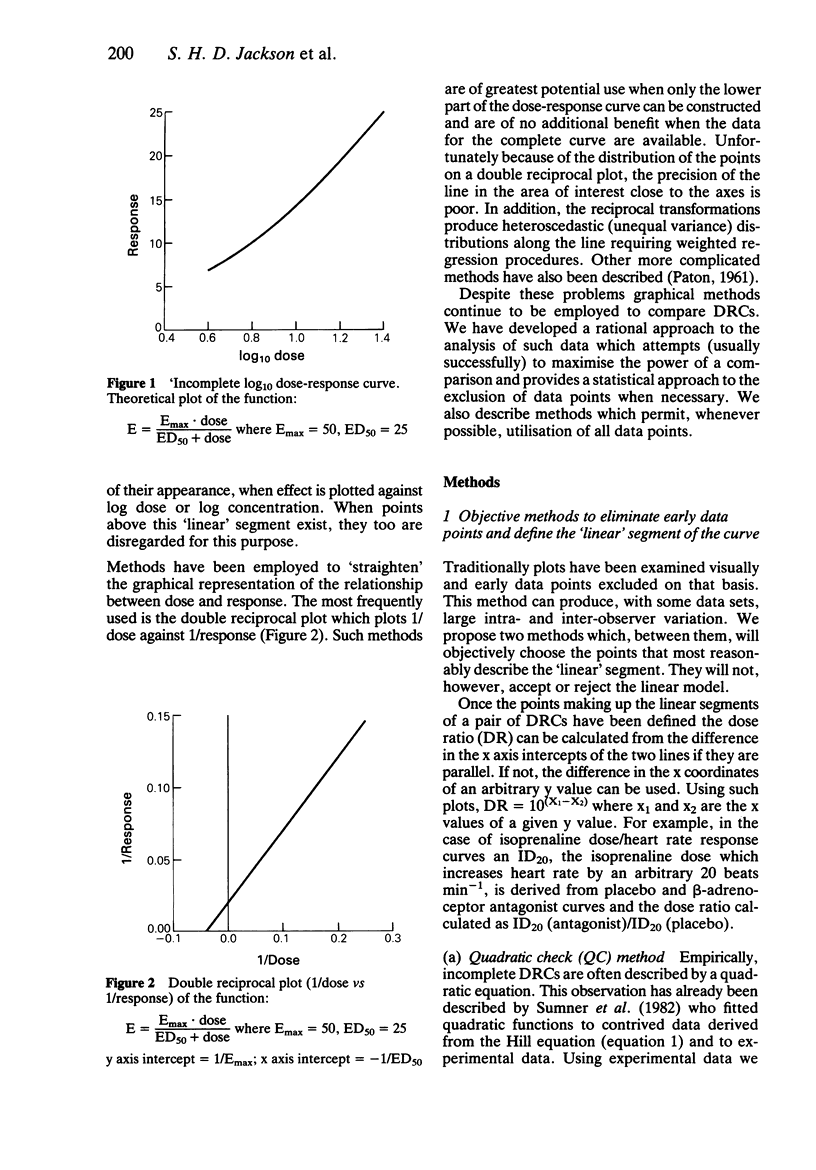
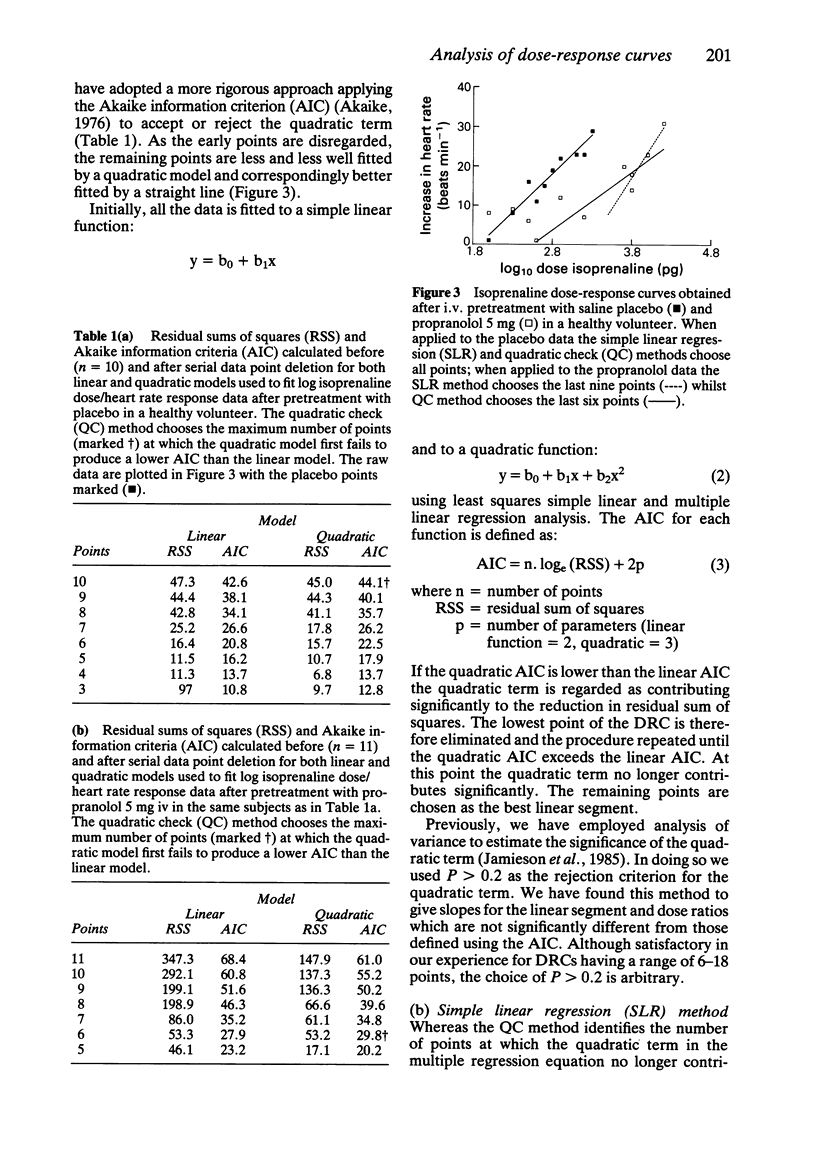
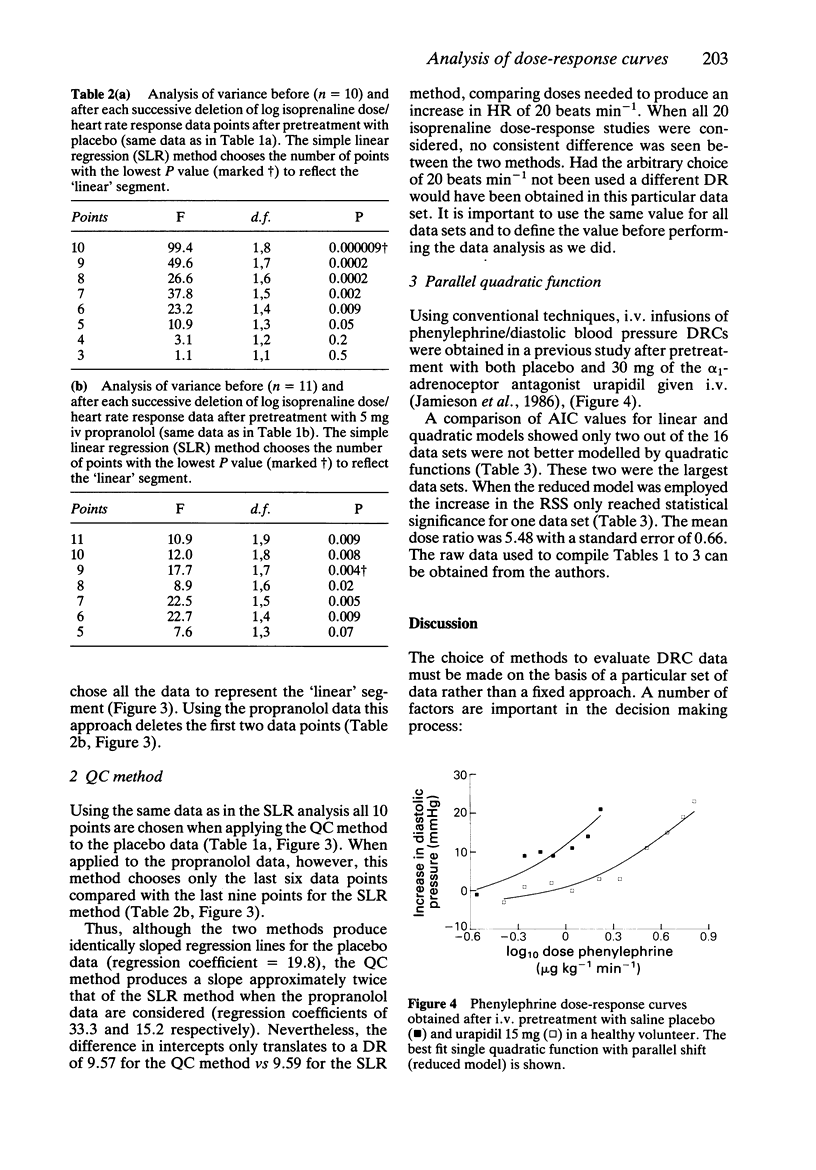
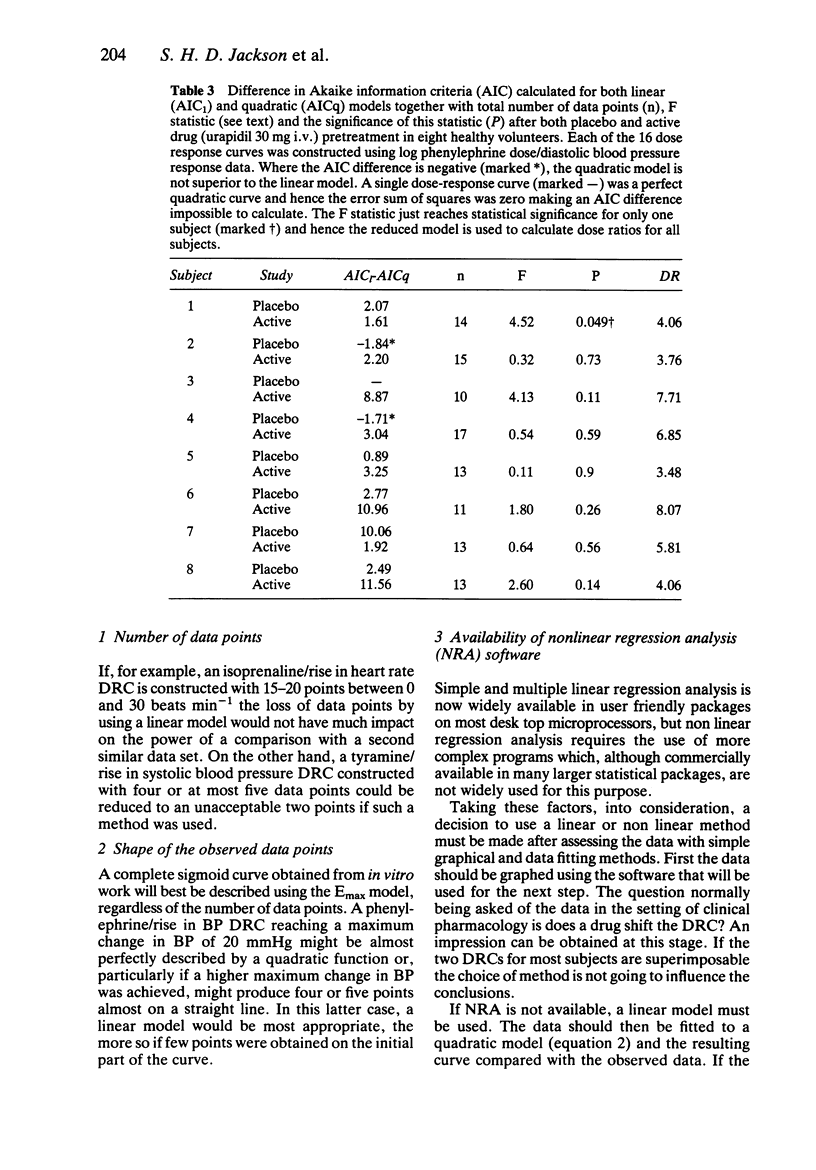
Abstract
The rationale for the objective assessment of dose-response curves (DRCs) is presented. Using data derived from isoprenaline/heart rate responses studies, two new statistical methods of objectively defining the terminal linear segment of an incomplete DRC are presented. Using data derived from phenylephrine/diastolic blood pressure response studies, the parallel shift quadratic model of Sumner et al. (1982) has been extended to include a measure of the suitability of the quadratic model for each individual data set using the Akaike information criterion. A parallel shift Emax model is proposed for complete DRCs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boxenbaum H. G., Riegelman S., Elashoff R. M. Statistical estimations in pharmacokinetics. J Pharmacokinet Biopharm. 1974 Apr;2(2):123–148. doi: 10.1007/BF01061504. [DOI] [PubMed] [Google Scholar]
- Johnston A. SIMP: a computer program in BASIC for nonlinear curve fitting. J Pharmacol Methods. 1985 Dec;14(4):323–329. doi: 10.1016/0160-5402(85)90008-7. [DOI] [PubMed] [Google Scholar]
- Sumner D. J., Elliott H. L., Reid J. L. Analysis of the pressor dose response. Clin Pharmacol Ther. 1982 Oct;32(4):450–458. doi: 10.1038/clpt.1982.188. [DOI] [PubMed] [Google Scholar]


