Abstract
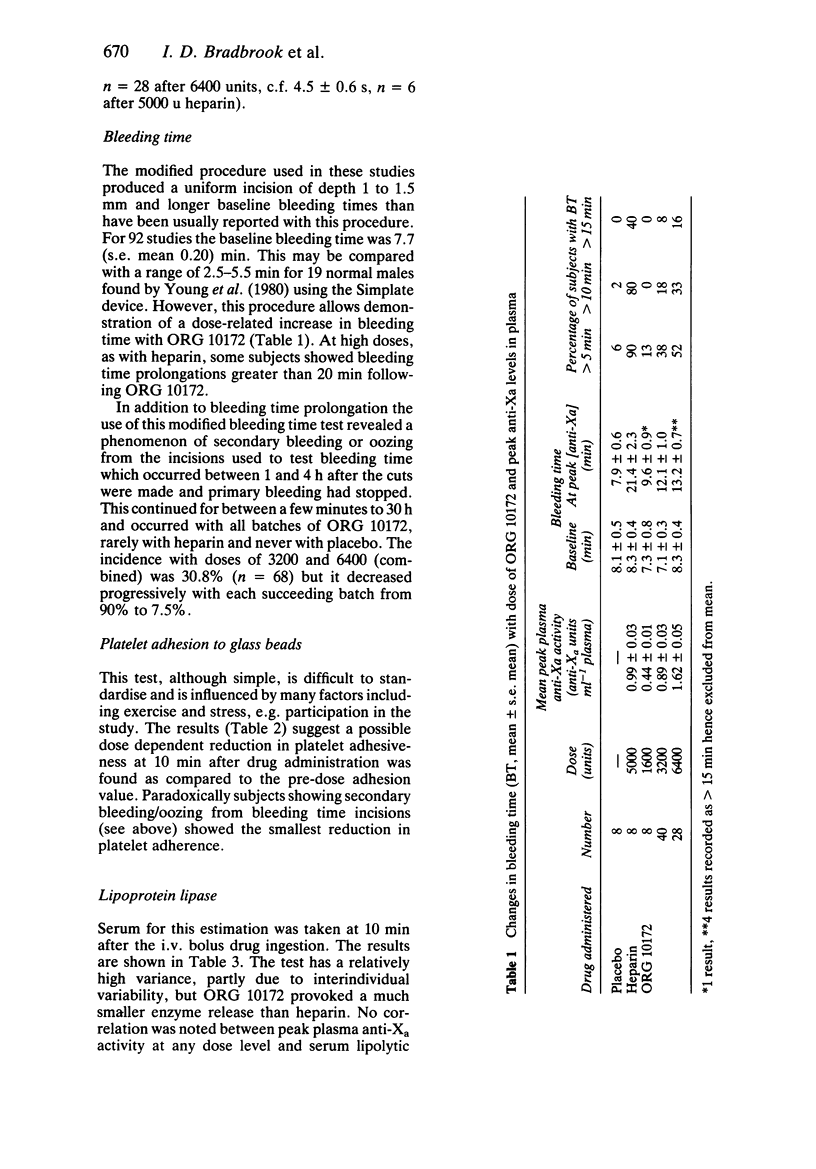
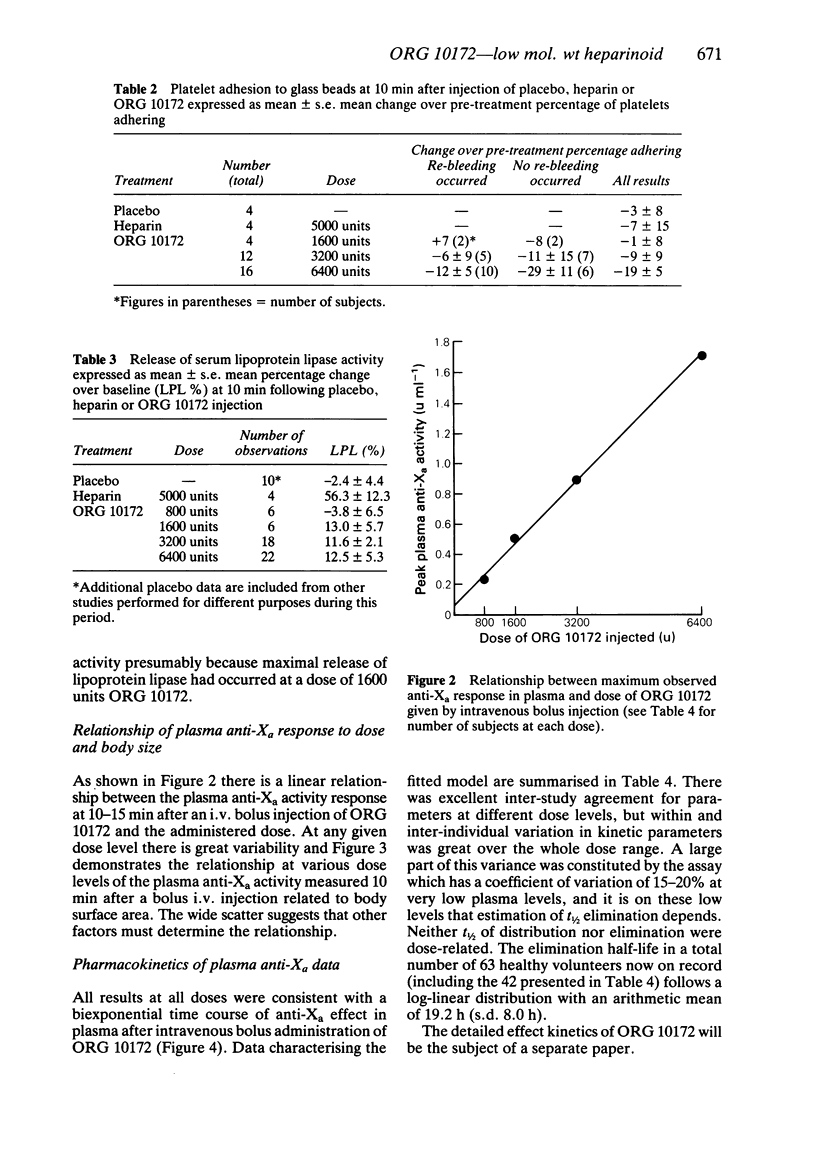
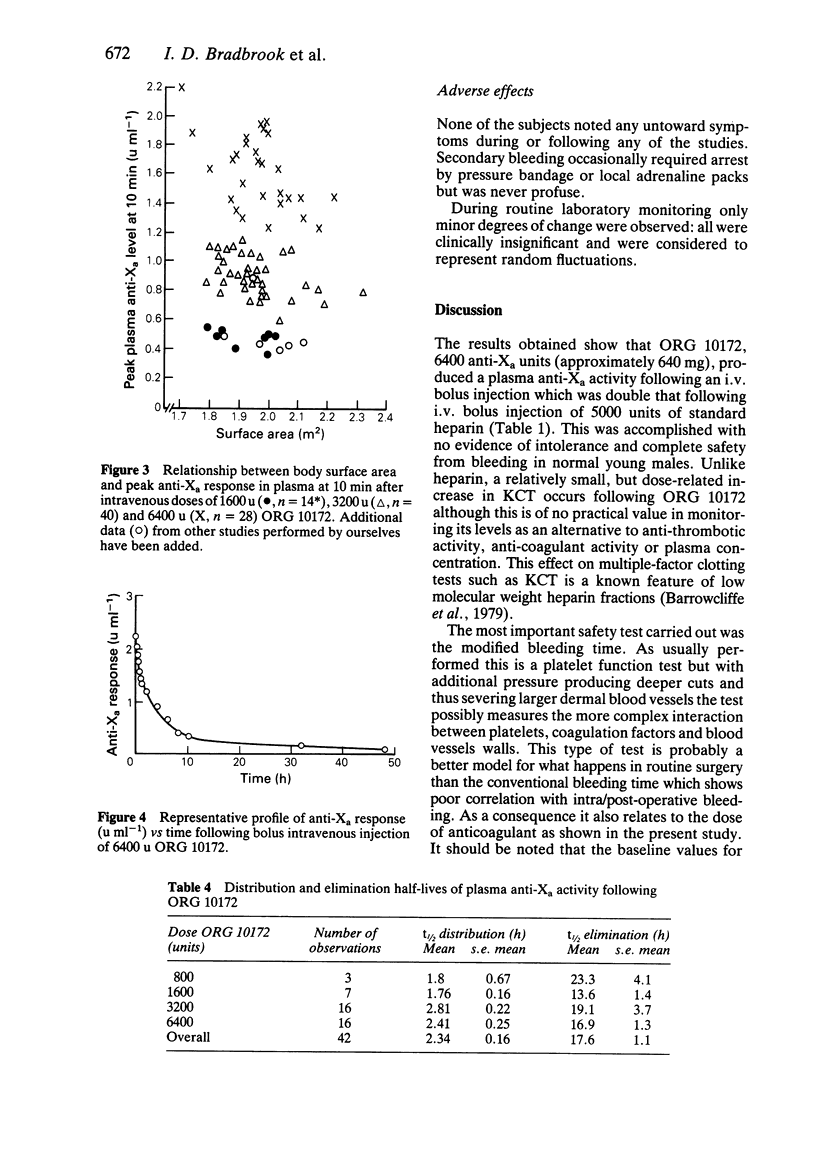
ORG 10172 is a heparinoid with mean molecular weight 6500 daltons. Intravenous bolus injections of ORG 10172 were compared with placebo and heparin injections in 91 separate studies in 83 healthy male subjects. 6400 units ORG 10172 produced a mean maximum change of 14.7 s in kaolin cephalin time (c.f. greater than 120 s for 5000 units heparin). Changes in prothrombin time were minimal (1.6 s for 6400 units ORG 10172 and 4.5 s after 5000 units heparin). A dose-related increase in bleeding time occurred after ORG 10172 and at high doses (greater than 3200 units) some secondary bleeding, which was never serious, occurred at between 1 and 4 h after incision. A dose-dependent reduction in ex vivo platelet adhesiveness was found at 10 min after ORG 10172 injection. ORG 10172 promoted a much smaller release of lipoprotein lipase as compared with heparin. The effect of ORG 10172 on plasma factor Xa activity (one measure of its action) was described by a biexponential decay with a mean distribution half-life of 2.34 (s.e. mean 0.16) h and mean disposition half-life of 17.6 (s.e. mean 1.1) h. It thus has a much longer duration of effect than heparin. There was a linear relationship of plasma anti-Xa response to increasing dose although there was some variability only partly explained by differences in body weight or surface area. ORG 10172 administration by bolus intravenous injection was well tolerated and there was no evidence of adverse effects on clinical chemistry or haematology tests.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrowcliffe T. W., Johnson E. A., Eggleton C. A., Kemball-Cook G., Thomas D. P. Anticoagulant activities of high and low molecular weight heparin fractions. Br J Haematol. 1979 Apr;41(4):573–583. doi: 10.1111/j.1365-2141.1979.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Carter C. J., Kelton J. G., Hirsh J., Gent M. Relationship between the antithrombotic and anticoagulant effects of low molecular weight heparin. Thromb Res. 1981 Jan 1;21(1-2):169–174. doi: 10.1016/0049-3848(84)90045-8. [DOI] [PubMed] [Google Scholar]
- Cipolle R. J., Seifert R. D., Neilan B. A., Zaske D. E., Haus E. Heparin kinetics: variables related to disposition and dosage. Clin Pharmacol Ther. 1981 Mar;29(3):387–393. doi: 10.1038/clpt.1981.53. [DOI] [PubMed] [Google Scholar]
- Decousus H. A., Croze M., Levi F. A., Jaubert J. G., Perpoint B. M., De Bonadona J. F., Reinberg A., Queneau P. M. Circadian changes in anticoagulant effect of heparin infused at a constant rate. Br Med J (Clin Res Ed) 1985 Feb 2;290(6465):341–344. doi: 10.1136/bmj.290.6465.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellem A. J. Platelet adhesiveness in von Willebrand's disease. A study with a new modification of the glass bead filter method. Scand J Haematol. 1970;7(5):374–382. [PubMed] [Google Scholar]
- Henny C. P., Ten Cate H., Ten Cate J. W., Surachno S., van Bronswijk H., Wilmink J. M., Ockelford P. A. Use of a new heparinoid as anticoagulant during acute haemodialysis of patients with bleeding complications. Lancet. 1983 Apr 23;1(8330):890–893. doi: 10.1016/s0140-6736(83)91326-0. [DOI] [PubMed] [Google Scholar]
- Hirsh J., van Aken W. G., Gallus A. S., Dollery C. T., Cade J. F., Yung W. L. Heparin kinetics in venous thrombosis and pulmonary embolism. Circulation. 1976 Apr;53(4):691–695. doi: 10.1161/01.cir.53.4.691. [DOI] [PubMed] [Google Scholar]
- Holmer E., Lindahl U., Bäckström G., Thunberg L., Sandberg H., Söderström G., Anderson L. O. Anticoagulant activities and effects on platelets of a heparin fragment with high affinity for antithrombin. Thromb Res. 1980 Jun 15;18(6):861–869. doi: 10.1016/0049-3848(80)90208-x. [DOI] [PubMed] [Google Scholar]
- Jaques L. B. Heparins--anionic polyelectrolyte drugs. Pharmacol Rev. 1979 Jun;31(2):99–166. [PubMed] [Google Scholar]
- Mant M. J., O'Brien B. D., Thong K. L., Hammond G. W., Birtwhistle R. V., Grace M. G. Haemorrhagic complications of heparin therapy. Lancet. 1977 May 28;1(8022):1133–1135. doi: 10.1016/s0140-6736(77)92388-1. [DOI] [PubMed] [Google Scholar]
- Meuleman D. G., Hobbelen P. M., van Dedem G., Moelker H. C. A novel anti-thrombotic heparinoid (Org 10172) devoid of bleeding inducing capacity: a survey of its pharmacological properties in experimental animal models. Thromb Res. 1982 Aug 1;27(3):353–363. doi: 10.1016/0049-3848(82)90082-2. [DOI] [PubMed] [Google Scholar]
- Mielke C. H., Jr, Kaneshiro M. M., Maher I. A., Weiner J. M., Rapaport S. I. The standardized normal Ivy bleeding time and its prolongation by aspirin. Blood. 1969 Aug;34(2):204–215. [PubMed] [Google Scholar]
- Mikhailidis D. P., Barradas M. A., Mikhailidis A. M., Magnani H., Dandona P. Comparison of the effect of a conventional heparin and a low molecular weight heparinoid on platelet function. Br J Clin Pharmacol. 1984 Jan;17(1):43–48. doi: 10.1111/j.1365-2125.1984.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman C. S., Provan J. L. Control and complications of intermittent heparin therapy. Surg Gynecol Obstet. 1977 Sep;145(3):338–342. [PubMed] [Google Scholar]
- Ockelford P. A., Carter C. J., Mitchell L., Hirsh J. Discordance between the anti-Xa activity and the antithrombotic activity in an ultra-low molecular weight heparin fraction. Thromb Res. 1982 Nov 1;28(3):401–409. doi: 10.1016/0049-3848(82)90121-9. [DOI] [PubMed] [Google Scholar]
- Ofosu F. A., Modi G., Cerskus A. L., Hirsh J., Blajchman M. A. Heparin with low affinity to antithrombin III inhibits the activation of prothrombin in normal plasma. Thromb Res. 1982 Nov 15;28(4):487–497. doi: 10.1016/0049-3848(82)90165-7. [DOI] [PubMed] [Google Scholar]
- Olivecrona T., Bengtsson G., Marklund S. E., Lindahl U., Hök M. Heparin-lipoprotein lipase interactions. Fed Proc. 1977 Jan;36(1):60–65. [PubMed] [Google Scholar]
- Salzman E. W., Deykin D., Shapiro R. M., Rosenberg R. Management of heparin therapy: Controlled prospective trial. N Engl J Med. 1975 May 15;292(20):1046–1050. doi: 10.1056/NEJM197505152922002. [DOI] [PubMed] [Google Scholar]
- Ten Cate H., Henny C. P., Büller H. R., Ten Cate J. W., Magnani H. N. Use of a heparinoid in patients with hemorrhagic stroke and thromboembolic disease. Ann Neurol. 1984 Mar;15(3):268–270. doi: 10.1002/ana.410150311. [DOI] [PubMed] [Google Scholar]
- Tietz N. W., Fiereck E. A. A specific method for serum lipase determination. Clin Chim Acta. 1966 Mar;13(3):352–358. doi: 10.1016/0009-8981(66)90215-4. [DOI] [PubMed] [Google Scholar]
- Wessler S., Gitel S. N. Heparin: new concepts relevant to clinical use. Blood. 1979 Apr;53(4):525–544. [PubMed] [Google Scholar]
- Wilson J. E., 3rd, Bynum L. J., Parkey R. W. Heparin therapy in venous thromboembolism. Am J Med. 1981 Apr;70(4):808–816. doi: 10.1016/0002-9343(81)90537-4. [DOI] [PubMed] [Google Scholar]
- Young V. P., Giles A. R., Pater J., Corbett W. E. Sex differences in bleeding time and blood loss in normal subjects following aspirin ingestion. Thromb Res. 1980 Dec 1;20(5-6):705–709. doi: 10.1016/0049-3848(80)90160-7. [DOI] [PubMed] [Google Scholar]


