Abstract
Toxoplasma gondii is an obligate intracellular parasite that contains a relic plastid, called the apicoplast, deriving from a secondary endosymbiosis with an ancestral alga. Metabolic labelling experiments using [14C]acetate led to a substantial production of numerous glycero- and sphingo-lipid classes in extracellular tachyzoites. Syntheses of all these lipids were affected by the herbicide haloxyfop, demonstrating that their de novo syntheses necessarily required a functional apicoplast fatty acid synthase II. The complex metabolic profiles obtained and a census of glycerolipid metabolism gene candidates indicate that synthesis is probably scattered in the apicoplast membranes [possibly for PA (phosphatidic acid), DGDG (digalactosyldiacylglycerol) and PG (phosphatidylglycerol)], the endoplasmic reticulum (for major phospholipid classes and ceramides) and mitochondria (for PA, PG and cardiolipid). Based on a bioinformatic analysis, it is proposed that apicoplast produced acyl-ACP (where ACP is acyl-carrier protein) is transferred to glycerol-3-phosphate for apicoplast glycerolipid synthesis. Acyl-ACP is also probably transported outside the apicoplast stroma and irreversibly converted into acyl-CoA. In the endoplasmic reticulum, acyl-CoA may not be transferred to a three-carbon backbone by an enzyme similar to the cytosolic plant glycerol-3-phosphate acyltransferase, but rather by a dual glycerol-3-phosphate/dihydroxyacetone-3-phosphate acyltransferase like in animal and yeast cells. We further showed that intracellular parasites could also synthesize most of their lipids from scavenged host cell precursors. The observed appearance of glycerolipids specific to either the de novo pathway in extracellular parasites (unknown glycerolipid 1 and the plant like DGDG), or the intracellular stages (unknown glycerolipid 8), may explain the necessary coexistence of both de novo parasitic acyl-lipid synthesis and recycling of host cell compounds.
Keywords: acyl-lipid metabolism, apicoplast, fatty acid synthesis, glycerol-3-phosphate, Toxoplasma gondii, Type II fatty acid synthase (FAS II)
Abbreviations: ACC, acetyl-CoA carboxylase; ACP, acyl-carrier protein; ANS, 8-anilinonaphthalene-1-sulphonic acid; BODIPY®, 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene; DAG, diacylglycerol; DGDG, digalactosyldiacylglycerol; DPG, diphosphatidylglycerol; 2D-TLC, two-dimensional TLC; ECL, enhanced chemiluminescence; FA, fatty acid; FAS II, Type II FA synthase; fop, aryloxyphenoxypropionate herbicide; GlcCer, glycosylcerebroside; HFF, human foreskin fibroblast; HsTfR, human transferrin receptor; IF, immunofluorescence; LacCer, lactosylcerebroside; mAb, monoclonal antibody; MGDG, monogalactosyldiacylglycerol; NEFA, non-esterified FA; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PS, phosphatidylserine; PV, parasitophorous vacuole; SQDG, sulphoquinovosyldiacylglycerol; TriHexCer, globotriosylcerebroside
INTRODUCTION
The unicellular eukaryote Toxoplasma gondii is an obligate intracellular parasite with an extremely broad host range, capable of infecting virtually all types of nucleated cells from warm-blooded vertebrate hosts [1]. It is an important opportunistic pathogen of humans, causing severe encephalitis in immunocompromised individuals and congenital birth defects when primary infection occurs during pregnancy [2,3]. About a decade ago, a major breakthrough was reached in the understanding of the parasite's evolution and biology, with the discovery that T. gondii and other parasites of the Apicomplexa phylum harboured a relic plastid-like organelle, named the apicoplast, that derives from a secondary endosymbiosis with an ancestral alga [4–7]. Plastids are semi-autonomous cellular organelles only described thus far in plants and algae. In plants, the embryonic proplastid can differentiate into the photosynthetically active chloroplast, or into numerous non-green plastids (amyloplasts, chromoplasts, etioplasts, leucoplasts etc.), which all have vital metabolic functions in different organs [8,9]. These plastids are foremost on the list of known targets for herbicides [10,11] and the discovery of a plastid in apicomplexan parasites, which are responsible for extremely serious diseases in humans (malaria and toxoplasmosis) and livestock (coccidioses, theilerioses, babesioses etc.), therefore raised hopes for the development of new innovative herbicide-based drugs [12].
Whereas the apicoplast's function has been discussed since its discovery [13], it became rapidly obvious that this organelle was essential for parasite survival, at least in Plasmodium and Toxoplasma [12,14–16]. The hypothesized function of the apicoplast is based on knowledge about metabolic pathways in non-photosynthetic plastids of plants such as FA (fatty acid), isoprenoid, haem, starch and aromatic amino acid synthesis [9].
It has long been held that apicomplexan parasites were incompetent for de novo FA synthesis [17–19], but the recent record of nuclear-encoded apicoplast-targeted genes for all enzymes of the FA biosynthesis pathway provided strong arguments in favour of a de novo FA biosynthesis in this organelle (for a review see [20]). As in bacteria and plants, the T. gondii genome contains the group of highly conserved proteins known as FAS II (Type II FA synthase), with distinct enzymes for the different reactions [21–24]. Incorporation of the precursor [14C]acetate into FA chains by Plasmodium falciparum [24] and T. gondii [25] demonstrated that the de novo FA biosynthetic pathway was functional. Furthermore, the existence of FAS II would explain the susceptibility of T. gondii to herbicides targeting plastid ACC (acetyl-CoA carboxylase) [22,26] or FabI [enoyl-ACP (acyl-carrier protein) reductase] [23,27]. This FAS II pathway is seen as a promising drug target, mostly because it is structurally and functionally distinct from the equivalent pathway in the vertebrate hosts, i.e. FAS I [28]. A tempting hypothesis to explain the importance of apicoplast FAS II would be that the produced FAs are used for de novo syntheses of essential membrane acyl-lipids (glycero- and sphingo-lipids).
Parasite development is characterized by intense membrane production and reorganization. Upon host cell invasion, T. gondii resides and divides within a unique specialized compartment, the PV (parasitophorous vacuole), which primarily derives from the host cell plasma membrane but is rapidly modified by the parasite [29,30]. The PV is devoid of host cell transmembrane proteins [31,32] and does not fuse with endocytic or exocytotic vesicles of the host cell [33,34]. It was hypothesized that the apicoplast might provide material for some of the membranes necessary for successful infection, the establishment and/or maturation of the PV and/or for parasite cell division [21]. Using lipids conjugated to fluorescent probes and radiolabelled precursors, Charron and Sibley [25] provided evidence that part of the lipid material required for membrane mass increase comes from scavenging of some host cell lipids. Diversion of host cell material was mostly of some phospholipids that were tentatively identified as different PC (phosphatidylcholine) species [25]. Lipid precursors such as polar head groups, PA (phosphatidic acid) and small FAs primarily introduced into host cells, are secondarily used to manufacture PC. A production of PC by extracellular Toxoplasma was also recorded after diffusion of radiolabelled acetic acid [25]. However, it remained unclear whether labelling was due to an incorporation of acetate into the FAs owing to the FAS II pathway or into other building blocks of the phospholipids, such as the glycerol moiety or the polar head groups, owing to other metabolic routes. Until now, all published analyses have concentrated on some specific lipids [25,35–39] and no detailed global analysis of T. gondii membrane acyl-lipid synthesis and metabolization is available.
In the present study, we investigated the capacity of T. gondii for de novo membrane glycero- and sphingo-lipid synthesis. Using metabolic labelling with [14C]acetate and a pharmacological knockout of the plastid FAS II, we showed that in extracellular Toxoplasma the apicoplast-generated FAs were used to synthesize numerous membrane acyl-lipids. The complex profile of de novo synthesized lipids was analysed using 2D-TLC (two-dimensional TLC) and co-migration with characterized lipids from Arabidopsis thaliana. Gene candidates for the corresponding activities in T. gondii were inventoried by similarity searches using A. thaliana genes as probes. Comparative analysis revealed that, whereas most of the lipid classes were synthesizable owing to either de novo synthesis or utilization of host cell compounds, selective synthesis of some lipids was also observed. Therefore the present study supports that a defect of the plastid FAS II pathway compromises the selective production of some acyl-lipids, which are possibly essential for the parasite and cannot be compensated for by import of precursors from the host cell.
EXPERIMENTAL
Materials
Cell culture media and fetal bovine serum were obtained from Life Technologies. The radiolabelled FA precursor [1-14C]acetic acid sodium salt (55 mCi·mmol−1) was from Amersham Biosciences. The herbicide haloxyfop and Merck silica gel 60 TLC plates were purchased from Sigma. Protease inhibitor cocktail tablets were from Roche Diagnostics, propidium iodide from Molecular Probes and the ECL (enhanced chemiluminescence) system for detection of Western-blot signals from Pierce Chemical. The pri-mary antibody mAb (monoclonal antibody) H68.4 reacting with the HsTfR (human transferrin receptor) was from Zymed Laboratories. Primary antibody mAb TG05-54 was used to detect the Toxoplasma SAG1 surface protein (TgSAG1) and mAb TG17-113 to detect GRA5 (TgGRA5), a protein of the PV membrane [40]. The peroxidase-conjugated secondary antibody for detection of proteins on Western blots and the BODIPY® (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene)-conjugated goat anti-mouse IgG for IF (immunofluorescence) detection were purchased from Jackson ImmunoResearch Laboratories.
Parasite culture
Tachyzoites of the RH strain were propagated under standard procedures, by serial passage in HFF (human foreskin fibroblast) monolayers in D10 medium (Dulbecco's modified Eagle's medium supplemented with 10%, v/v, heat-inactivated fetal bovine serum, 1 mM glutamine, 500 units·ml−1 penicillin and 50 μg·ml−1 streptomycin) at 37 °C and under 5% CO2.
Metabolic labelling
For metabolic labelling, freshly lysed parasites were purified through a column of silicon-treated glass wool to eliminate host cell debris and washed three times in PBS. Parasites were then incubated with 5–10 μCi·ml−1 [14C]acetic acid at 37 °C in D10 medium, subsequently collected by centrifugation, washed four times with PBS to eliminate non-incorporated radioactivity and used for lipid extraction (Figure 1). When required, a 1 h treatment with 300 μM haloxyfop [22] preceded the metabolic labelling. To determine lipid acquisition from host cells, HFF monolayers were incubated in the presence of 5 μCi·ml−1 [14C]acetic acid for 8 h prior to tachyzoite infection. After an overnight infection, intracellular parasites were collected by scraping the monolayers and were mechanically released from host cells by sequential passage through 20, 23, 25 and 27G needles. Parasites were further purified through a column of silicon-treated glass wool to eliminate host cell debris prior to lipid extraction and chromatography (Figure 1).
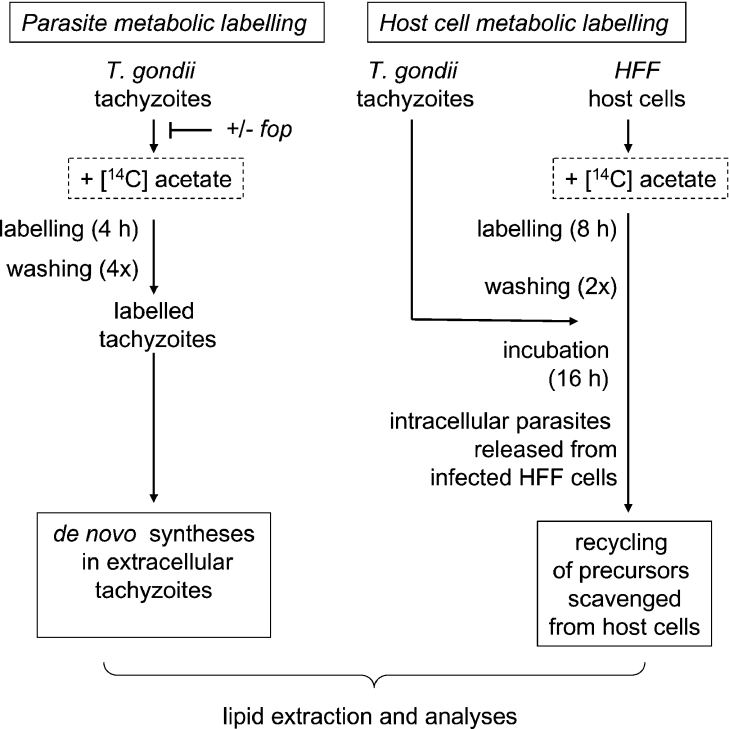
Figure 1. Scheme for metabolic labelling of T. gondii extra- and intra-cellular tachyzoites.
Extracellular Toxoplasma cells were labelled for 4–6 h in the presence of [14C]acetate (left panel) and the lipid profile was subsequently analysed. When required, a 1 h treatment with the aryloxyphenoxypropionate herbicide haloxyfop (fop) preceded the metabolic labelling. HFF cells were labelled in the presence of [14C]acetate und subsequently infected by Toxoplasma tachyzoites (right panel). Lipid analyses of intracellular parasites were then carried out to detect diversion of labelled carbons from host cell compounds for the parasite acyl-lipid metabolism.
Viability assay
Tachyzoite viability was routinely determined by propidium iodide staining. Parasites were incubated for 15 min in the presence of 5 μg·ml−1 propidium iodide in PBS and observed with a Zeiss Axioplan 2 fluorescence microscope. Non-viable parasites take up the dye and show a red fluorescence.
IF microscopy
IF microscopy was used to determine if isolated parasites were free of PV membrane fractions. Aliquots of parasitic preparations collected before and after filtration through a glass wool column were spotted on glass slides, dried and fixed in 2.5% (v/v) formaldehyde. The slides were further incubated with the mAb TG17-113 and then with the BODIPY®-conjugated goat anti-mouse IgG. Slides were mounted in 50% (v/v) glycerol and examined with a Zeiss Axioplan 2 fluorescence microscope using a ×200 magnification to provide a general view of the preparations.
Lipid extraction and chromatographic analyses
Lipids from [14C]acetic acid-labelled parasites or HFF cells were extracted as described by Bligh and Dyer [41] and analysed by 2D-TLC. To discriminate between synthesized glycerolipids (KOH-sensitive) and sphingolipids (KOH-resistant), radiolabelled parasite- or HFF-synthesized lipids were incubated in 0.1 M KOH in methanol/water (1:1, v/v) for 4 h at 50 °C so as to clear esterlinked FAs of glycerolipids. Hydrolysis-resistant sphingolipids were subsequently resolved by 2D-TLC. Lipids were co-migrated on silica gel plates along with an established mixture of total A. thaliana lipids as an internal standard for identification. The solvent system for the first dimension was chloroform/methanol/water (65:25:4, by vol.) and chloroform/acetone/methanol/acetic acid/water (100:40:20:20:10, by vol.) for the second, allowing the plates to dry between each development. Finally, after a careful drying, TLC plates were sprayed with 0.2% ANS (8-anilinonaphthalene-1-sulphonic acid) in methanol and stained lipids were visualized with UV light. Radiolabelled Toxoplasma lipids were detected by exposition of TLC plates with erasable screens for 24–72 h. Screens were scanned with a phosphoimager FLA 8000 (Fuji). The software used for reading screens and further image analyses was ImageReader and ImageGauge (Fuji). Radiolabelled Toxoplasma sphingolipids were identified by their sensitivity to alkaline treatment and co-migration with ANS-visualized A. thaliana lipids. Glycosphingolipids from the HFF lipid mixture were detected owing to their purple coloration after α-naphtol staining and their resistance to alkaline treatment.
SDS/PAGE and immunoblot analysis
Proteins were separated by SDS/PAGE (13% polyacrylamide) and transferred to nitrocellulose by liquid transfer. Membranes were blocked in 5% (w/v) non-fat dry milk in PBS, incubated with the appropriate primary antibody, rinsed and incubated with the peroxidase-conjugated goat secondary antibody. Signals were detected by using the ECL system.
Census of T. gondii gene candidates possibly involved in acyl-lipid metabolism
The list of acyl-lipid gene candidates from the Arabidopsis Lipid Gene Database (620 entries, November 2004; [42]; http://www.plantbiology.msu.edu/lipids/genesurvey/) was used as a probe to seek gene candidates in the preliminary T. gondii genomic database via http://ToxoDB.org. These genomic data were provided by The Institute for Genomic Research (supported by the NIH grant no. AI05093) and by the Sanger Center (Wellcome Trust). A first list of gene candidates was selected after comparison between the Arabidopsis sequences and the Toxoplasma predicted proteins (20878 entries, including possibly redundant annotations provided by TigrScan, GlimmerHMM and Twinscan, November 2004) using both BLASTP [43] and the BLOSUM 62 similarity matrix [44] implemented in the BIOFACET software package [45]. A first criterion for a strong similarity was set with a Score threshold of 100 and an E-value cutoff of 1×10−8. The second criterion for sequence selection was the ‘double-click’, i.e. confirmation of the best alignment with homologues of the Arabidopsis probes in the best hits obtained after a comparison of the Toxoplasma sequences with the Swiss-Prot molecular database (http://www.expasy.org/sprot/). Prediction of a cleavable signal peptide (Sp) or signal anchor sequence (Sa) was analysed using the SignalP method (version 3.0; [46]; http://www.cbs.dtu.dk/services/SignalP/). In the absence of any bioinformatic apicoplast-targeting prediction tool for Toxoplasma protein sequences, prediction of a chloroplast-like transit peptide (Ctp) downstream a signal peptide was sought using the ChloroP method designed for a broad range of species (version 1.1; [47]; http://www.cbs.dtu.dk/services/ChloroP/). Prediction of a mitochondrial transit peptide (Mtp) was achieved using the MitoProt method [48] (http://ihg.gsf.de/ihg/mitoprot.html). Eventually, any sequence sharing homology with two Arabidopsis isoenzymes localized in distinct organelles, was listed in the compartment that corresponds to the target prediction. As a positive control of the method, we checked that all the apicoplast FAS II pathway and ACP genes could be recovered.
RESULTS
Metabolic labelling strategies to dissect the 14C-route from provided acetate to Toxoplasma polar lipids
To investigate whether T. gondii tachyzoites were able to synthesize polar lipids from apicoplast-synthesized FAs, we analysed lipid extracts obtained after metabolic labelling with the FA precursor [14C]acetate. Figure 1 summarizes the labelling and analysis strategies to investigate the metabolic routes of the labelled carbons from acetate to lipids. Haloxyfop was added to block FAS II and to investigate whether the lipid labelling was downstream of apicoplast FA synthesis. To analyse de novo syntheses in extracellular parasites, tachyzoites were incubated in the presence of [14C]acetate for 4 h (labelling was linear for at least 6 h, results not shown) and washed thoroughly to eliminate non-incorporated radioactivity. These radiolabelled parasites were then directly processed for lipid analysis. Addition of haloxyfop [fop (aryloxyphenoxypropionate herbicide)] abolished the polar lipid labelling (see below, and Figure 2). The herbicide sensitivity indicated that a de novo synthesis of FAs, owing to the apicoplast FAS II, was required for incorporation of radioactivity in complex lipids. Therefore the labelling was attributable to a 14C-route from acetate to the FA moiety of the produced lipids. Eventually, HFF labelling with [14C]acetate prior to T. gondii infection was also carried out to investigate lipid synthesis that recycled host cell precursors (Figures 1 and 4).
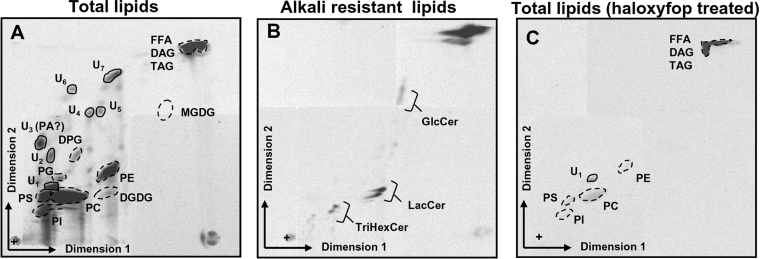
Figure 2. Identification of de novo synthesized lipids by T. gondii tachyzoites.
Parasites were labelled with the FA precursor [14C]acetate and extracted lipids were analysed by 2D-TLC along with total A. thaliana lipids as markers for identification. Lipids extracted from the same number of parasites (6×108) were deposited on the chromatography plates. (A) Parasites were labelled for 4 h extracellularly prior to lipid extraction and TLC analysis. (B) Same labelling conditions as in (A), but radiolabelled lipids were subjected to an alkaline treatment (KOH in methanolic phase) to identify sphingolipids amongst the parasite-synthesized lipids. (C) Same conditions as in (A), but the labelling was preceded by a 2 h treatment with the herbicide haloxyfop; spots represent the radiolabelled lipids de novo synthesized by the parasite, broken line circled positions correspond to the positions of the co-migrated Arabidopsis lipids identified by coloration with ANS, and solid line circled spots indicate positions of lipids not found in the Arabidopsis lipid mixture. TAG, triacylglycerol; U1–U7, unknown lipids in the Arabidopsis lipid mixture. U3 was tentatively identified as PA; +, deposit point of lipids. FFA, NEFA.
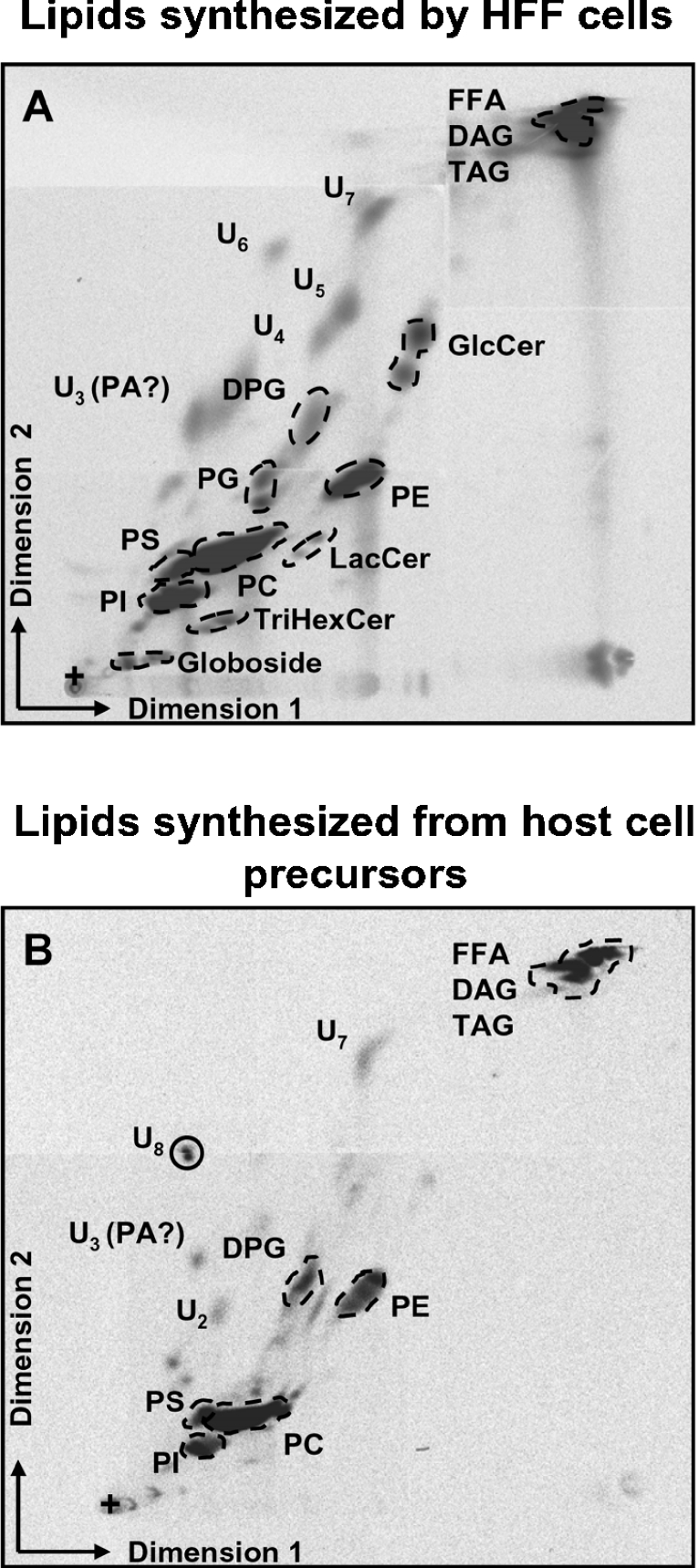
Figure 4. Lipid acquisition from the host cell.
HFF host cells were labelled with [14C]acetate prior to infection with unlabelled parasites. Intracellular tachyzoites were purified and their radiolabelled lipid content was analysed by 2D-TLC along with total Arabidopsis lipids as markers for identification. (A) HFF radiolabelled lipids. (B) Toxoplasma radiolabelled lipids produced from 14C-labelled host cell components. U2–U8, unknown lipids in the Arabidopsis lipid mixture. U3 was tentatively identified as PA; +, deposit point of lipids. FFA, NEFA.
Metabolic profiles of extracellular Toxoplasma polar lipids produced with apicoplast-synthesized FAs
In a first set of experiments, we analysed lipids metabolically labelled after incubation of extracellular tachyzoites in the presence of [14C]acetate. A representative lipid profile is shown in Figure 2(A). Spots correspond to lipids synthesized by the parasites with radiolabelled acetate, while positions circled with broken lines indicate the positions of ANS-visualized A. thaliana lipids co-migrated as markers. The radiolabelled profile shows that the parasite is capable of synthesizing the major phospholipids: PC, PE (phosphatidylethanolamine), PS (phosphatidylserine), PI (phosphatidylinositol) and, albeit to a lesser extent, PG (phosphatidylglycerol). Concerning organellar lipids, the mitochondrial DPG (diphosphatidylglycerol or cardiolipid) was weakly labelled (Figure 2A). Although the incorporation of tritiated galactose from UDP-galactose into chloroplastic galactolipids was previously reported in Toxoplasma partly lysed cells [49], no MGDG (monogalactosyldiacylglycerol) could be detected under our experimental conditions. However, a radiolabelled glycerolipid co-migrated with Arabidopsis chloroplast DGDG (digalactosyldiacylglycerol; Figure 2A). In addition, seven major unidentified glycerolipids (U1–U7) were synthesized. Their characterization by MS was restricted by lack of biological material. However, the failure to identify these lipids may be an indication of rare or intermediary structures, U3 being tentatively identified as a PA molecular species (according to the two-dimensional Rf (retardation factor) position [50]). U1 is possibly unique to the parasite (undetected in Arabidopsis standard lipids or in HFF-synthesized lipids shown in Figure 4). Three series of spots among the parasite-synthesized lipids were identified as sphingolipids by their resistance to alkaline treatment: GlcCer (glycosylcerebroside), LacCer (lactosylcerebroside) and TriHexCer (globotriosylcerebroside) (Figure 2B).
Metabolic labelling of Toxoplasma lipids was nearly completely abolished after incubation of tachyzoites with haloxyfop (Figure 2C). The effect of this fop herbicide, which targets plastid ACC, was investigated at a concentration previously shown to affect Toxoplasma tachyzoites but not its host cells (300 μM; [22]). A 1 h preincubation of extracellular tachyzoites with the herbicide before [14C]acetate addition was the minimal time required to see an effect on lipid synthesis. When both components were added simultaneously, [14C]acetate incorporation started before the herbicide could have reached its target (results not shown). This very rapid incorporation is similar in isolated plant chloroplasts, where free acetate is the most efficient substrate for FAS II [51]. Thus, after a 1 h incubation with the herbicide at 37 °C, 5 μCi·ml−1 [14C]acetate was added and parasites were incubated for another 4 h. During this time period, the herbicide did not affect parasite viability, as monitored by propidium iodide staining of parasites just before lipid extraction. Viability was found to be higher than 90% (results not shown) as in non-herbicide treated parasites. As shown in Figure 2(C), the fop herbicide abolished almost totally the FA and polar lipid biosyntheses in Toxoplasma and we had to lengthen exposure of the chromatography over an additional 2 weeks to assess the labelling background. This collapse of polar lipid biosyntheses induced by haloxyfop in viable cells demonstrates that the lipid production is fully dependent on the FAS II pathway.
Metabolic profile of intracellular Toxoplasma polar lipids, synthesized after mobilization of labelled precursors from host cells
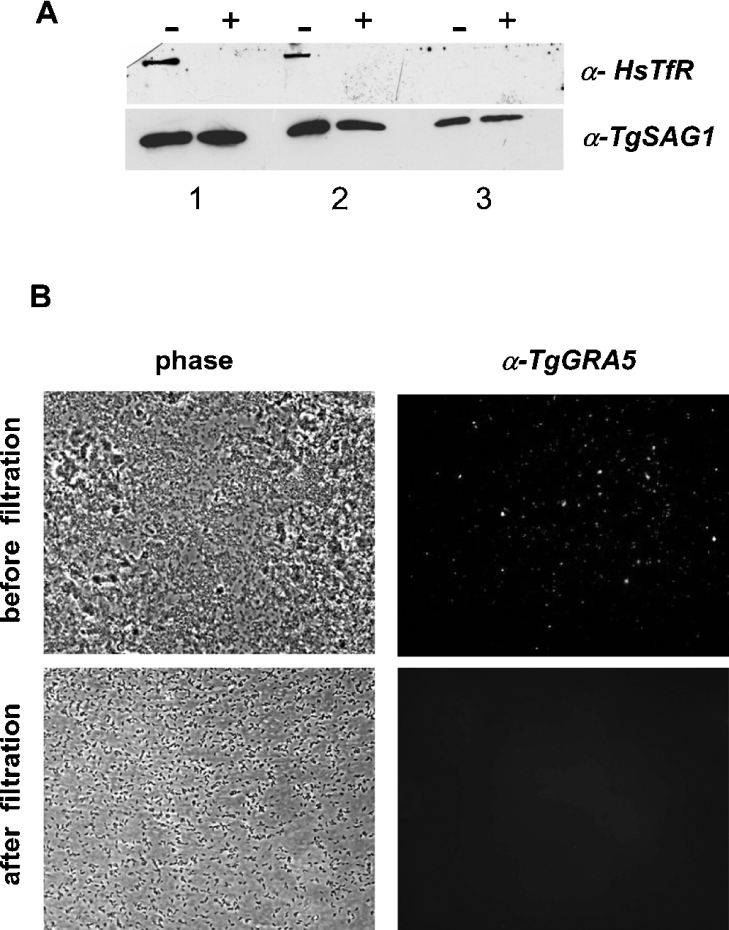
T. gondii is incompetent to divide in vitro in the absence of host cells, indicating that the intracellular way of life not only protects the parasite from extracellular immune attacks, but also provides one or more essential factor(s) to the parasite. Previous studies reported the acquisition and metabolization of host cell lipids by Toxoplasma [25,35–39]; however, these studies did not provide a detailed global analysis of the lipids produced. Furthermore, these analyses used specific radioisotopic and/or fluorophore-conjugated lipids and were, most of the time, performed by microscopic observations. But specific labelling of parasite compartments by exogenous, artificially labelled lipids does not necessarily reflect the lipid scavenging and metabolization by the parasite. Therefore, in a second set of experiments, HFF monolayers were labelled with [14C]acetic acid 8 h prior to infection with unlabelled tachyzoites. After an overnight intracellular growth within labelled HFF host cells, parasites were purified and their radiolabelled lipid profile was analysed. As the purity of this intracellular parasite fraction is essential for the interpretation of our results, we conducted a non-radioactive control experiment under the same conditions and analysed the parasites before and after glass-wool filtration for the presence of contaminating host cell or PV membranes. Western-blot analysis showed that the HsTfR was not detected in the parasite preparation after glass-wool filtration, whereas a positive staining was observed in the parasite preparation before filtration (Figure 3A). In addition, microscope examination of the same fractions revealed that the filtered preparation was highly enriched in parasites and free of visible membranous debris (phase contrast). IF microscopy showed that the PV membrane protein GRA5 was only detected in the parasite preparation prior to filtration (Figure 3B). These two control experiments led us to conclude that our intracellular parasite fractions are essentially free of contaminating PV and host cell membranes.
Figure 3. Purity control experiment of the intracellular parasite fraction.
After an overnight intracellular growth within HFF cells, parasites were collected by scraping the monolayer and were mechanically released from host cells by sequential passage through 20, 23, 25 and 27G needles. Parasites were further purified through a column of silicon-treated glass wool. (A) Western-blot analysis of the parasite fraction before (−) and after (+) filtration; detection of host cell membranes was done using the anti-HsTfR (α-HsTfR) mAb H68.4. Protein extracts correspond to 107 (1), 106 (2) and 105 (3) parasites. The α-TgSAG1 antibody that is directed against the T. gondii major surface protein SAG1 was used as an internal control of protein load in each lane. (B) IF microscopy of the PV membrane protein GRA5 (α-TgGRA5) in the same fractions before and after filtration.
Figure 4(A) shows HFF lipids synthesized after [14C]acetate labelling. The five major phospholipids PC, PE, PI, PS and PG were detected as well as the four main cerebrosides: GlcCer, LacCer, TrihexCer and globoside. The mitochondrial cardiolipid (DPG) was also strongly labelled. Figure 4(B) shows the labelled lipids that tachyzoites imported and/or manufactured using radiolabelled lipids or lipid precursors diverted from host cells. The profile is far less complex than the one obtained after de novo synthesis in free tachyzoites (Figure 2A). As reported by Charron and Sibley [25], the major ubiquitous phospholipid is PC, but it only represented about half of the labelled phospholipids in our experiment; PE, PS and PI are also substantially produced. PG was not detected. Charron and Sibley [25] also showed that intracellular Toxoplasma did not mobilize fluorophore-conjugated PC from host cells; PC was therefore most likely generated from metabolized precursors diverted from the host cell, rather than directly imported. The radiolabelled NEFAs (non-esterified FAs) we detected in our TLC analyses (Figure 4B, FFA) might result from a direct import from host cells and/or from a degradation/metabolization of other scavenged lipids. Some of the uncharacterized glycerolipids listed earlier [U3 (PA?), U2 and U7] appeared to be also generated from host cell compounds (Figure 4B). In the list of minor uncharacterized glycerolipids, one could notice U8 (Figure 4B), which was detected neither in extracellularly labelled tachyzoites nor in HFF cells. U8 is very likely generated from modified host cell compounds and may be specific to the intracellular parasitic life stage. In contrast, the major U1 glycerolipid synthesized by extracellular tachyzoites (Figure 2A) as well as the chloroplast-like DGDG were not synthesized from host cell precursors. U1 and DGDG might not be synthesizable outside the apicoplast-dependent de novo biosynthetic pathways. Interestingly, intracellular tachyzoites exhibited a high labelling of the mitochondrial cardiolipid (DPG; Figure 4B). DPG is generated from PG, which could not be detected in intracellular parasites; it is therefore likely to be imported directly from the host cell.
Table 1 summarizes the lipid profiles obtained in the present study. The use of host cell components for parasite lipid synthesis is apparently a very complex process, either following routes of direct import of lipids (DPG?), of close intermediates for polar lipid syntheses [PA (U3?), NEFAs], or of pools of unrelated imported molecules that are largely degraded before serving as building blocks for lipid production. Furthermore, the lipid metabolism of the parasite shifts from an autonomous de novo process to a parasitic recycling of host cell material upon invasion. However, at least some of the Toxoplasma-specific glycerolipids (U1, DGDG) may not be produced from host cell component scavenging and may require functional apicoplast FA synthesis.
Table 1. Polar lipid metabolic labelling profiles in extracellular and intracellular Toxoplasma.
Lipid assessment was based on 2D-TLC and co-migration with characterized lipids from A. thaliana. Sphingolipids were identified by their resistance to alkaline hydrolysis with KOH in methanolic phase. U1–U8, unknown glycerolipids 1–8; (+), (++), (+++), qualitative detection scale; nd, not detected. In the case of U3–U7, occurrence in HFF cells was supposed from co-migrating spots in Toxoplasma samples.
| Toxoplasma tachyzoites | |||
|---|---|---|---|
| Lipid | Extracellular | Intracellular | Human HFF cells |
| Membrane glycerolipids | |||
| Ubiquitous phospholipids | |||
| PC | +++ | +++ | +++ |
| PE | +++ | ++ | +++ |
| PS | +++ | + | +++ |
| PI | + | + | ++ |
| PG | ++ | nd | ++ |
| Mitochondrial phospholipids | |||
| DPG | + | + | ++ |
| Chloroplast-like glycolipids | |||
| MGDG | nd | nd | nd |
| DGDG | + | nd | nd |
| SQDG | nd | nd | nd |
| Uncharacterized glycerolipids | |||
| U1 | ++ | nd | nd |
| U2 | ++ | + | nd |
| U3 (PA?) | ++ | + | + |
| U4 | + | nd | + |
| U5 | + | nd | + |
| U6 | + | nd | + |
| U7 | ++ | + | + |
| U8 | nd | + | nd |
| Membrane sphingolipids | |||
| GlcCer | + | + | ++ |
| LacCer | + | nd | + |
| TriHexCer | + | nd | + |
| Globoside | + | nd | + |
Census of Toxoplasma gene candidates possibly involved in acyl-lipid metabolism
Table 2 gives the list of 26 Toxoplasma gene candidates for the major steps in membrane lipid production, using either acyl-ACP (acyl-carrier protein) or acyl-CoA as substrates. In this census, gene candidates for enzymes involved in glycerolipid synthesis are likely to occur in the apicoplast, endoplasmic reticulum and mitochondria. In the apicoplast, first transfer of an acyl to a three-carbon backbone would occur due to a glycerol-3-phosphate acyltransferase candidate, using acyl-ACP as a substrate. Subsequent production of PA may occur because of a putative 2-lysophosphatidate acyltransferase.
Table 2. Gene candidates for acyl-lipid metabolism in T. gondii.
EC number: enzyme nomenclature following recommendation of the International Union of Biochemistry and Molecular Biology. Genes whose function is based on sequence similarity are in italic characters; genes whose function is based on experimental data are in normal characters. Absence of a gene candidate is indicated by (nc), except in case of absence of experimental evidence for the gene occurrence (no evidence). Plants having no known dual dihydroxyacetone-phosphate/glycerol-3-phosphate acyltransferase, the corresponding sequence from yeast (Uniprot accession no. GPT1_YEAST P32784) was used as a probe. In the case of the plastidial CDP-DAG synthetase gene candidate, prediction of an Sp (*) could be assessed when computed with the second methionine as a start. Ctp, chloroplast-like transit peptide; Mtp, mitochondrial transit peptide; Sa, signal anchor sequence; Sp, signal peptide.
| Gene candidates | ||||
|---|---|---|---|---|
| Acyl-ACP- or acyl-CoA-dependent lipid metabolism | EC number | A. thaliana | T. gondii | Target prediction |
| Synthesis of membrane lipids in plastids (PA, MGDG, DGDG, SQDG and PG) | ||||
| using acyl-ACP | ||||
| Glycerol-phosphate acyltransferase | 2.3.1.15 | At1g32200 | TgTigrScan_3735 | Sp Ctp |
| 2-Lysophosphatidate acyltransferase (PA synthase) | 2.3.1.51 | At4g30580 | TgTigrScan_0603 | Sp Ctp |
| Plastidial phosphatidic acid phosphatase | 3.1.3.4 | At2g01180 | nc | – |
| MGDG synthase | 2.4.1.46 | At2g11810, At4g31780, | nc | – |
| At5g20410 | ||||
| DGDG synthase | 2.4.1.184 | At3g11670, At4g00550 | nc | – |
| Galactolipid:galactolipid galactosyltransferase | nc | nc | – | |
| Sulpholipid synthase (SQDG synthase) | At5g01220 | No evidence | – | |
| CDP-DAG synthetase | 2.7.7.41 | At2g45150, At3g60620, | TgTigrScan_4999 | Sp Ctp* |
| At4g26770 | ||||
| PG-phosphate synthase | 2.7.8.5 | At2g39290 | TgTigrScan_4540 | Sp Ctp |
| PG-phosphate phosphatase (PG synthase) | nc | nc | – | |
| Synthesis of membrane lipids in endomembrane systems (PA, APC, PE, PS, PI, PG and Cer) | ||||
| using acyl-Co | ||||
| Plant-like endomembrane glycerol-phosphate acyltransferase | 2.3.1.15 | At5g06090, At3g11430 | nc | – |
| Dual dihydroxyacetone-phosphate/glycerol-phosphate | No evidence | TgTwinScan_6545 | Sp | |
| acyltransferase (none in plants; in yeast: GPT1_YEAST) | ||||
| Peroxisomal dihydroxyacetone-phosphate; ether lipid | No evidence | TgTwinScan_3802 | Sp | |
| syntheses (none in plants; in human: GNPAT-HUMAN) | ||||
| 2-Lysophosphatidate acyltransferase (PA synthase) | 2.3.1.51 | At1g80950, At3g18850, | nc | – |
| At1g75020 | ||||
| Phosphatidate phosphatase | 3.1.3.4 | At1g15080 | TgTigrScan_1948 | Sa |
| Phosphatidylserine synthase (PS synthase) | 2.7.8.8 | nc | nc | – |
| Phosphatidylinositol synthase (PI synthase) | 2.7.8.11 | At1g68000, At4g38570 | TgTigrScan_5902 | Sa |
| Phosphatidylserine decarboxylase | 4.1.1.65 | At4g25970, At5g57190 | TgTigrScan_0325 | Sp |
| Ethanolamine/serine base-exchange enzyme | At1g15110 | TgTigrScan_7983 | Sp | |
| DAG cholinephosphotransferase (PC synthase) | 2.7.8.2 | At3g25585 | TgTigrScan_0833 | Sp |
| CDP-DAG synthetase | 2.7.7.41 | At1g62430, At4g22340 | TgTigrScan_1888 | ? |
| PG-phosphate synthase | 2.7.8.5 | At3g55030 | nc | – |
| PG-phosphate phosphatase (PG synthase) | nc | nc | – | |
| Serine palmitoyltransferase (LCB1-3) | 2.3.1.50 | At4g36480, At3g48780, | TgTigrScan_1389 | Sp |
| At5g23670 | ||||
| Ceramide synthase (sphingosine N-acyltransferase) | 2.3.1.24 | At3g25540, At3g19260, | TgTigrScan_2444 | Sp |
| At1g13580 | ||||
| Synthesis of membrane lipids in mitochondria (PA, PG and DPG) using acyl-CoA | ||||
| Glycerol-phosphate acyltransferase | 2.3.1.15 | At1g02390, At1g06520 | nc | – |
| 2-Lysophosphatidate acyltransferase | 2.3.1.51 | At4g30580 | TgTigrScan_8310 | Mtp |
| Phosphatidate phosphatase | 3.1.3.4 | At3g02600 | nc | – |
| PG-phosphate synthase | 2.7.8.5 | At4g04870 | nc | – |
| PG-phosphate phosphatase (PG synthase) | nc | nc | – | |
| Glycerol-3-phosphate dehydrogenase | 1.1.99.5 | At3g10370 | TgTigrScan_1884 | Mtp |
| Cardiolipid synthase (DPG synthase) | nc | nc | – | |
| Phosphatidylserine decarboxylase | 4.1.1.65 | At4g16700 | TgTigrScan_0325 | Mtp |
| Synthesis and storage of triacylglycerols using acyl-CoA | ||||
| Acyl-CoA: DAG acyltransferase | 2.3.1.20 | At2g19450 | TgTigrScan_5720 | ? |
| Hydrolysis of acylglycerols and acyl-hydrolases | ||||
| Acyl-CoA oxidase | 1.3.3.6 | At1g06290, At1g06310, | TgTigrScan_7878 | Sa |
| At2g35690, At4g16760, | ||||
| At5g65110 | ||||
| 3-Hydroxyacyl-CoA dehydrogenase | 1.1.1.35 | At3g15290 | TgTigrScan_3778 | ? |
| Ketoacyl-CoA thiolase | 2.3.1.16 | At1g04710, At2g33150, | TgTigrScan_7504, | Mtp |
| At5g48880 | TgTigrScan_8000 | Sp | ||
| Lysophospholipase | 3.1.1.5 | At1g52700, At3g15650, | TgTigrScan_5386 | ? |
| At4g22300, At5g20060 | ||||
| Carboxylic ester hydrolases (patatin-like) | 3.1.1.* | At1g33270, At3g57140, | TgTigrScan_2912, | ? |
| At5g04040 | TgTigrScan_4606 | Sa | ||
| Phospholipase A1 | 3.1.1.32 | At1g31480 | TgTigrScan_5764 | ? |
| Acyl-CoA thioesterase | 3.1.2.20 | At2g30720, At5g48370 | TgTigrScan_1278 | ? |
No gene candidates for chloroplast-like galactolipid syntheses could be identified. In contrast, two sequences possibly encoding enzymes of the PG synthesis pathway could be predicted as apicoplast-targeted (Table 2). In the endoplasmic reticulum, transfer of an acyl to a three-carbon backbone may not involve a glycerol-3-phosphate acyltransferase as it occurs in plants. An alternative pathway, known in animal endoplasmic reticulum, utilizes glycerol-3-phosphate or dihydroxyacetone-3-phosphate as a substrate for the three-carbon backbone. Using the yeast dual dihydroxyacetone-3-phosphate/glycerol-3-phosphate acyltransferase as a probe, we could indeed identify, in the Toxoplasma genome annotation release [43–45], a gene candidate predicted to be targeted to the endoplasmic reticulum. Genes possibly encoding important steps of endoplasmic reticulum phospholipids (PC, PE, PI, PS and PG) and ceramide metabolism were also predicted (Table 2).
Table 2 shows gene candidates for acyl-lipid catabolism. In this latter section of the Table, the few genes characterized might occur in various cell compartments, as judged from the automated target prediction. Thus, in addition to the inventory of possible components of the membrane lipid synthetic machineries, we could record some clues about lipid degradation enzymes that may be responsible for diversion of some host cell lipids.
DISCUSSION
In the present study, metabolic labelling profiles of membrane lipids of Toxoplasma tachyzoites were analysed (Figure 1). Our results highlighted for the first time the production of numerous glycero- and sphingo-lipid classes in extracellular tachyzoites (Figure 2, Table 1). More importantly, syntheses of all these lipids were affected by haloxyfop, demonstrating that their de novo syntheses necessarily required a functional apicoplast FAS II pathway.
The major ubiquitous phospholipids (PC, PE, PS, PI and PG) were abundantly synthesized under all conditions, consistent with the massive need for such components in most membranes. Mitochondrial membrane biogenesis seems also to occur during the extracellular life stage, as DPG synthesis was also detected. Part of the labelled PG might serve as an immediate precursor for DPG synthesis. Interestingly, although the chloroplast-like galactolipids MGDG and DGDG had been previously measured in suspensions of partly lysed Toxoplasma cells, no synthesis of MGDG could be observed under any of our experimental labelling conditions. In contrast, we detected production of DGDG (Figure 2A). The de novo synthesis of DGDG seems therefore likely to be the result of a channelled process, with no accumulation of the MGDG intermediate. A similar process has been shown to occur in Euglena gracilis whose plastid also finds its origin from a secondary endosymbiosis. The E. gracilis chloroplastic MGDG is so rapidly transformed into DGDG that its synthesis is nearly not detectable [52]. In plants, plastids are also characterized by their content of SQDG (sulphoquinovosyldiacylglycerol), a sulphonated glycolipid. We could never detect any synthesis of SQDG, either in the present metabolic labelling study or in independent attempts to assay an SQDG synthesis in partly lysed parasites (results not shown). Consistently, no gene candidate involved in SQDG synthesis could be inventoried (Table 2).
If the above results show that T. gondii is capable of synthesizing all lipids required for its viability, including parasite-specific glycerolipids, the profile of lipids synthesized by intracellular tachyzoites using 14C-labelled material from HFF host cells is less complex (Figure 4B). The parasite diverts host cell precursors to contribute to the syntheses of major phospholipids, mostly PC, PE, PS, PI and the mitochondrial DPG, as well as some of the unknown lipids (Table 1), suggesting that both de novo production and recycling of building blocks diverted from host cells are likely to coexist. This lipid acquisition would be facilitated by the intimate and specific apposition of the membrane of the PV with the host cell lipid biosynthesis apparatus, i.e. the endoplasmic reticulum and the mitochondria [53].
Therefore most of the lipid metabolism would be a quantitative shift from an autonomous to a partly dependent process. This conclusion raises two important questions. First, why is haloxyfop lethal, if most of the parasite lipids could be diverted from host components? Part of the answer may lie in the characterization and understanding of the biological roles of U1 and DGDG glycerolipids that are specific to the parasite and strictly dependent on the apicoplast FAS II pathway. Alternatively, apicoplast FAS II may produce unique and vital FA molecular species, used for dedicated purposes other than glycero- or sphingo-lipid manufacturing. Secondly, why would Toxoplasma be dependent on host cell lipids to divide, when it is capable of synthesizing most of its lipids autonomously? Obviously, processes other than membrane biogenesis may be involved; nevertheless part of the answer may also lie in the biological function of U8, a glycerolipid that we only detected after invasion and incorporation of host cell labelled carbons (Figure 3B, Table 1).
In eukaryotic cells, redundant and branched pathways of production and transformation of glycerolipids occur in various membrane compartments. A given class of lipids can be scattered in diverse pools and metabolized owing to distinct compartmentalized pathways. To that extent, no detailed conclusion on the subcellular metabolism of lipids can be driven from the global metabolic profile analyses presented here. However, we can deduce an important hypothesis based on general considerations regarding glycerolipid metabolism, particularly when addressing the question of apicoplast-synthesized versus host cell-derived FA sources. FAs are thiol-esterified either to ACP or to CoA. In plant cells, acyl-ACPs are primarily generated in the chloroplast owing to the FAS II pathway. They can be transported outside the plastid after exchange with CoA, supplying the cytosol with acyl-CoA. In most glycerolipid synthesis sites, the transfer of acyl to a three-carbon backbone implies a glycerol-3-phosphate substrate. It occurs either in the plastid inner membrane, using stromal acyl-ACP, or in the endoplasmic reticulum and mitochondria membranes, utilizing acyl-CoA. PA produced can be consequently hydrolysed into DAG (diacylglycerol) due to a phosphatidate phosphatase. These two compounds are key intermediates for glycerolipid syntheses in the plant plastid membranes (for MGDG, DGDG and PG) [54], the endoplasmic reticulum (for PC, PS, PE, PI and PG) and the mitochondrial membranes (for PG and DPG). Occurrence of glycerolipids outside their biosynthetic sites further requires vesicular and non-vesicular trafficking systems. If the lipid metabolism of Toxoplasma was indeed plant-like, one would deduce from our results that apicoplast-synthesized FAs should be primarily coupled with ACP and be either directly available for apicoplast glycerolipid syntheses or transported to other sites after exchange of ACP by CoA. Alternatively, FAs imported from the host cell might be coupled with CoA, producing acyl-CoA and be available for glycerolipid syntheses in endomembranes and mitochondria. The lack of U1 and DGDG synthesis in intracellular parasites might indicate an incapacity for generating acyl-ACP from host cell material. Thus the acyl-ACP→acyl-CoA route might be non-reversible, a phenomenon that would explain the necessary conservation of the apicoplast FAS II pathway during evolution.
The complex profile of lipids that the parasite can synthesize from apicoplast acyl-ACP suggests that Toxoplasma contains the entire corresponding enzymatic factories. Table 2 gives an inventory of gene candidates from the Toxoplasma genome that would contribute to de novo glycerophospholipid syntheses. No complete apicoplast, endoplasmic reticulum or mitochondrial pathway could be recovered, but gene candidates for most important steps were predicted. The accuracy of this list probed in the early draft genomic annotation [43–45] will undoubtedly benefit from the advances of the collaborative annotation project in the near future. Some sequences may be confirmed, others rejected; however, important trends may be deduced. In this enquiry, four gene candidates might be targeted to the apicoplast, further supporting that, like plant plastids, this organelle is possibly an acyl-ACP-dependent glycerolipid factory. Transfer from acyl-ACP probably involves a glycerol-3-phosphate acyltransferase, as in plant plastids. Whereas in plants a glycerol-3-phosphate acyltransferase isoenzyme is responsible for the first transfer of an acyl from acyl-CoA in the endoplasmic reticulum, we could not identify such a gene in Toxoplasma. In animal cells and yeast, the substrate for the three-carbon backbone can alternatively be dihydroxyacetone-3-phosphate. In the present census, we could identify a gene candidate corresponding to an endoplasmic reticulum bifunctional glycerol-3-phosphate/dihydroxyacetone-3-phosphate acyltransferase (Table 2). This composite metabolic picture would reflect the inheritance of an acyl-ACP/glycerol-3-phosphate glycerolipid synthetic pathway from the ancestral alga and of an acyl-CoA/glycerol-3-phosphate/dihydroxyacteone-3-phosphate pathway from the ancestral protozoa. Since the apicoplast is connected peripherally to the endomembrane system, the four membranes that surround the organelle may function as ‘centrifugally specialized’ glycerolipid machinery, with the acyl-ACP/glycerol-3-phosphate pathway in innermost membranes and the acyl-CoA/dihydroxyacetone-3-phosphate/glycerol-3-phosphate pathway in outermost membranes.
Acknowledgments
We thank Karine Musset for skilled technical support, especially for host cell and parasite propagation, Nahid Azzouz (Institut für Virologie, Philipps-Universität, Marburg, Germany) and Jacques Joyard (Laboratoire de Physiologie Cellulaire Végétale, UMR 5168, Grenoble, France) for helpful advice on metabolic labelling during the initial phases of this work, and Jean Gagnon (Lab. Transmission and Pathogenesis of Prion Diseases, FRE 2685, Grenoble, France) for a critical reading of this paper. This work was funded by the CNRS through the programme ‘Microbiologie Fondamentale’, by the ANVAR grant no. A0106220V/AT assigned to E.M. and a grant ‘Region Rhône-Alpes’ assigned to M.-F.C.-D. Genomic data were obtained from http://ToxoDB.org.Genomic provided by The Institute for Genomic Research (supported by NIH grant no. AI05093) and by the Sanger Center (Wellcome Trust).
References
- 1.Dubey J. P. Toxoplasma, Hammondia, Besnoitia, Sarcocystis, and other tissue cyst-forming coccidian of man and animals. In: Kreier J. P., editor. Parasitic Protozoa, vol. III. New York: Academic Press; 1977. pp. 101–237. [Google Scholar]
- 2.Luft B. J., Hafner R., Korzun A. H., Leport C., Antoniskis D., Bosler E. M., Bourland D. D., Uttamchandani R., Fuhrer J., Jacobson J., et al. Toxoplasmic encephalitis in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 1993;329:995–1000. doi: 10.1056/NEJM199309303291403. [DOI] [PubMed] [Google Scholar]
- 3.Wong S. Y., Remington J. S. Toxoplasmosis in pregnancy. Clin. Infect. Dis. 1994;18:853–862. doi: 10.1093/clinids/18.6.853. [DOI] [PubMed] [Google Scholar]
- 4.Wilson R. J. M., Williamson D. H., Preiser P. Malaria and other apicomplexans: the ‘plant’ connection. Infect. Agents Dis. 1994;3:29–37. [PubMed] [Google Scholar]
- 5.Wilson R. J. M., Denny P. W., Preiser P. R., Rangachari K., Roberts K., Roy A., Whyte A., Strath M., Moore D. J., Moore P. W., et al. Complete gene map of the plastid-like DNA of the malaria parasite Plasmodium falciparum. J. Mol. Biol. 1996;261:155–172. doi: 10.1006/jmbi.1996.0449. [DOI] [PubMed] [Google Scholar]
- 6.McFadden G. I., Reith M. E., Munholland J., Lang-Unnasch N. Plastid in human parasites. Nature (London) 1996;381:482. doi: 10.1038/381482a0. [DOI] [PubMed] [Google Scholar]
- 7.Maréchal E., Cesbron-Delauw M.-F. The apicoplast: a new member of the plastid family. Trends Plant Sci. 2001;6:200–205. doi: 10.1016/s1360-1385(01)01921-5. [DOI] [PubMed] [Google Scholar]
- 8.Kirk J. T. O., Tilney-Bassett R. A. E. Their Chemistry, Structure, Growth and Inheritance. North-Holland: Elsevier; 1978. The plastids. [Google Scholar]
- 9.Neuhaus H. E., Emes M. J. Nonphotosynthetic metabolism in plastids. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000;51:111–140. doi: 10.1146/annurev.arplant.51.1.111. [DOI] [PubMed] [Google Scholar]
- 10.Duke S. O. Overview of herbicide mechanisms of action. Environ. Health Perspect. 1990;87:263–271. doi: 10.1289/ehp.9087263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maréchal E. Chloroplast biogenesis and function are first in the list of essential Arabidopsis genes. Trends Plant Sci. 2002;7:99–100. doi: 10.1016/s1360-1385(02)02248-3. [DOI] [PubMed] [Google Scholar]
- 12.Fichera M. E., Roos D. S. A plastid organelle as a drug target in apicomplexan parasites. Nature (London) 1997;390:407–409. doi: 10.1038/37132. [DOI] [PubMed] [Google Scholar]
- 13.Gleeson M. T. The plastid in Apicomplexa: what use is it? Int. J. Parasitol. 2000;30:1053–1070. doi: 10.1016/s0020-7519(00)00100-4. [DOI] [PubMed] [Google Scholar]
- 14.Weissig V., Vetro-Widenhouse T. S., Rowe T. C. Topoisomerase II inhibitors induce cleavage of nuclear and 35-kb plastid DNAs in the malarial parasite Plasmodium falciparum. DNA Cell Biol. 1997;16:1483–1492. doi: 10.1089/dna.1997.16.1483. [DOI] [PubMed] [Google Scholar]
- 15.Ralph S. A., D'Ombrain M. C., McFadden G. I. The apicoplast as an antimalarial drug target. Drug Resist. Update. 2001;4:145–151. doi: 10.1054/drup.2001.0205. [DOI] [PubMed] [Google Scholar]
- 16.He C. Y., Shaw M. K., Pletcher C. H., Striepen B., Tilney L. G., Roos D. S. A plastid segregation defect in the protozoan parasite Toxoplasma gondii. EMBO J. 2001;20:330–339. doi: 10.1093/emboj/20.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haldar K., Ferguson M. A., Cross G. A. Acylation of a Plasmodium falciparum merozoite surface antigen via sn-1,2-diacyl glycerol. J. Biol. Chem. 1985;260:4969–4974. [PubMed] [Google Scholar]
- 18.Holz G. G., Jr Lipids and the malarial parasite. Bull. WHO. 1977;55:237–248. [PMC free article] [PubMed] [Google Scholar]
- 19.Matesanz F., Duran-Chica I., Alcina A. The cloning and expression of Pfacs1, a Plasmodium falciparum fatty acyl coenzyme A synthetase-1 targeted to the host erythrocyte cytoplasm. J. Mol. Biol. 1999;291:59–70. doi: 10.1006/jmbi.1999.2964. [DOI] [PubMed] [Google Scholar]
- 20.Surolia A., Ramya T. N., Ramya V., Surolia N. 'FAS't inhibition of malaria. Biochem. J. 2004;383:401–412. doi: 10.1042/BJ20041051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waller R. F., Keeling P. J., Donald R. G. K., Striepen B., Handman E., Lang-Unnasch N., Cowman A. F., Besra G. S., Roos D. S., McFadden G. I. Nuclear-encoded proteins target to the apicoplast in Toxoplasma gondii and Plasmodium falciparum. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12352–12357. doi: 10.1073/pnas.95.21.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zuther E., Johnson J. J., Haselkorn R., McLeod R., Gornicki P. Growth of Toxoplasma gondii is inhibited by aryloxyphenoxypropionate herbicides targeting acetyl-CoA carboxylase. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13387–13392. doi: 10.1073/pnas.96.23.13387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLeod R., Muench S. P., Rafferty J. B., Kyle D. E., Mui E. J., Kirisits M. J., Mack D. G., Roberts C. W., Samuel B. U., Lyons R. E., et al. Triclosan inhibits the growth of Plasmodium falciparum and Toxoplasma gondii by inhibition of apicomplexan Fab I. Int. J. Parasitol. 2001;31:109–113. doi: 10.1016/s0020-7519(01)00111-4. [DOI] [PubMed] [Google Scholar]
- 24.Surolia N., Surolia A. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 2001;7:167–173. doi: 10.1038/84612. [DOI] [PubMed] [Google Scholar]
- 25.Charron A. J., Sibley L. D. Host cells: mobilizable lipid resources for the intracellular parasite Toxoplasma gondii. J. Cell Sci. 2002;115:3049–3059. doi: 10.1242/jcs.115.15.3049. [DOI] [PubMed] [Google Scholar]
- 26.Jelenska J., Sirikhachornkit A., Haselkorn R., Gornicki P. The carboxylase activity of the apicoplast acetyl-CoA carboxylase of Toxoplasma gondii is the target of aryloxyphenoxypropionate inhibitors. J. Biol. Chem. 2002;277:23208–23215. doi: 10.1074/jbc.M200455200. [DOI] [PubMed] [Google Scholar]
- 27.Levy C. W., Roujeinikova A., Sedelnikova S., Baker P. J., Stuitje A. R., Slabas A. R., Rice D. W., Rafferty J. B. Molecular basis of triclosan activity. Nature (London) 1999;398:383–384. doi: 10.1038/18803. [DOI] [PubMed] [Google Scholar]
- 28.Smith S. The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. FASEB J. 1994;8:1248–1259. [PubMed] [Google Scholar]
- 29.Lingelbach K., Joiner K. A. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: an unusual compartment in infected cells. J. Cell Sci. 1998;111:1467–1475. doi: 10.1242/jcs.111.11.1467. [DOI] [PubMed] [Google Scholar]
- 30.Ralph S. A., Van Dooren G. G., Waller R. F., Crawford M. J., Fraunholz M. J., Foth B. J., Tonkin C. J., Roos D. S., McFadden G. I. Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat. Rev. Microbiol. 2004;2:203–216. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 31.Mordue D. G., Sibley L. D. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J. Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- 32.Mordue D. G., Hakansson S., Niesman I., Sibley L. D. Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Exp. Parasitol. 1999;92:87–99. doi: 10.1006/expr.1999.4412. [DOI] [PubMed] [Google Scholar]
- 33.Sibley L. D., Weidner E., Krahenbuhl J. L. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature (London) 1985;315:416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- 34.Sinai A. P., Joiner K. A. Safe haven: the cell biology of nonfusogenic pathogen vacuoles. Annu. Rev. Microbiol. 1997;51:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- 35.Coppens I., Sinai A. P., Joiner K. A. Toxoplasma gondii exploits host low-density lipoprotein receptor-mediated endocytosis for cholesterol acquisition. J. Cell Biol. 2000;149:167–180. doi: 10.1083/jcb.149.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonda S., Ting L.-M., Novak S., Kim K., Maher J. J., Farese R. V., Jr, Ernst J. D. Cholesterol esterification by host and parasite is essential for optimal proliferation of Toxoplasma gondii. J. Biol. Chem. 2001;276:34434–34440. doi: 10.1074/jbc.M105025200. [DOI] [PubMed] [Google Scholar]
- 37.Robibaro B., Stedman T. T., Coppens I., Ngô H. M., Pypaert M., Bivona T., Nam H. W., Joiner K. A. Toxoplasma gondii Rab5 enhances cholesterol acquisition from host cells. Cell. Microbiol. 2002;4:139–152. doi: 10.1046/j.1462-5822.2002.00178.x. [DOI] [PubMed] [Google Scholar]
- 38.Coppens I., Joiner K. A. Host but not parasite cholesterol controls Toxoplasma cell entry by modulating organelle discharge. Mol. Biol. Cell. 2003;14:3804–3820. doi: 10.1091/mbc.E02-12-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quittnat F., Nishikawa Y., Stedman T. T., Voelker D. R., Choi J. Y., Zahn M. M., Murphy R. C., Barkley R. M., Pypaert M., Joiner K. A., et al. On the biogenesis of lipid bodies in ancient eukaryotes: synthesis of triacylglycerols by a Toxoplasma DGAT1-related enzyme. Mol. Biochem. Parasitol. 2004;138:107–122. doi: 10.1016/j.molbiopara.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Lecordier L., Mercier C., Sibley L. D., Cesbron-Delauw M.-F. Transmembrane insertion of the Toxoplasma gondii GRA5 protein occurs after soluble secretion into the host cell. Mol. Biol. Cell. 1999;10:1277–1287. doi: 10.1091/mbc.10.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 42.Beisson F., Koo A. J. K., Ruuska S., Schwender J., Pollard M., Thelen J. J., Paddock T., Salas J. J., Savage L., Milcamps A., et al. Arabidopsis genes involved in acyl lipid metabolism. A 2003 census of the candidates, a study of the distribution of expressed sequence tags in organs, and a web-based database. Plant Physiol. 2003;132:681–697. doi: 10.1104/pp.103.022988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 44.Henikoff S., Henikoff J. G. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10915–10919. doi: 10.1073/pnas.89.22.10915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Codani J. J., Comet J. P., Aude J. C., Glémet E., Wozniak A., Risler J. L., Hénaut A., Slonimski P. P. Automatic analysis of large-scale pairwise alignments of protein sequences. Methods Microbiol. 1999;28:229–244. [Google Scholar]
- 46.Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. Improved predicition of signal peptides: SignalP 3.0. J. Mol. Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 47.Emanuelsson O., Nielsen H., von Heijne G. ChloroP, a neural network-based method for predicting chloroplast transit peptides and their cleavage sites. Protein Sci. 1999;8:978–984. doi: 10.1110/ps.8.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Claros M. G., Vincens P. Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 1996;241:779–786. doi: 10.1111/j.1432-1033.1996.00779.x. [DOI] [PubMed] [Google Scholar]
- 49.Maréchal E., Azzouz N., Santos de Macedo C., Block M. A., Feagin J. E., Schwarz R. T., Joyard J. Synthesis of chloroplast galactolipids in apicomplexan parasites. Euk. Cell. 2002;1:653–656. doi: 10.1128/EC.1.4.653-656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Douce R. Site of biosynthesis of galactolipids in spinach chloroplasts. Science. 1974;183:852–853. doi: 10.1126/science.183.4127.852. [DOI] [PubMed] [Google Scholar]
- 51.Ohlrogge J., Pollard M., Bao X., Focke M., Girke T., Ruuska S., Mekhedov S., Benning C. Fatty acid synthesis: from CO2 to functional genomics. Biochem. Soc. Trans. 2000;28:567–573. [PubMed] [Google Scholar]
- 52.Matson R. S., Fei M., Chang S. B. Comparative studies of biosynthesis of galactolipids in Euglena gracilis strain Z. Plant Physiol. 1970;45:531–532. doi: 10.1104/pp.45.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sinai A. P., Webster P., Joiner K. A. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J. Cell Sci. 1997;110:2117–2128. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- 54.Benning C., Ohta H. Three enzyme systems for galactoglycerolipid biosynthesis are coordinately regulated in plants. J. Biol. Chem. 2005;280:2397–2400. doi: 10.1074/jbc.R400032200. [DOI] [PubMed] [Google Scholar]