Abstract
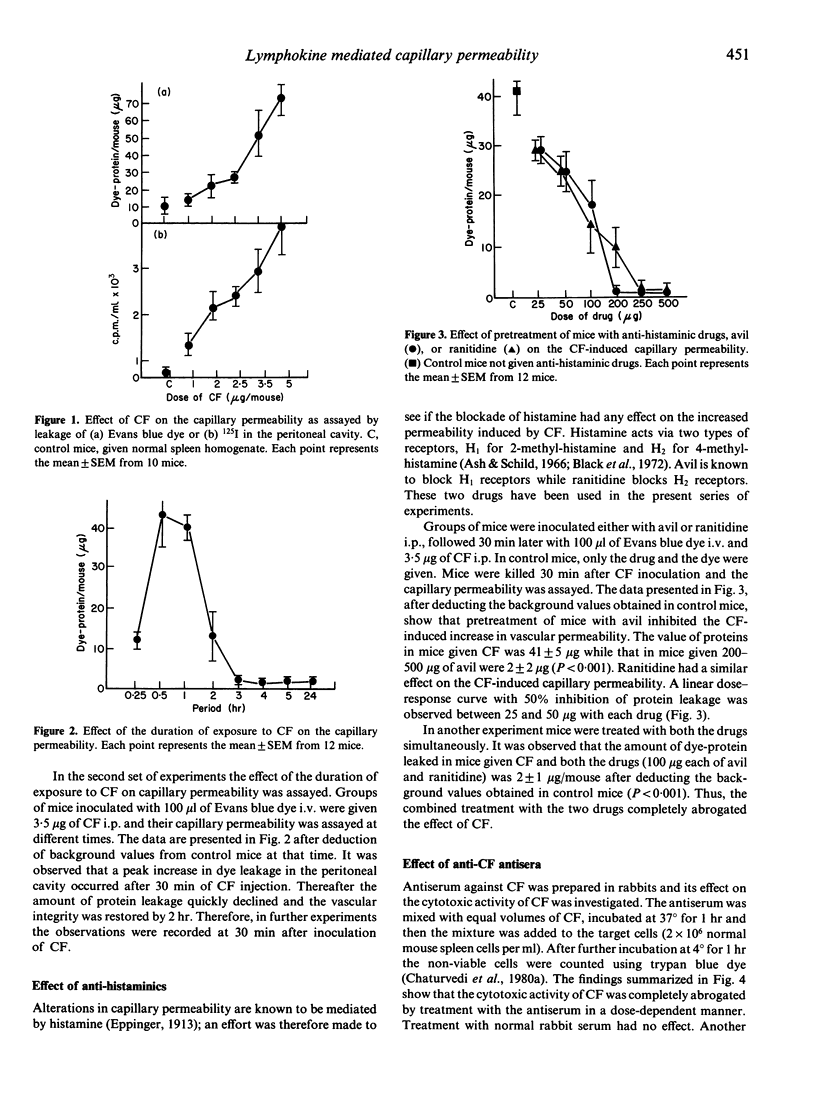
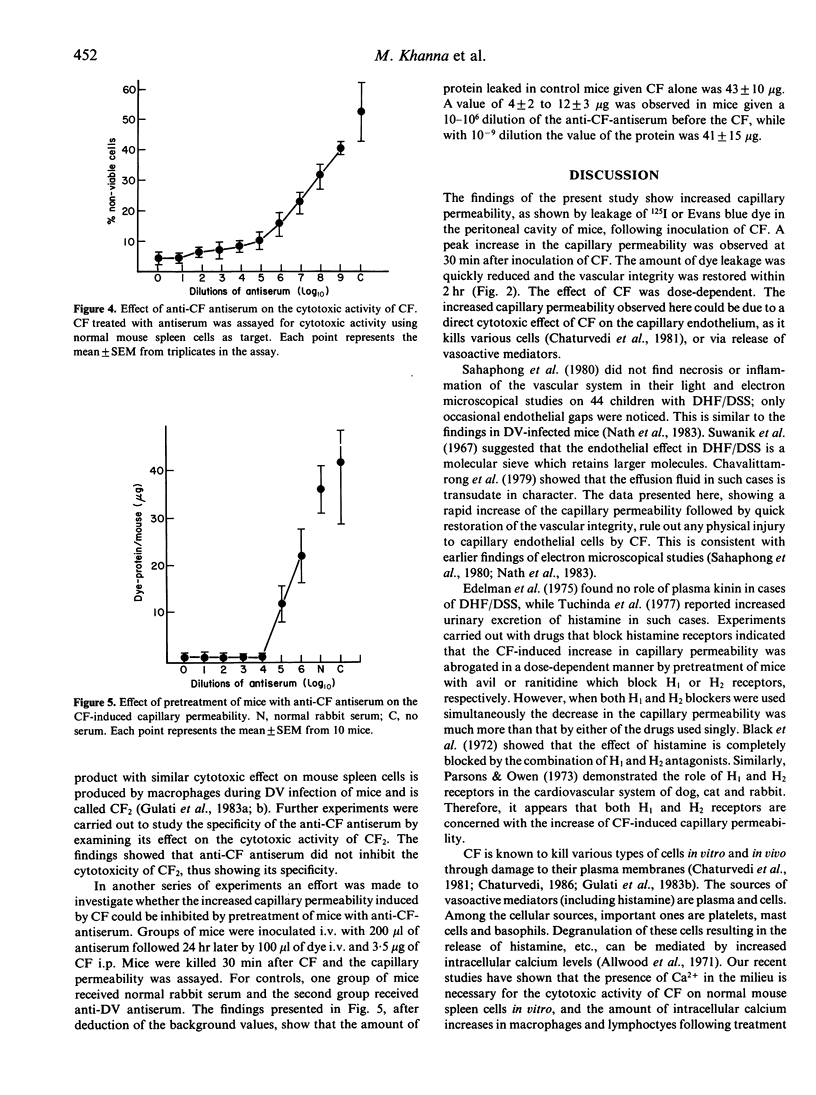
The mechanism of increased capillary permeability, seen in cases of dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS), is not known. Dengue type 2 virus (DV) is known to induce production of a lymphokine, the cytotoxic factor (CF), by the T lymphocytes of mouse spleen. The data presented here show that intraperitoneal inoculation of CF in mice results in increased capillary permeability in a dose-dependent manner, as shown by leakage of intravenously injected radiolabelled iodine (125I) or Evans blue dye. Peak leakage occurred 30 min after inoculation of CF and the vascular integrity was restored by 2 hr. The increase in capillary permeability was abrogated by pretreatment of mice with anti-CF antibodies, avil (H1 receptor blocker) or ranitidine (H2 receptor blocker). The findings thus show that a DV-induced lymphokine, the CF, increases the capillary permeability via release of histamine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allwood G., Asherson G. L., Davey M. J., Goodford P. J. The early uptake of radioactive calcium by human lymphocytes treated with phytohaemagglutinin. Immunology. 1971 Sep;21(3):509–516. [PMC free article] [PubMed] [Google Scholar]
- Black J. W., Duncan W. A., Durant C. J., Ganellin C. R., Parsons E. M. Definition and antagonism of histamine H 2 -receptors. Nature. 1972 Apr 21;236(5347):385–390. doi: 10.1038/236385a0. [DOI] [PubMed] [Google Scholar]
- Chaturvedi U. C., Bhargava A., Mathur A. Production of cytotoxic factor in the spleen of dengue virus-infected mice. Immunology. 1980 Aug;40(4):665–671. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Dalakoti H., Mathur A. Characterization of the cytotoxic factor produced in the spleen of dengue virus-infected mice. Immunology. 1980 Oct;41(2):387–392. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Nagar R., Gulati L., Mathur A. Variable effects of dengue virus-induced cytotoxic factors on different subpopulations of macrophages. Immunology. 1987 Jul;61(3):297–301. [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi U. C., Tandon P., Mathur A. Effect of immunosuppression on dengue virus infection in mice. J Gen Virol. 1977 Sep;36(3):449–458. doi: 10.1099/0022-1317-36-3-449. [DOI] [PubMed] [Google Scholar]
- Chavalittamrong B., Pidetcha P., Pongpipat D., Habanananda S., Tuchinda M. Composition of pleural fluid in dengue hemorrhagic fever. J Med Assoc Thai. 1979 Feb;62(2):55–58. [PubMed] [Google Scholar]
- Edelman R., Nimmannitya S., Colman R. W., Talamo R. C., Top F. H., Jr Evaluation of the plasma kinin system in dengue hemorrhagic fever. J Lab Clin Med. 1975 Sep;86(3):410–421. [PubMed] [Google Scholar]
- Gulati L., Chaturvedi U. C., Mathur A. Characterization of the cytotoxin produced by macrophages in response to dengue virus-induced cytotoxic factor. Br J Exp Pathol. 1983 Apr;64(2):185–190. [PMC free article] [PubMed] [Google Scholar]
- Gulati L., Chaturvedi U. C., Mathur A. Dengue virus-induced cytotoxic factor induces macrophages to produce a cytotoxin. Immunology. 1983 May;49(1):121–130. [PMC free article] [PubMed] [Google Scholar]
- Halstead S. B. Immune enhancement of viral infection. Prog Allergy. 1982;31:301–364. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Litman D., Hsu D. J., Marchesi V. T. Evidence that spectrin binds to macromolecular complexes on the inner surface of the red cell membrane. J Cell Sci. 1980 Apr;42:1–22. doi: 10.1242/jcs.42.1.1. [DOI] [PubMed] [Google Scholar]
- Macaraeg P. V., Jr, Bianchine J. R., Lasagna L. Tolerance to drug-induced writhing in mice. J Pharmacol Exp Ther. 1968 May;161(1):130–140. [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath P., Tandon P., Gulati L., Chaturvedi U. C. Histological & ultrastructural study of spleen during dengue virus infection of mice. Indian J Med Res. 1983 Jul;78:83–90. [PubMed] [Google Scholar]
- Sahaphong S., Riengrojpitak S., Bhamarapravati N., Chirachariyavej T. Electron microscopic study of the vascular endothelial cell in dengue hemorrhagic fever. Southeast Asian J Trop Med Public Health. 1980 Jun;11(2):194–204. [PubMed] [Google Scholar]
- WHITTLE B. A. THE USE OF CHANGES IN CAPILLARY PERMEABILITY IN MICE TO DISTINGUISH BETWEEN NARCOTIC AND NONNARCOTIC ALALGESICS. Br J Pharmacol Chemother. 1964 Apr;22:246–253. doi: 10.1111/j.1476-5381.1964.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


