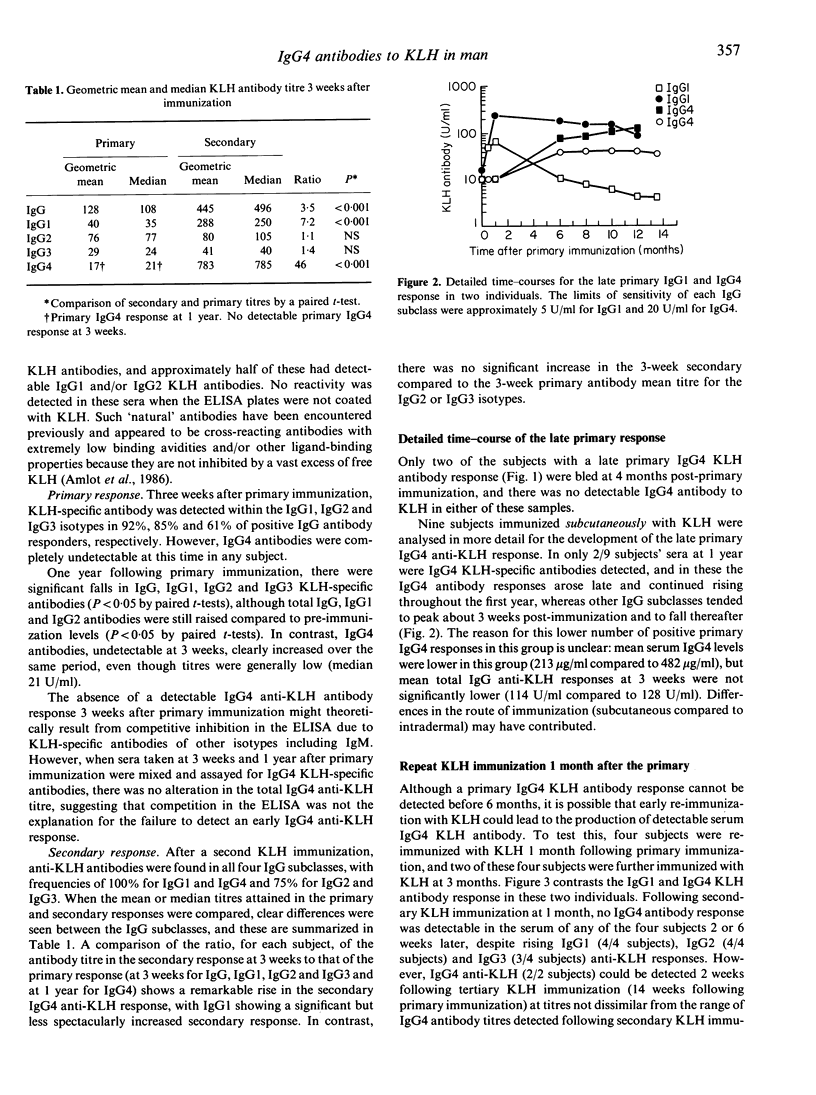
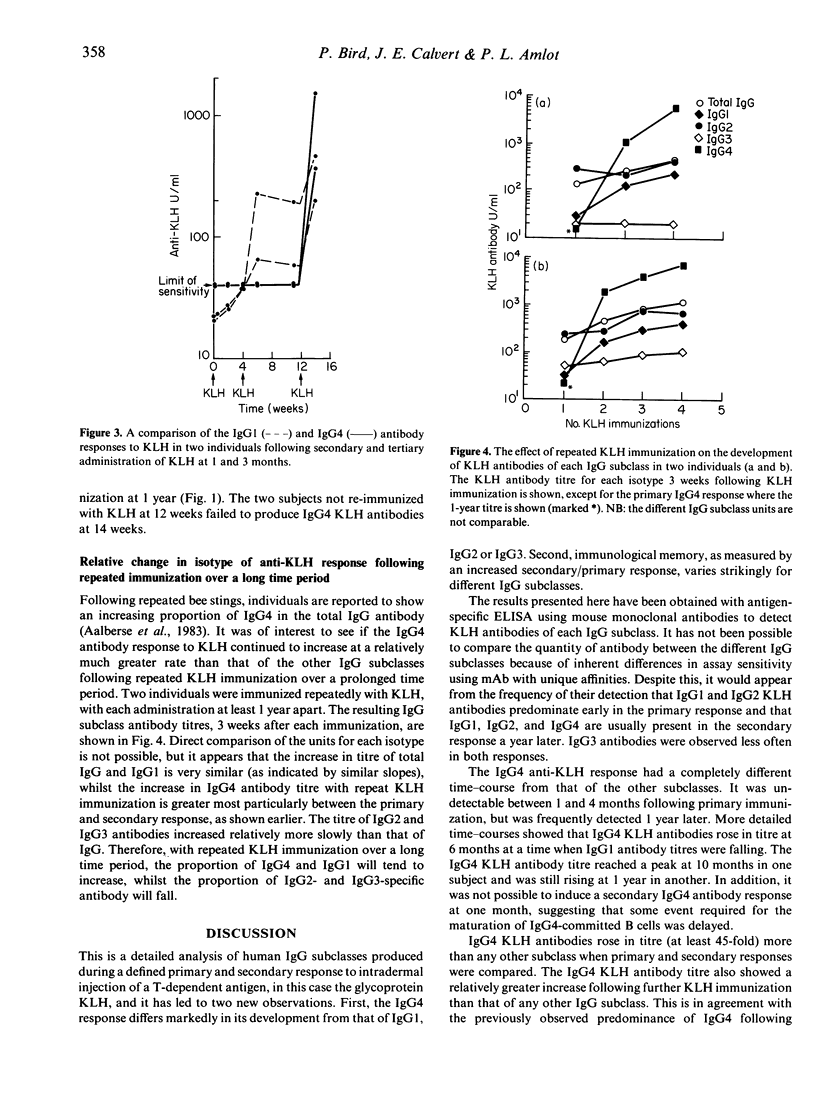
The human primary and secondary IgG subclass antibody responses to keyhole limpet haemocyanin (KLH) have been measured by ELISA using IgG subclass-specific monoclonal antibodies. KLH-specific IgG1 and IgG2 antibodies were detected 3 weeks after primary immunization, and IgG1, IgG2 and IgG4 antibodies after secondary immunization. IgG3 antibodies were observed less frequently in both primary and secondary responses. Unlike the other subclasses, IgG4 antibodies developed very slowly during the primary response, with no antibody detected at 3 weeks and often with only low titres 1 year after immunization. In one individual, this IgG4 primary response peaked around 10 months, but there was considerable variation between individuals. Comparing primary and secondary responses, the greatest increase in KLH antibody was for the IgG4 subclass (45-fold rise), followed by IgG1 (7.3-fold rise), whilst IgG2 and IgG3 KLH-specific antibodies did not show a significantly increased secondary response. There was no detectable IgG4 antibody response when secondary immunization was performed 1 month after the primary, even though IgG1, IgG2 and IgG3 antibodies were present. Reasons for the different time-course of IgG4 anti-KLH development and the isotype-related differences in 'memory' responses are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aalberse R. C., van der Gaag R., van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4-restricted response. J Immunol. 1983 Feb;130(2):722–726. [PubMed] [Google Scholar]
- Amlot P. L., Hayes A. E., Gray D., Gordon-Smith E. C., Humphrey J. H. Human immune responses in vivo to protein (KLH) and polysaccharide (DNP-Ficoll) neoantigens: normal subjects compared with bone marrow transplant patients on cyclosporine. Clin Exp Immunol. 1986 Apr;64(1):125–135. [PMC free article] [PubMed] [Google Scholar]
- Amlot P. L., Hayes A. E. Impaired human antibody response to the thymus-independent antigen, DNP-Ficoll, after splenectomy. Implications for post-splenectomy infections. Lancet. 1985 May 4;1(8436):1008–1011. doi: 10.1016/s0140-6736(85)91613-7. [DOI] [PubMed] [Google Scholar]
- Bergstedt-Lindqvist S., Sideras P., MacDonald H. R., Severinson E. Regulation of Ig class secretion by soluble products of certain T-cell lines. Immunol Rev. 1984 Apr;78:25–50. doi: 10.1111/j.1600-065x.1984.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Bird P., Lachmann P. J. The regulation of IgG subclass production in man: low serum IgG4 in inherited deficiencies of the classical pathway of C3 activation. Eur J Immunol. 1988 Aug;18(8):1217–1222. doi: 10.1002/eji.1830180811. [DOI] [PubMed] [Google Scholar]
- Brüggemann M., Williams G. T., Bindon C. I., Clark M. R., Walker M. R., Jefferis R., Waldmann H., Neuberger M. S. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987 Nov 1;166(5):1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Seymour B. W., Lebman D. A., Hiraki D. D., Christiansen J. A., Shrader B., Cherwinski H. M., Savelkoul H. F., Finkelman F. D., Bond M. W. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988 Feb;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Curtis J. E., Hersh E. M., Harris J. E., McBride C., Freireich E. J. The human primary immune response to keyhole limpet haemocyanin: interrelationships of delayed hypersensitivity, antibody response and in vitro blast transformation. Clin Exp Immunol. 1970 Apr;6(4):473–491. [PMC free article] [PubMed] [Google Scholar]
- Del Prete G., Maggi E., Parronchi P., Chrétien I., Tiri A., Macchia D., Ricci M., Banchereau J., De Vries J., Romagnani S. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988 Jun 15;140(12):4193–4198. [PubMed] [Google Scholar]
- Devey M. E., Wilson D. V., Wheeler A. W. The IgG subclasses of antibodies to grass pollen allergens produced in hay fever patients during hyposensitization. Clin Allergy. 1976 May;6(3):227–236. doi: 10.1111/j.1365-2222.1976.tb01901.x. [DOI] [PubMed] [Google Scholar]
- Dohlsten M., Hedlund G., Sjögren H. O., Carlsson R. Two subsets of human CD4+ T helper cells differing in kinetics and capacities to produce interleukin 2 and interferon-gamma can be defined by the Leu-18 and UCHL1 monoclonal antibodies. Eur J Immunol. 1988 Aug;18(8):1173–1178. doi: 10.1002/eji.1830180805. [DOI] [PubMed] [Google Scholar]
- Flanagan J. G., Rabbitts T. H. Arrangement of human immunoglobulin heavy chain constant region genes implies evolutionary duplication of a segment containing gamma, epsilon and alpha genes. Nature. 1982 Dec 23;300(5894):709–713. doi: 10.1038/300709a0. [DOI] [PubMed] [Google Scholar]
- Klaus G. G., Humphrey J. H., Kunkl A., Dongworth D. W. The follicular dendritic cell: its role in antigen presentation in the generation of immunological memory. Immunol Rev. 1980;53:3–28. doi: 10.1111/j.1600-065x.1980.tb01038.x. [DOI] [PubMed] [Google Scholar]
- Layton G. T., Stanworth D. R. The quantitation of IgG4 antibodies to three common food allergens by ELISA with monoclonal anti-IgG4. J Immunol Methods. 1984 Oct 26;73(2):347–356. doi: 10.1016/0022-1759(84)90410-1. [DOI] [PubMed] [Google Scholar]
- Lew A. M. The effect of epitope density and antibody affinity on the ELISA as analysed by monoclonal antibodies. J Immunol Methods. 1984 Aug 3;72(1):171–176. doi: 10.1016/0022-1759(84)90445-9. [DOI] [PubMed] [Google Scholar]
- Linde G. A., Hammarström L., Persson M. A., Smith C. I., Sundqvist V. A., Wahren B. Virus-specific antibody activity of different subclasses of immunoglobulins G and A in cytomegalovirus infections. Infect Immun. 1983 Oct;42(1):237–244. doi: 10.1128/iai.42.1.237-244.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde G. A. Subclass distribution of rubella virus-specific immunoglobulin G. J Clin Microbiol. 1985 Jan;21(1):117–121. doi: 10.1128/jcm.21.1.117-121.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe J., Bird P., Hardie D., Jefferis R., Ling N. R. Monoclonal antibodies (McAbs) to determinants on human gamma chains: properties of antibodies showing subclass restriction or subclass specificity. Immunology. 1982 Oct;47(2):329–336. [PMC free article] [PubMed] [Google Scholar]
- Mandel T. E., Phipps R. P., Abbot A., Tew J. G. The follicular dendritic cell: long term antigen retention during immunity. Immunol Rev. 1980;53:29–59. doi: 10.1111/j.1600-065x.1980.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Poppema S., Visser L., De Leij L. Reactivity of presumed anti-natural killer cell antibody Leu 7 with intrafollicular T lymphocytes. Clin Exp Immunol. 1983 Dec;54(3):834–837. [PMC free article] [PubMed] [Google Scholar]
- Powers G. D., Abbas A. K., Miller R. A. Frequencies of IL-2- and IL-4-secreting T cells in naive and antigen-stimulated lymphocyte populations. J Immunol. 1988 May 15;140(10):3352–3357. [PubMed] [Google Scholar]
- Pène J., Rousset F., Brière F., Chrétien I., Wideman J., Bonnefoy J. Y., De Vries J. E. Interleukin 5 enhances interleukin 4-induced IgE production by normal human B cells. The role of soluble CD23 antigen. Eur J Immunol. 1988 Jun;18(6):929–935. doi: 10.1002/eji.1830180615. [DOI] [PubMed] [Google Scholar]
- Péterfy F., Kuusela P., Mäkelä O. Affinity requirements for antibody assays mapped by monoclonal antibodies. J Immunol. 1983 Apr;130(4):1809–1813. [PubMed] [Google Scholar]
- Rajewsky K., Förster I., Cumano A. Evolutionary and somatic selection of the antibody repertoire in the mouse. Science. 1987 Nov 20;238(4830):1088–1094. doi: 10.1126/science.3317826. [DOI] [PubMed] [Google Scholar]
- Seppälä I. J., Routonen N., Sarnesto A., Mattila P. A., Mäkelä O. The percentages of six immunoglobulin isotypes in human antibodies to tetanus toxoid: standardization of isotype-specific second antibodies in solid-phase assay. Eur J Immunol. 1984 Sep;14(9):868–875. doi: 10.1002/eji.1830140918. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Paul W. E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987 May 22;236(4804):944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Umetsu D. T., Jabara H. H., DeKruyff R. H., Abbas A. K., Abrams J. S., Geha R. S. Functional heterogeneity among human inducer T cell clones. J Immunol. 1988 Jun 15;140(12):4211–4216. [PubMed] [Google Scholar]
- Yount W. J., Dorner M. M., Kunkel H. G., Kabat E. A. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968 Mar 1;127(3):633–646. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]


