Abstract
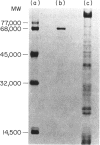
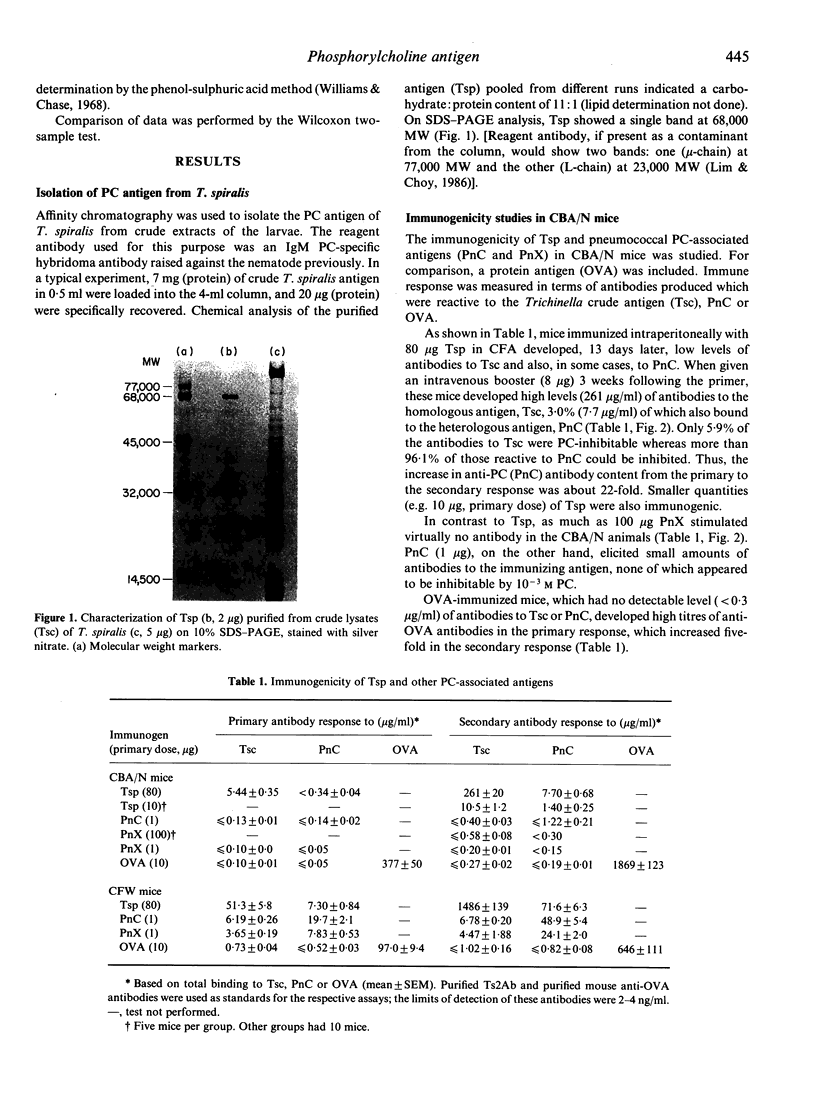
A phosphorylcholine (PC)-containing glycoprotein of 68,000 molecular weight (MW) was isolated from Trichinella spiralis. The potential of this antigen (Tsp) as a species-specific vaccine against Streptococcus pneumoniae was studied in both immunologically deficient (CBA/N) and normal (CFW) mice. Unlike the PC determinant found in S. pneumoniae, Tsp is a type 1 thymus-independent (TI-1) antigen, as it was able to stimulate PC-specific antibody production in CBA/N animals, though less well than in CFW mice. Immunological memory to this antigen was observed in both strains of mice, and the predominant class of antibodies formed was IgM. In further studies, Tsp-immunized CFW mice were protected against a fatal challenge of S. pneumoniae type 3. Protection in these animals is probably mediated by the PC-specific antibodies present, which comprised 87.9% of antibodies reactive to S. pneumoniae, or 58.7% of total antibodies formed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrosino D. M., Siber G. R., Chilmonczyk B. A., Jernberg J. B., Finberg R. W. An immunodeficiency characterized by impaired antibody responses to polysaccharides. N Engl J Med. 1987 Mar 26;316(13):790–793. doi: 10.1056/NEJM198703263161306. [DOI] [PubMed] [Google Scholar]
- Anderson P., Insel R. A., Smith D. H., Cate T. R., Couch R. B., Glezen W. P. A polysaccharide-protein complex from Haemophilus influenzae type b. III. Vaccine trial in human adults. J Infect Dis. 1981 Dec;144(6):530–538. doi: 10.1093/infdis/144.6.530. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Forman C., Hudak S., Claflin J. L. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med. 1982 Oct 1;156(4):1177–1185. doi: 10.1084/jem.156.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy W. F., Lim P. L., Ng M. H. A comparison of immunological methods for the detection of Trichinella spiralis antigen. J Immunol Methods. 1988 Oct 4;113(1):17–24. doi: 10.1016/0022-1759(88)90377-8. [DOI] [PubMed] [Google Scholar]
- Fairchild R. L., Sterner K. E., Braley-Mullen H. Primary murine immunoglobulin M responses to certain pneumococcal capsular polysaccharides consist primarily of anti-pneumococcal cell wall carbohydrate antibodies. Infect Immun. 1986 Jun;52(3):867–871. doi: 10.1128/iai.52.3.867-871.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray B. M., Dillon H. C., Jr, Briles D. E. Epidemiological studies of Streptococcus pneumoniae in infants: development of antibody to phosphocholine. J Clin Microbiol. 1983 Nov;18(5):1102–1107. doi: 10.1128/jcm.18.5.1102-1107.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Apicella M. A., Greenwood B., Mäkelä P. H. Vaccines against encapsulated bacteria: a global agenda. Rev Infect Dis. 1987 Jan-Feb;9(1):176–188. doi: 10.1093/clinids/9.1.176. [DOI] [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Young N. M. Structure of the complex polysaccharide C-substance from Streptococcus pneumoniae type 1. Biochemistry. 1980 Sep 30;19(20):4712–4719. doi: 10.1021/bi00561a026. [DOI] [PubMed] [Google Scholar]
- Käyhty H., Karanko V., Peltola H., Sarna S., Mäkelä P. H. Serum antibodies to capsular polysaccharide vaccine of group A Neissera meningitidis followed for three years in infants and children. J Infect Dis. 1980 Dec;142(6):861–868. doi: 10.1093/infdis/142.6.861. [DOI] [PubMed] [Google Scholar]
- Köhler H., Smyk S., Fung J. Immune response to phosphorylcholine. VIII. The response CBA/N mice to PC-LPS. J Immunol. 1981 May;126(5):1790–1793. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lim P. L., Choy W. F. Monoclonal IgM/A hybrid antibodies: artifacts due to anti-idiotype (T15) antibodies in commercial anti-alpha sera. Mol Immunol. 1986 Aug;23(8):909–916. doi: 10.1016/0161-5890(86)90077-5. [DOI] [PubMed] [Google Scholar]
- Lim P. L., Ho M. Y. Diagnosis of enteric fever by inhibition assay using peroxidase-labelled monoclonal antibody and Salmonella typhi lipopolysaccharide. Aust J Exp Biol Med Sci. 1983 Dec;61(Pt 6):687–704. doi: 10.1038/icb.1983.65. [DOI] [PubMed] [Google Scholar]
- Lim P. L. Isolation of specific IgM monoclonal antibodies by affinity chromatography using alkaline buffers. Mol Immunol. 1987 Jan;24(1):11–15. doi: 10.1016/0161-5890(87)90106-4. [DOI] [PubMed] [Google Scholar]
- Moreno C., Lifely M. R., Esdaile J. Immunity and protection of mice against Neisseria meningitidis group B by vaccination, using polysaccharide complexed with outer membrane proteins: a comparison with purified B polysaccharide. Infect Immun. 1985 Feb;47(2):527–533. doi: 10.1128/iai.47.2.527-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Chapman A. J., Goree A., Jonsson S., Briles D., Baughn R. E. Natural and vaccine-related immunity to Streptococcus pneumoniae. J Infect Dis. 1986 Aug;154(2):245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Peltola H., Mäkelä H., Käyhty H., Jousimies H., Herva E., Hällström K., Sivonen A., Renkonen O. V., Pettay O., Karanko V. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977 Sep 29;297(13):686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- Potter M. Antigen-binding myeloma proteins of mice. Adv Immunol. 1977;25:141–211. [PubMed] [Google Scholar]
- Scher I. The CBA/N mouse strain: an experimental model illustrating the influence of the X-chromosome on immunity. Adv Immunol. 1982;33:1–71. doi: 10.1016/s0065-2776(08)60834-2. [DOI] [PubMed] [Google Scholar]
- Snippe H., van Houte A. J., van Dam J. E., De Reuver M. J., Jansze M., Willers J. M. Immunogenic properties in mice of hexasaccharide from the capsular polysaccharide of Streptococcus pneumoniae type 3. Infect Immun. 1983 Jun;40(3):856–861. doi: 10.1128/iai.40.3.856-861.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen U. B., Agger R., Bennedsen J., Henrichsen J. Phosphorylcholine determinants in six pneumococcal capsular polysaccharides detected by monoclonal antibody. Infect Immun. 1984 Mar;43(3):876–878. doi: 10.1128/iai.43.3.876-878.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeira F. M., Leiro J., Santamarina M. T., Villa T. G., Sanmartín-Durán M. L. Immune response to Trichinella epitopes: the antiphosphorylcholine plaque-forming cell response during the biological cycle. Parasitology. 1987 Jun;94(Pt 3):543–553. doi: 10.1017/s0031182000055888. [DOI] [PubMed] [Google Scholar]
- Wallick S., Claflin J. L., Briles D. E. Resistance to Streptococcus pneumoniae is induced by a phosphocholine-protein conjugate. J Immunol. 1983 Jun;130(6):2871–2875. [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]