Abstract
In secretory carrier membrane proteins (SCAMPs), the most conserved structural segment is between transmembrane spans 2 and 3, facing the cytosol. A synthetic peptide, CWYRPIYKAFR (E peptide), from this segment of SCAMP2 potently inhibits exocytosis in permeabilized neuroendocrine (PC12) cells. E peptide blocked discharge of 35S-labeled secretogranin with the same structural selectivity and potency as observed for hexosaminidase secretion in mast cells. SCAMPs 1 and 2 are concentrated primarily on intracellular membranes in PC12 cells. Both, however, are found on plasma membranes, but neither is present on large dense-core vesicles. Yet, large dense-core vesicles marked by secretogranin attach to plasma membranes at foci containing SCAMP2 along with syntaxin1 and complexin at putative cell-surface docking/fusion sites. Regulated overexpression of SCAMP2 with point mutations in its E peptide but not of normal SCAMP2 caused dose-dependent inhibition of depolarization-induced secretion. The SCAMP2 mutants also inhibited secretion stimulated by elevated calcium. Inhibition was largely overcome by adding lysophosphatidylcholine to the medium at concentrations that do not otherwise affect secretion. Although overexpression of normal or mutant SCAMP2 slightly inhibits endocytosis, this effect does not appear to be related to the specific effect of the mutant SCAMP on stimulated exocytosis. Thus, SCAMP2 not only colocalizes with fusion sites but also appears to have an essential function in granule exocytosis through actions mediated by its E peptide–containing domain.
INTRODUCTION
In the field of intracellular trafficking, it is now widely believed that fusion among membranes at their cytoplasmic surfaces is achieved by linking soluble N-ethylmaleimide–sensitive factor attachment protein (SNAP) receptor (SNARE) proteins between the partner membranes, which then drive the merger of associated bilayers by formation of stable SNARE complexes (McNew et al., 2000). Although analysis of these events using purified and reconstituted SNARE proteins has provided strong support for the possibility that the SNAREs represent the minimum machinery required for fusion (Weber et al., 1998; Parlati et al., 1999; McNew et al., 2000), several studies have suggested that other proteins support the fusion of biological membranes in ways that are complementary to the function of SNAREs. For example, certain proteins act in facilitating linkage of SNAREs (Sato and Wickner, 1998; Betz et al., 2001; Voets et al., 2001), whereas others promote assembly of SNARE oligomers or stabilize the primed state, which may be essential for the ensuing fusion (Littleton et al., 2001; Reim et al., 2001; Tokumaru et al., 2001; Chen et al., 2002). It remains questionable, however, whether SNARE complexes are sufficient for formation and stable expansion of aqueous pores that complete the fusion event. Indeed, studies of vacuole fusion in yeast have suggested that SNARE complexes may act together with a vacuolar transporter complex to facilitate assembly of an intermembrane proteolipid complex (containing the V0 portion of vacuolar ATPase) and its subsequent function in formation and expansion of fusion pores (Peters et al., 2001; Muller et al., 2002). These findings are complemented by recent reports implying a rate-limiting role of VAMP2/SNARE complexes proximal to final fusion and demonstrating function of synaptotagmins in regulating fusion pore expansion (Schoch et al., 2001; Wang et al., 2001).
We have been studying the role of secretory carrier membrane proteins (SCAMPs) in membrane trafficking. These integral membrane proteins with four transmembrane spans are conserved across animal and plant kingdoms and are widely distributed among membranes of the cell-surface recycling system, including secretory organelles and endosomes (Brand et al., 1991; Brand and Castle, 1993; Laurie et al., 1993; Fernandez-Chacon and Sudhof, 2000; Hubbard et al., 2000). Although they were not originally recognized to be present at significant levels in the plasma membrane, SCAMPs were recently detected in plasma membranes of CHO and NRK cell lines (our unpublished data) and are colocalized with the SNAREs SNAP-23 and syntaxin4 in the mast cell surface (Guo et al., 2002). Since their discovery, the function of SCAMPs has remained elusive, although two studies now point to their possible participation in membrane fusion. First, mast cells from mice in which the gene for SCAMP1 was ablated appear to have a defect in forming stabilized fusion pores during exocytosis (Fernandez-Chacon et al., 1999). Second, exocytosis is blocked at a very late step by a peptide (known as E peptide) (Hubbard et al., 2000) that is thought to compose part of the functional domain of SCAMP2 (Guo et al., 2002). Although these observations are quite interesting, the gene knockout study has not provided direct evidence that SCAMP1 is involved in exocytosis and has acknowledged the possibility that absence of this SCAMP could either impair exocytosis or accelerate endocytosis. In addition, even though the blockade of exocytosis by E peptide of SCAMP2 exhibited impressive sequence specificity and the sequence is unique to SCAMP, the direct relationship of the perturbations to the function of SCAMP2 was not assessed.
In the present study, we have provided a link between the inhibition of secretion by E peptide and the function of SCAMP2 in exocytosis using the rat pheochromocytoma (PC12) cell line, in which E peptide blocks large dense-core vesicle release. We have shown that SCAMP2 is concentrated at putative docking/fusion sites for large dense-core vesicles at the cell surface, and point mutants of full-length SCAMP2 within the E peptide segment exhibit a dose-dependent dominant-inhibitory effect on exocytosis when overexpressed in tetracycline-regulated cells. The data imply a function of SCAMP2 late in exocytosis that is manifest from its location in the plasma membrane rather than from an association with the secretory granule.
MATERIALS AND METHODS
Materials
Doxycycline (dox), poly-d-lysine, and phorbol dibutyrate were from Sigma (St. Louis, MO); calcium ionophore A23187 was from Calbiochem (San Diego, CA). Lysophosphatidylcholine (LPC; α-palmitoyl) was from Avanti, Inc. (Birmingham, AL). All SCAMP peptides were synthesized, purified, and analyzed by high-performance liquid chromatography and mass spectroscopy at the University of Virginia Biomolecular Research Facility. Monoclonal antibody (mAb) 9E10 against the myc epitope was obtained as described (Wu and Castle, 1997), and rabbit anti-human growth hormone was provided by Dr. A. Parlow, National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases. Mouse monoclonal antibodies to syntaxin1 (HPC1) and complexin were obtained from Sigma and BD-Biosciences Transduction Laboratories (Lexington, KY), respectively. Rabbit anti-secretogranin II was from Biodesign International (Saco, ME); portions of this antibody were biotinylated using NHS-biotin (Pierce Endogen, Rockford, IL) according to the manufacturer's instructions. Rabbit anti-syntaxin1 antibody was obtained from Synaptic Systems (Göttingen, Germany), and anti-SNAP25 was previously described (Guo et al., 1998). Fluorescence-labeled secondary antibodies and neutravidin conjugates were from Molecular Probes (Eugene, OR); rat transferrin (Sigma) was covalently labeled with Alexa 594 by use of a kit supplied by the manufacturer (Molecular Probes). 125I-goat anti-rabbit IgG was from NEN (Boston, MA), Life Science Products. Goat anti-rabbit IgG conjugated to 5-nm gold was from EY Laboratories (San Mateo, CA). Rabbit anti-SCAMP1 (1ω) was described previously (Wu and Castle, 1997), and anti-SCAMP2 (2τ) against a synthetic peptide (C)FSQGIFSSRTFHR of SCAMP2 conjugated to keyhole limpet hemocyanin was raised at Covance (Denver, PA)and affinity-purified on peptide covalently linked to Sulfolink (Pierce Endogen). Enzyme-linked immunosorbent assay (ELISA) kits for assay of human growth hormone were from Roche Molecular Biochemicals (Hertfordshire, UK). LipofectAmine Plus was from Life Technologies (Paisley, UK), pTRE2 plasmid from Clontech (BD-Biosciences, Palo Alto, CA), and cDNA encoding human growth hormone (hGH) inserted in pXGH5 from Nichols Institute Diagnostics (San Clemente, CA).
Cell Culture, Transfection, and Tetracycline-regulated Expression of SCAMP2 Constructs
Rat pheochromocytoma PC12 cells were a gift of Dr. Sam Green (University of Virginia), and tetracycline-regulated PC12 cells (tet-off) were obtained from Clontech. Both were cultured at 37°C and 10% CO2 in DMEM containing 10% horse serum (Hyclone, Logan, UT), 5% fetal bovine serum (Clontech). Tet-off PC12 cells were plated (1 × 106 cells/well) in poly-d-lysine coated 6-well dishes and incubated overnight in serum-containing medium followed by 2 h in serum-free DMEM. For cotransfection with hGH and SCAMP2, duplicate or triplicate wells received 2 μg pXGH5, 2 μg pTRE2 (either empty vector or vector containing N-myc–tagged, full-length wild-type or mutant SCAMP2 DNA), and LipofectAmine Plus according to the manufacturer's instructions. After 6 h, 2 ml of DMEM with serum and antibiotics was added. Various levels of expression of SCAMP2 constructs were obtained by adding dox (2 μg/ml) at the indicated times after beginning the transfection and continuing incubation to 72 h in the presence of dox.
To determine the level of expression of SCAMP2 polypeptides, the cells were lysed in 0.5% NP-40, 0.1% deoxycholate (DOC) in PBS containing protease inhibitors, and clarified lysates were subjected to SDS-PAGE, Western blotting with anti-SCAMP 2ω, followed by 125I-labeled secondary antibody and phosphorimager analysis using Image Quant software. N-myc tagged SCAMP is readily resolved from untagged SCAMP2, allowing quantification of exogenous and endogenous protein. The level of overexpression was calculated by correcting for the transfection efficiency, which was evaluated from immunofluorescence experiments.
Perturbation of Exocytosis in Permeabilized and Intact PC12 Cells
PC12 cells were incubated overnight in 24-well plates in DMEM containing 250 μCi/ml [35S]Na2SO4 (DuPont/NEN), washed, and chased 90 min at 37°C. The labeled cells were rinsed once in ice-cold Na-GB buffer (137 mM Na-glutamate, 2 mM MgCl2, 20 mM PIPES, pH 6.8, 1 mg/ml BSA) and incubated 15 min on ice in 3 U/ml streptolysin-0 (SLO) in Na-GB. After replacement of the medium with Na-GB, the cells were warmed to 37°C for 3 min and then returned to ice. The medium was replaced with ice-cold K-GB (K-glutamate replacing Na-glutamate in GB) containing 1 mM ATP and either 3 mM EGTA (control) or 3 mM Ca-EGTA (pCa 5; stimulated) and supplemented (or not) with SCAMP peptides. After 30 min on ice, the samples were warmed for 10 min at 37°C to elicit secretion. After incubation, media and resuspended cells were precipitated with 10% trichloroacetic acid, and pellets were extracted with cold acetone, solubilized in sample buffer, and subjected to SDS-PAGE. Fixed gels were dried and analyzed by phosphorimaging using Image Quant software to evaluate fractional discharge of 35S-labeled secretogranin.
Transfected PC12 cells expressing hGH and different levels of SCAMP2 polypeptide were assayed for hGH secretion essentially as described previously (Chung et al., 1999). At 72 h after transfection, the wells were washed with low-K+ buffer (in mM: 5.6 KCl, 145 NaCl, 2.2 CaCl2, 0.5 MgCl2, 15 HEPES, pH 7.4, 5.6 glucose) and then incubated in succession (10 min each incubation) with low-K+ buffer and high-K+ buffer (in mM: 56 KCl, 95 NaCl, 2.2 CaCl2, 0.5 MgCl2, 15 HEPES, pH 7.4, 5.6 glucose). LPC was included in some incubations in either low- or high-K+ buffer to test its effect on unstimulated and depolarization-induced secretion. After incubation, the cells were lysed 10 min on ice in NP-40 buffer containing protease inhibitors, and clarified supernatants were used along with the secretions for assay of hGH by ELISA. As an alternative to stimulation by depolarization, transfected cells were stimulated in low-K+ buffer containing calcium ionophore A23187 (0.5 μM) and phorbol dibutyrate (0.1 μM). Both drugs were dissolved in dimethyl sulfoxide, which was present at a final concentration of 0.001% during incubation. This level of solvent neither enhanced nor inhibited unstimulated or stimulated secretion (our unpublished results).
Mutagenesis and Cloning of SCAMP2 cDNA
Recombinant DNA encoding full-length SCAMP2 with an NH2-terminal myc epitope tag was constructed by PCR using SCAMP2 cDNA cloned into the EcoRI and XhoI sites of BlueScript SK−, pBS (Stratagene, La Jolla, CA). Mutations within the E peptide segment of N-myc-SCAMP2 were generated by sequence overlap extension PCR using overlapping pairs of primers that encode both base pair changes (Ho et al., 1989). Two mutations were used. In mutant A, the N-terminal cysteine in the E peptide segment (CWYRPIYKAFR) was changed to A, and in mutant B, the subsequent tryptophan was changed to A. The 5′ to 3′ primers of the pairs were mutant A: 5′-TTCCTTGCTTGGTACCGA-3′ and mutant B: 5′-TTCCTTTGTGCGTACCGA-3′. Mutations were confirmed by sequencing (University of Virginia Biomolecular Research Facility), and cDNAs were subcloned into pTRE2 using the NheI and SalI sites.
Cell Fractionation and Immunolabeling of PC12 Cells and Plasma Membrane Sheets
Purification of large dense-core vesicles from PC12 cells was performed by a previously published procedure (Stinchcombe and Huttner, 1994) with minor modifications. Cells from two or three 15-cm plates were scraped and transferred to 0.27 M sucrose plus protease inhibitors and homogenized by 20 passes through a chilled ball bearing homogenizer (0.0004-in clearance). The resulting homogenate was cleared of large organelles and subjected to successive velocity and equilibrium centrifugations as prescribed, and 0.45-ml fractions of the final gradient were used for chloroform-methanol extraction and SDS-PAGE/Western blotting. Antigens were detected by enhanced chemiluminescence or with 125I-labeled secondary antibodies and phosphorimaging.
For immunofluorescence, PC12 cells were plated on poly-d-lysine–coated coverslips. Intact cells were fixed 15 min with 3% formaldehyde, permeabilized 5 min in PIPES-buffered PBS, 0.05% saponin, and then incubated 10 min in PBS, 10 mM glycine and 30 min in PBS, 1% goat serum. Alternatively, cells were chilled on ice and then briefly sonicated (single pulse) to prepare plasma membrane sheets. The sonication medium was either 25 mM HEPES, pH 7.0, 25 mM KCl, 2.5 mM magnesium acetate, 0.2 mM dithiothreitol (Hussain et al., 1999) or 20 mM HEPES, pH 7.2, 120 mM K-glutamate, 20 mM K-acetate, 10 mM EGTA, 2 mM Mg-ATP, and 0.5 mM dithiothreitol (Lang et al., 2001). After fixation, washing, blocking, and incubation with primary and secondary antibodies (Wu and Castle, 1997), the specimens were mounted and examined in a Zeiss microscope. Most images were collected in 0.1-μm stacks and were digitally deconvolved using OpenLab software.
To examine the distribution of SCAMP in the plasma membrane of PC12 cells at the electron microscopic level, we immunolabeled sheets of plasma membrane that had been ripped away from the upper cell surface after adsorption to polylysine-coated electron microscope grids (Sanan and Anderson, 1991; Wilson et al., 2000). The cells cultured on polylysine-coated coverslips were washed in chilled buffer (in mM: 20 HEPES, pH 7.2, 120 K-glutamate, 20 K-acetate, 10 EGTA, 2 Mg-ATP, 0.5 dithiothreitol), adsorbed to the grids with light pressure, and torn manually by use of a forceps to lift the grids. After a brief wash in the same buffer, the samples were fixed for 15 min in 3% formaldehyde, blocked with 5% goat serum in PBS, and immunostained with anti-SCAMP 2 (in 5% goat serum, PBS) and goat anti-rabbit antibody conjugated to 5 nm colloidal gold (diluted 1:20 from the commercial stock in 5% goat serum in PBS). Subsequent processing through osmium tetroxide, tannic acid, and uranyl acetate was carried out as described (Wilson et al., 2000).
Transferrin Uptake
To examine the effect of exogenous SCAMP2, PC12 cells expressing myc-tagged SCAMP2 (wild-type or mutant) were incubated 1 h in serum-free medium and then for 5 min at 37°C in the presence of 20 μg/ml Alexa 594–labeled transferrin. The cells were then fixed and processed for microscopy, and both nontransfected and transfected (myc-stained) cells were assessed for concentration of fluorescent transferrin in perinuclear recycling endosomes. Images of cells were recorded without digital processing.
RESULTS
E Peptide Inhibits Stimulated Exocytosis in Permeabilized PC12 Cells
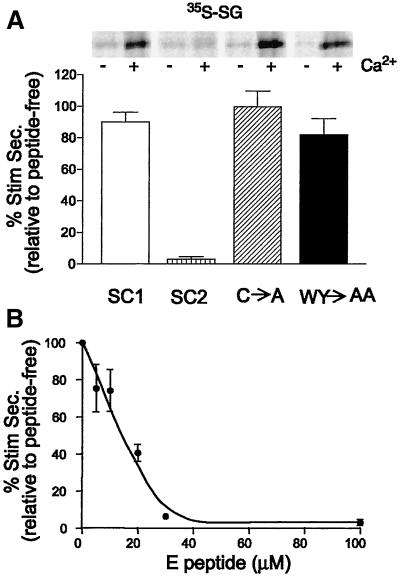
Recently, we demonstrated that synthetic E peptide corresponding to a portion of the short cytoplasm-facing segment linking the second and third transmembrane spans of SCAMP2 is a potent and sequence-specific inhibitor of exocytosis in permeabilized mast cells (Guo et al., 2002). The apparently unique association of the peptide segment with SCAMPs and the colocalization of SCAMPs with selected SNARE proteins at the mast cell surface suggested a possible role of SCAMP2 in exocytotic membrane fusion. However, the inability to easily transfect and culture highly differentiated mast cells was an obstacle to testing SCAMP2 function more directly. To complement these observations and to overcome the limitations in experimental approach, we examined whether E peptide inhibited regulated secretion in neuroendocrine PC12 cells, in which expression of mutant SCAMPs would be possible. PC12 cells were labeled with [35S]sulfate, and the discharge of 35S-labeled secretogranin from large dense-core vesicles was tested after SLO permeabilization, equilibration with peptide, and stimulation with buffered 10 μM Ca2+. As shown in Figure 1, E peptide from SCAMP2 (CWYRPIYKAFR) strongly inhibited secretion. In contrast, the corresponding peptide from SCAMP1 (G replacing K8; L6 replacing I6) and two structural variants of SCAMP2 E peptide (A replacing C1; AA replacing W2Y3) had little or no inhibitory effect. Autoradiographs included in Figure 1A illustrate the effect and also show that unstimulated secretion is not affected by any of the peptides. Half-maximal inhibition by SCAMP2 E peptide occurred at ∼15 μM (Figure 1B). Thus, the sequence specificity and potency of inhibition are the same as in mast cells (Guo et al., 2002).
Figure 1.
E peptide inhibits stimulated release of 35S-labeled secretogranin from permeabilized PC12 cells. Cells in 24-well plates were labeled overnight with [35S]sulfate, chased, and permeabilized with SLO. They were then equilibrated 30 min on ice with or without (control) EGTA-buffered Ca2+ (10 μM) and with or without E peptide. Secretion of 35S-labeled SG was evaluated as in MATERIALS AND METHODS. (A) E peptides from normal SCAMP2, normal SCAMP1, and structural variants of SCAMP2 in which residues 1, and 2 and 3 together, were changed from C to A and WY to AA, respectively, were compared at a concentration of 100 μM. Stimulated secretion of 35S-labeled secretogranin ranged from 35 to 45% of total above an unstimulated secretion of 1–2% of total. The inset presents a sample autoradiograph that demonstrates clearly the effects of the peptide on secretion. (B) Dose-response curve for inhibition of secretion by E peptide of SCAMP2. The results shown are normalized to stimulated secretion from peptide-free samples from three independent experiments and are plotted as mean ± SEM.
Distribution of SCAMPs 1 and 2 in PC12 Cells
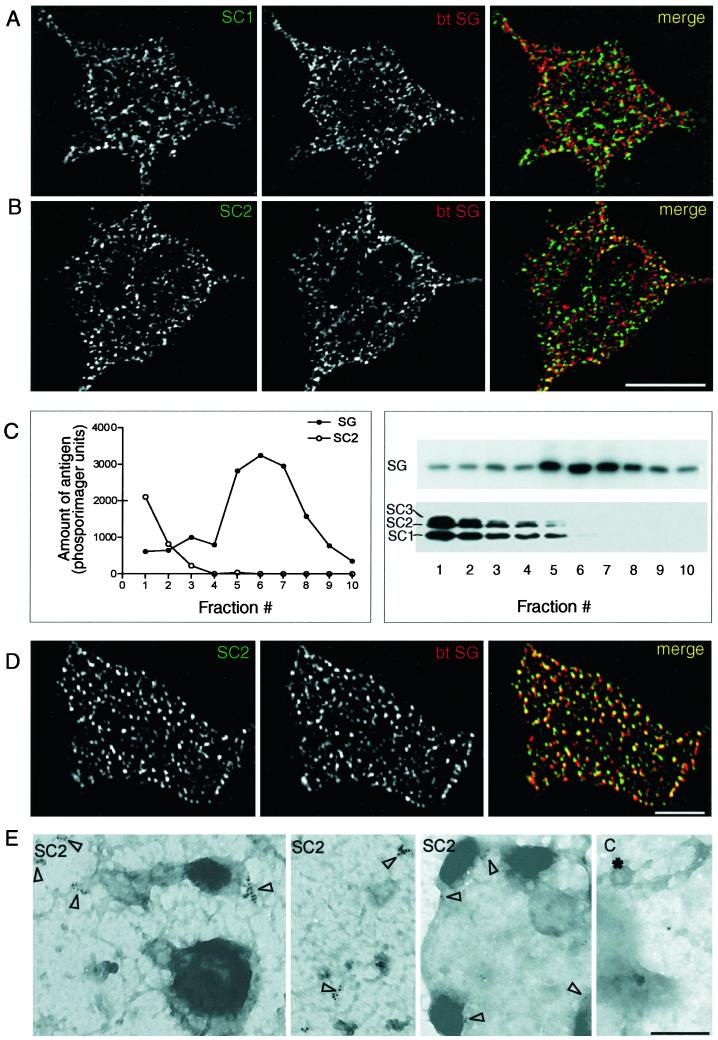
We conducted a series of studies to examine the localization of SCAMPs 1 and 2 in PC12 cells with particular interest in the extent to which the distribution of SCAMP2 reiterated or differed from that characterized in other cell types, especially mast cells (Guo et al., 2002). By immunofluorescence, both SCAMPs 1 and 2 are observed in foci throughout the cytoplasm and extending to the borders of the cells, consistent with a primary localization to endosomes and other recycling organelles. However, the distribution of secretogranin II (SG), a marker of large dense-core vesicles, was largely distinct (Figure 2, A and B). This observation immediately raised the question of whether the two SCAMPs are significant components of granule membranes. Notably, at occasional points near the cell periphery, the patterns of SG and SCAMP2 staining were similar, and their overlap in a merged image seemed likely (Figure 2B). To address the presence or absence of SCAMPs 1 and 2 in the granules more directly, we purified the granules by subcellular fractionation and compared the distributions of the SCAMPs and SG across gradient fractions by Western blotting. As shown in Figure 2C, SCAMPs 1 and 2 (and also 3) were concentrated together in low-density fractions, with negligible amounts codistributing with SG. Thus, surprisingly and in contrast to mast cells and other regulated secretory cell types that we have examined previously (Brand et al., 1991; Guo et al., 2002; our unpublished observations), SCAMPs 1 and 2 do not seem to be significant components of secretory granule membranes in PC12 cells.
Figure 2.
.Localization of SCAMPs 1 and 2 in PC12 cells. (A and B) Double-label immunofluorescence images showing the distributions of the two SCAMPs (stained with antibodies 1ω and 2τ and Alexa 488-tagged secondary antibody) compared with secretogranin (SG; stained with biotinylated anti-SG and avidin-Alexa 594). A digitally deconvolved section (0.2 μm) including an unstained nuclear profile is shown in each case. Bar, 10 μm. (C) Distribution of SCAMPs compared with secretogranin in large dense-core vesicles purified by gradient centrifugation. Plots of SG and SC2 are intensity profiles of bands from Western blots probed with anti-SG and anti-SC2 (2τ), detected with 125I-labeled secondary antibody, and analyzed by phosphorimaging. The accompanying Western blots show the distribution of SG and of SCAMPs 1–3 as detected by mAb 7C12 and enhanced chemiluminescence. (D) Distributions of SCAMP2 and SG as observed on plasma membrane sheets (Lang et al., 2001) using double-label immunofluorescence (antibodies as in B). The bar corresponds to 5 μm. (E) Immunogold-stained electron microscopic images showing multiply labeled SCAMP2 foci (open arrowheads) on plasma membranes torn from the upper cellular surface. Primary antibody 2τ was used. The fourth panel, labeled C, is a negative control showing an occasional solitary gold particle when primary antibody staining was omitted. * identifies a clathrin-coated pit; bar, 0.2 μm.
The absence of significant amounts of SCAMP2 in large dense-core vesicles yet its apparent overlap with SG at the cell periphery suggested possible colocalization with docked granules. To examine this possibility with minimal interference from SCAMP-containing intracellular organelles, we prepared plasma membrane sheets by light sonication of cells cultured on polylysine-coated coverslips and double-labeled them with antibodies to SCAMP2 and SG. As seen in the fluorescence micrographs in Figure 2D, SCAMP2 was indeed present throughout the surface in small foci, and the majority of the SG staining, marking large dense-core vesicles associated with the sheets, overlapped the SCAMP staining significantly. To confirm that the population of SCAMP2 observed on the plasma membranes was indeed in the cell surface, we carried out immunogold labeling on plasma membrane sheets prepared by adhering polylysine-coated electron microscope grids to the upper cell surface and ripping away from the rest of the cell. As seen in Figure 2E, immunogold particles representing SCAMP2 appeared in multiple discrete clusters within the surface, which were nearly always restricted to patches that were more intensely stained in the images. In a few instances, we visualized surfaces on which attachment of large dense-core vesicles appeared to have been preserved, and gold particles were present near the attachment sites (Figure 2E). We were unable to discern whether SCAMP2 was concentrated directly beneath the granules. When the primary anti-SCAMP2 antibody was omitted, labeling of the membrane surfaces was insignificant and appeared primarily in the form of occasional solitary gold particles (Figure 2E, control).
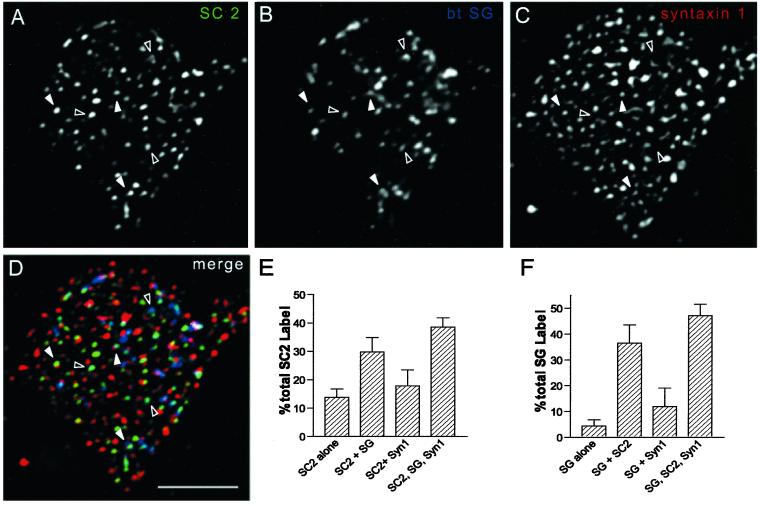
The overlap of SCAMP2 and SG on plasma membrane sheets and apparent association with sites of granule attachment (Figure 2D) seemed reminiscent of the association of large dense-core vesicles with foci of syntaxin1 at sites of exocytosis on PC12 cell plasma membranes (Lang et al., 2001). Consequently, we wondered to what extent SCAMP2 might be localized to prospective docking/fusion sites. To examine this possibility, we conducted triple labeling on the plasma membrane sheets prepared by sonication using rabbit anti-SCAMP2, mouse monoclonal anti-syntaxin1, and a biotinylated rabbit antibody against SG. The results are shown in Figure 3. Comparison of the individual labeling patterns and the tricolor overlay shows that there is significant colocalization of SCAMP2 with large dense-core vesicles and syntaxin1. Because the intensities of the three colors were not usually matched to give white spots, we quantified the distributions of SCAMP2 and SG foci (Figure 3, E and F). Strikingly, 70% of SCAMP2 was colocalized with SG or with SG together with syntaxin1, whereas 85% of SG localized either with SCAMP2 alone or together with syntaxin1. These findings strongly suggest that SCAMP2 is present at sites at which large dense-core vesicles dock at the plasma membrane and especially at sites at which these granules are likely to undergo syntaxin1-mediated fusion. We have confirmed the association of SG-containing large dense-core vesicles with SCAMP foci in the plasma membranes using double labeling with anti-SG and anti-SCAMP mAb 7C12 (data not shown).
Figure 3.
(A–F) Comparison of the distributions of SCAMP2, syntaxin1, and secretogranin by triple-immunostaining of plasma membrane sheets prepared according to Lang et al. (2001). (A–C) Individual images of a sheet stained with anti-SCAMP2 (2τ)/Alexa 488–tagged secondary antibody (A), biotinylated anti-SG/avidin-pacific blue (B), and anti-syntaxin 1 (mAb HPC1)/Alexa 594–tagged secondary antibody (C). (D) Merged image of A–C. Open arrowheads identify several examples of SCAMP2 overlapping SG but not syntaxin 1, and filled arrowheads identify overlapping SCAMP2, SG, and syntaxin 1. (E and F) Quantitative comparison of the distributions of SCAMP2 (E) and SG (F) with respect to the other labeled antigens obtained from counts of five separate plasma membrane sheets differing from the one shown in the figure. D: Bar, 5 μm.
Colocalization of Complexin with SCAMP2
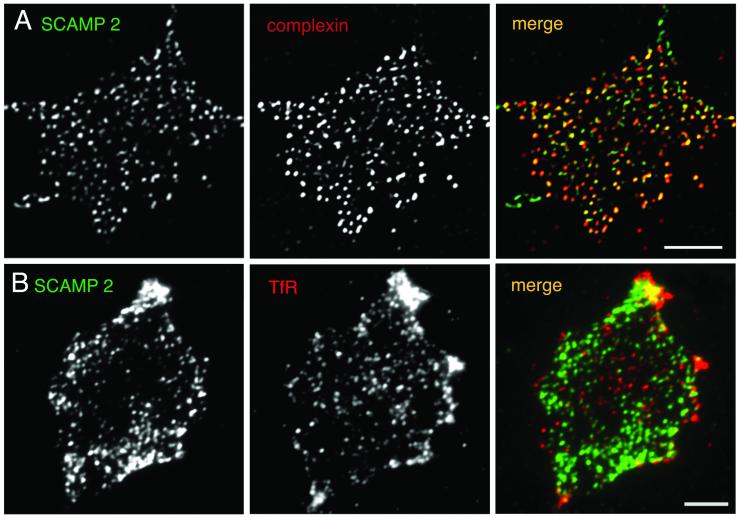
To evaluate further the close proximity of the plasma membrane portion of SCAMP2 to the fusion machinery of regulated exocytosis, we compared the distributions of SCAMP2 and complexin. Most studies of complexin have focused on its role in synaptic vesicle exocytosis, in which it binds and stabilizes synaptic SNARE complexes in supporting the final fusion event (Pabst et al., 2000; Reim et al., 2001; Tokumaru et al., 2001; Chen et al., 2002). Because the same SNAREs act in large dense-core vesicle exocytosis in PC12 cells (Chen et al., 1999; Lang et al., 2001), we assumed that complexin might have an analogous role in rapid granule fusion and would be concentrated at fusion sites. As shown in Figure 4, A–C, complexin is extensively colocalized with SCAMP2 on plasma membranes of PC12 cells. Quantification of immunostained foci on three different membrane sheets indicated that complexin and SCAMP2 were fully overlapped (or nearly so) on 85% of the foci and exhibited closely apposed staining on 5% of the foci, with 8 and 2% of foci showing staining for SCAMP2 and complexin alone, respectively. These results strongly support the view that SCAMP2 is colocalized with cell-surface fusion machinery.
Figure 4.
Comparison of the distribution of SCAMP2 with complexin and with the transferrin receptor (TfR) on plasma membrane sheets by double-label immunofluorescence. Images of a sheet stained with anti-SCAMP2 (2τ)/Alexa 488–tagged secondary antibody (A), anti-complexin mAb/Alexa 594–tagged secondary antibody (B), and a merged image (C) are shown at the top, and images of a sheet stained with anti-SCAMP2 (2τ)/Alexa 488–tagged secondary antibody (D), anti-TfR mAb/Alexa 594–tagged secondary antibody (E), and a merged image (F) are shown beneath. C: Bar, 5 μm.
As a control for the specificity of the colocalizations observed on the plasma membrane sheets, we compared the distribution of SCAMP2 with the transferrin receptor. Figure 4, D–F, shows that there is little overlap of SCAMP and receptor staining. The distributions of SCAMP2 and the GPI-anchored protein Thy1 were also compared, but as already shown for syntaxin1–Thy1 comparative staining (Lang et al., 2001), the abundant diffuse Thy1 staining was not concentrated with SCAMP2 (our unpublished observations).
Overexpression of Normal and Mutated Versions of Full-Length SCAMP2 in PC12 Cells
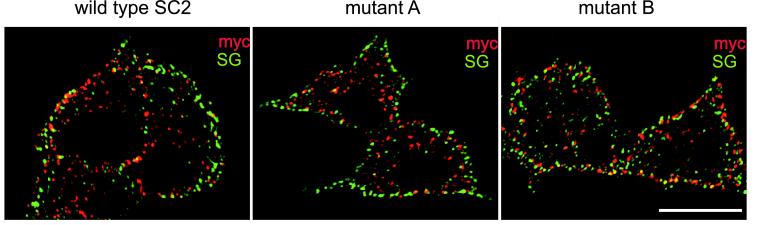
Having established that E peptide of SCAMP2 blocks large dense-core vesicle release and that a portion of SCAMP2 is concentrated at prospective granule docking/fusion sites, we addressed the role of SCAMP2 in exocytosis using overexpression of mutants of the full-length polypeptide. We hypothesized that if E peptide were part of the functional domain of SCAMP, overexpression of dysfunctional SCAMP2 mutated in the E peptide, but not normal SCAMP2, would inhibit regulated exocytosis by competitively binding to other essential components of the exocytotic machinery. Using tetracycline-regulated (Tet-off) PC12 cells, we compared the effects of two mutations within the E peptide segment of the N-myc–tagged full-length SCAMP2 with the effects of N-myc–tagged normal (wild-type) SCAMP2 and of mock transfection with the empty pTRE2 vector. The two mutations were C→A (mutant A) and W→A (mutant B) within the E peptide segment (see MATERIALS AND METHODS). In each case, the cells were cotransfected with cDNA encoding hGH, and the expressed hormone was used to assay secretion in the transfected cells. After transfection, dox was added at different times to generate a variety of levels of overexpression (relative to endogenous SCAMP2), and incubation was continued to 72 h. In two independent experiments in which dox was administered at 48 h, immunofluorescence microscopy showed that 12% of total cells were transfected with each myc-tagged SCAMP. Of the myc-SCAMP–expressing cells, 40–50% also expressed hGH, and ∼20% of transfected cells expressed hGH alone. To address the localizations of expressed proteins, we compared the distribution of each myc-tagged SCAMP2 to SG (Figure 5). In general, the exogenous SCAMP2 (normal or mutant) exhibits a distribution that is quite similar to what is observed for endogenous SCAMP2 in nontransfected cells (Figure 2B). The nuclear envelope/rough endoplasmic reticulum is unstained, indicating that the exogenous SCAMPs passed quality control. Also, none of the exogenous SCAMPs affected the distribution of SG. Large dense-core vesicles accumulate beneath the plasma membrane, and overexpression did not lead to enhanced costaining of these granules by the SCAMPs. The latter was also true when dense-core vesicles were discharged by depolarization before transfection to accelerate granule turnover (our unpublished observations).
Figure 5.
Expression and localization of myc-tagged SCAMP2 in Tet-regulated PC12 cells. Dox was added at 48 h after transfection, and cells were double-labeled with mouse anti-myc antibody and anti-secretogranin. Deconvolved images were prepared from five cells of each type expressing wild-type SCAMP2, mutant A (C→A), or mutant B (W→A). Representative optical sections (0.2 μm) including profiles of nuclei are shown. Bar, 10 μm.
Dose-dependent Inhibition of Depolarization-induced Exocytosis in PC12 Cells by Mutant SCAMP2
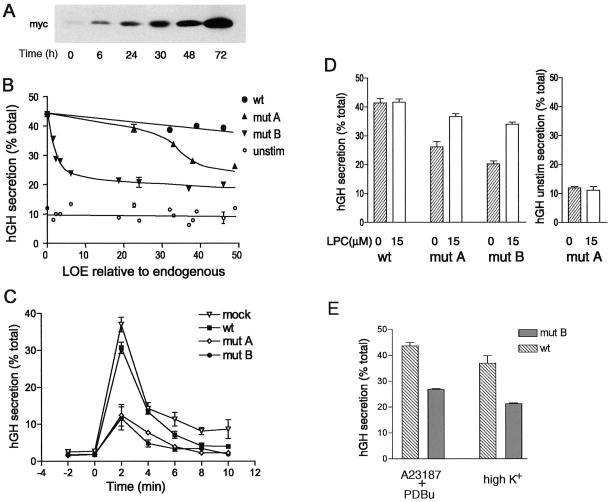
The effects of different levels of expression of exogenous SCAMP2s on secretion are presented in Figure 6. In preliminary experiments, we added dox at different times after transfection to validate that regulated expression of each exogenous SCAMP2 was achieved (Figure 6A) and to select time points that would provide comparable levels of expression of each construct. We then carried out several independent experiments in each of which replicate cell samples were transfected in parallel with DNAs encoding wild-type SCAMP2, mutants A (C→A) and B (W→A), or empty pTRE2 vector and in all cases with DNA encoding hGH. For each sample, the levels of endogenous and exogenous myc-tagged SCAMP2 were quantified by Western blotting, and secretion of hGH, initially in low-K+ medium and subsequently in high-K+ medium, was quantified by ELISA. As shown in Figure 6B, expression of myc-tagged wild-type SCAMP2 at levels up to 50-fold higher than endogenous SCAMP2 had very little effect on high K+-stimulated secretion compared with cells transfected with empty pTRE2. In contrast, mutants A and B both exhibited clear dose-dependent inhibition of regulated secretion that reached a maximal extent of 70%. In the case of mutant A, inhibition was apparent at 30-fold overexpression and was near maximal at 50-fold overexpression (on the basis of comparison with 120-fold overexpression; data not shown). Mutant B was much more potent, showing detectable inhibition at 1.6-fold overexpression and near-maximal inhibition at fivefold or greater overexpression.
Figure 6.
Tet-regulated expression of myc-tagged SCAMP2 and its mutants in PC12 cells and dose-dependent inhibition of depolarization-induced secretion of hGH. (A) Western blots with anti-myc antibody 9E10 illustrating the change in expression of exogenous SCAMP (mutant A used as an example) when dox is added at specified times after transfection. (B) Plot of hGH secretion (percentage of total) stimulated by depolarization (●, ▴, ▾) or unstimulated (○) as a function of the level of expression (LOE) of exogenous SCAMP2 relative to endogenous SCAMP2. Secretion of hGH was determined by ELISA. Mock transfection (pTRE vector) is shown at zero LOE with 42% hGH secretion (32% net stimulated). The data are compiled from eight independent transfection experiments. Error bars indicate the range obtained when independent experiments resulted in the same LOE relative to endogenous. (C) Time course of depolarization-induced hGH secretion by PC12 cells that had been transfected with pTRE2 vector, wild-type SCAMP2, mutant A, or mutant B. Samples of tet-regulated PC12 cells cotransfected in duplicate with cDNAs encoding hGH and either empty pTRE2 vector (mock), wild-type (wt) SCAMP2, mutant (mut) A, or mutant B were treated with 2 μg/ml dox at times that achieve comparable levels of expression. At 72 h after transfection, the samples were used to assay the rate of secretion at 2-min intervals. High-K+ medium was added at 0 min to initiate depolarization. (D) Addition of LPC to the medium substantially overcomes the inhibition of secretion caused by overexpression of SCAMP2 mutants A and B. Samples of tet-regulated PC12 cells cotransfected in duplicate with cDNAs encoding hGH and either wild-type SCAMP2, mutant A, or mutant B were treated with 2 μg/ml dox at times that achieve comparable levels of expression. At 72 h after transfection, the samples were incubated 10 min in low-K+ medium and then 10 min in high-K+ medium in the absence or presence of 15 μM LPC, and the media and cell lysates were assayed for hGH by ELISA and expressed as percentage of total. The results are from five independent experiments. For hGH unstimulated secretion (right), the results for mutant A are plotted as an example. For wild-type SCAMP2, the results are identical; for mutant B, hGH unstimulated secretion is 14.5 and 12% for 0 and 15 μM LPC, respectively. Error bars indicate mean ± SEM. (E) Overexpression of mutant B inhibits secretion of hGH stimulated by a combination calcium ionophore A23187 and phorbol dibutyrate. Samples of tet-regulated PC12 cells were cotransfected with cDNAs encoding hGH and either wild-type SCAMP2 or mutant B and were induced exactly as in D. Duplicate samples in three independent experiments were incubated 10 min in low-K+ medium (with or without dimethyl sulfoxide, the solvent for A23187 and phorbol dibutyrate; data with dimethyl sulfoxide shown) and then 10 min in either low-K+ medium with 0.5 μM A23187 and 0.1 μM phorbol dibutyrate (PDBu) or high-K+ medium. Media and cell lysates were assayed for hGH, and results are expressed as in D. Unstimulated secretion of hGH was 12–15% of total in all cases.
To check whether inhibition of secretion by the mutants of SCAMP2 was accompanied by a change in the kinetics of secretion, we compared the time courses of hGH discharge in response to K+ depolarization over a 10-min period. As shown in Figure 6C, inhibition by either mutant occurred outright, without any change in the rate of secretion.
Finally, to assess whether inhibition of secretion by overexpression of mutants A and B might reflect interference with molecular events of exocytosis that are manifest at the plasma membrane, we tested whether addition of LPC to the medium would rescue depolarization-driven hGH release. LPC, an inverted cone-shaped lipid, has been identified as a transbilayer fusogen in model systems (Chernomordik et al., 1998), and its addition to yeast expressing geranylgeranylated SNAREs was able to partially overcome a late block in exocytosis (Grote et al., 2000). Initially, we conducted a titration and established that concentrations of LPC <20 μM had no effect on hGH secretion from unstimulated cells. Subsequently, five separate experiments were performed using 15 μM LPC, a level similar to that used previously to perturb exocytosis in mammalian cells (Chernomordik et al., 1993), to check its effect on both unstimulated and stimulated secretion. As expected, LPC had no effect on unstimulated secretion (low-K+ medium; Figure 6D, right). Furthermore, LPC had no effect on K+-stimulated secretion of hGH by cells transfected with pTRE2 or myc-tagged wild-type SCAMP2. However, the treatment reversed the inhibition caused by overexpression of mutant A by 75% and of mutant B by 65% (Figure 6D, left). The level of restoration exceeds that obtained in yeast (Grote et al., 2000).
Mutant B of SCAMP2 Also Blocks Exocytosis Stimulated by Calcium Ionophore and Phorbol Ester
Exocytosis of large dense-core vesicles in PC12 cells in response to depolarization requires influx of extracellular calcium through plasma membrane channels (e.g., Taylor and Peers, 1999). Thus, it is possible that overexpressed mutant SCAMP might block exocytosis indirectly by interfering with calcium delivery and that addition of LPC reverses inhibition at this level rather than more distally. To evaluate this possibility, we bypassed depolarization-driven calcium influx and used calcium ionophore A23187 and phorbol ester (phorbol dibutyrate) to elevate intracellular calcium and stimulate secretion. We compared the discharge of hGH from cells expressing wild-type SCAMP2 and mutant B at levels comparable to those used in Figure 6D. As shown in Figure 6E, secretion stimulated by ionophore and phorbol ester in cells expressing wild-type SCAMP2 was slightly more robust than secretion stimulated by depolarization. However, expression of mutant B inhibited secretion by both types of stimuli to the same extent. Thus, the inhibition of ionophore/phorbol ester–stimulated secretion by mutant B argues that its perturbation is downstream of calcium delivery.
Effects of Exogenous SCAMP2 on Endocytosis
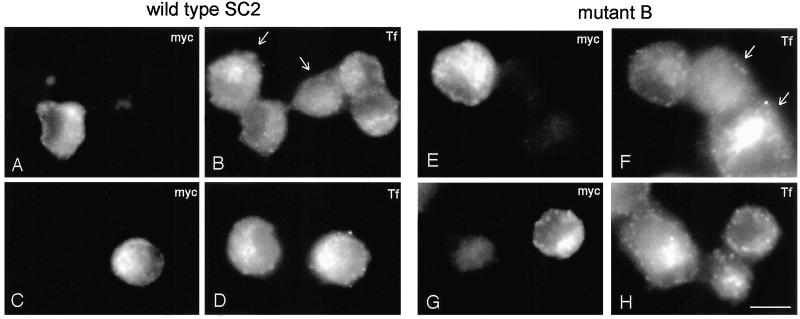
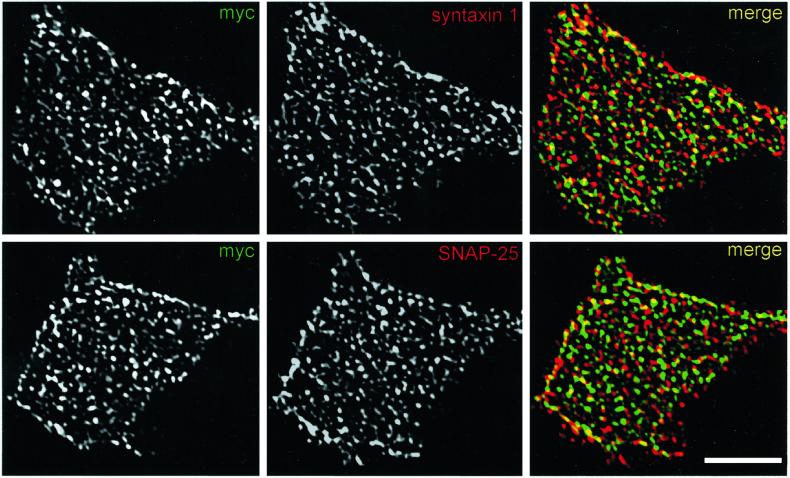
Because SCAMPs are concentrated in membranes of the endocytic pathway (Brand and Castle, 1993; Wu and Castle, 1998), another manner in which exogenous SCAMPs might alter exocytosis indirectly is by perturbing the trafficking of membrane proteins that are part of the exocytic machinery during endocytosis and recycling. Indeed, it was shown previously that robust transient overexpression of SCAMP1 decreases uptake of transferrin in Cos cells (Fernandez-Chacon et al., 2000). To check whether a similar perturbation might occur after tet-regulated overexpression of SCAMP2 in PC12 cells, we examined the endocytosis of fluorescently labeled transferrin in cells expressing comparable levels (approximately fivefold overexpression) of wild-type SCAMP2 and mutant B (Figure 7). We found that neither wild-type nor mutant forms of SCAMP2 blocked endocytosis outright; however, both appeared to decrease uptake and concentration of transferrin in recycling endosomes, with mutant B showing a stronger effect than wild-type. Notably, the level of the inhibitory effect seemed to be variable from cell to cell, probably reflecting differences in the level of expression of exogenous SCAMP2, but the variations were generally limited in that they fell within the range of variation in uptake of transferrin by nontransfected cells (see Figure 7, B and F). Thus, our results indicate that expression of exogenous SCAMP2 potentially could have indirect and negative effects on exocytosis. We suspect, however, that such effects are unlikely to be a major source of the perturbation that we have observed, for two reasons. First, endocytic trafficking is only slowed and not fully inhibited. Second, the levels of plasma membrane–associated t-SNAREs, syntaxin 1 and SNAP25 are not noticeably perturbed by expressing exogenous SCAMP2, especially mutant B (Figure 8).
Figure 7.
Effects of overexpressed SCAMP2 on endocytosis of transferrin. PC12 cells were transfected with wild-type SCAMP2 (A–D) or mutant B (E–H), each myc-tagged, using the same conditions as in Figure 6, C–E. After incubation for 5 min at 37°C with Alexa 594–labeled transferrin (Tf), cells were fixed and immunostained with anti-myc and Alexa 488–labeled secondary antibody. Full-thickness images are shown, allowing visual comparison of extents of Tf uptake and concentration by transfected and nontransfected cells. Although uptake is somewhat reduced in transfected cells relative to nontransfected neighbors, the range of the inhibitory effect is difficult to distinguish from the range in uptake by nontransfected cells (e.g., the cells marked by arrows in B and F). Lower right: Bar, 10 μm.
Figure 8.
Overexpression of SCAMP2, particularly mutant B, does not noticeably affect the distribution of plasma membrane SNAREs, syntaxin 1, and SNAP25. PC12 cells transfected with myc-tagged mutant B under the same conditions as in Figure 6, C–E, were examined by double-label immunofluorescence at the whole-cell level and as plasma membrane sheets prepared by light sonication. Representative images of plasma membrane sheets stained with antibodies against myc epitope plus syntaxin 1 and myc epitope plus SNAP-25 are shown and illustrate the similar and partially overlapping distributions of SCAMP2 with the SNAREs. Lower right: Bar, 5 μm.
DISCUSSION
The present studies have shown that SCAMP2 is partially localized to the plasma membrane, where it is concentrated at docking/fusion sites in neuroendocrine PC12 cells, and that it is quite likely to participate in the final events of large dense-core vesicle exocytosis. In combination with our findings made with permeabilized mast cells (Guo et al., 2002) and evidence that this SCAMP is broadly expressed in various cell types (Singleton et al., 1997), the observations suggest a general role of SCAMP2 in regulated granule exocytosis. Furthermore, because SCAMP1 may act in exocytosis and endocytosis (Fernandez-Chacon et al., 1999, 2000) and E peptides of other SCAMPs inhibit secretion (although some not as well as E peptide of SCAMP2) (Guo et al., 2002), the results suggest that SCAMPs collectively may function in cytoplasmic membrane fusion, either individually or collaboratively.
Whereas previous characterization of SCAMPs focused on their principal localization on vesicular carriers within the cell-surface recycling system, we have now shown quite clearly that some SCAMP2 is also present in the plasma membrane. From other studies in progress, it is apparent that this is a general feature of mammalian SCAMP isoforms that holds in multiple types of mammalian cells and cell lines (our unpublished results). Moreover, the evidence presented in this study strongly points to function of SCAMP2 in vesicular trafficking from sites that are in the plasma membrane (Figures 3, 4, and 6). Notably, concentration on intracellular membranes and function at the cell surface are characteristics of other four-transmembrane-spanning proteins (Maeker et al., 1997). Thus, we are inclined to refine previous functional implications stemming from the presence of SCAMPs in vesicular carriers. These SCAMPs might serve within the carriers as targets for vesicle-to-vesicle fusion (as in compound exocytosis) or sites of vesicle budding and fission (in analogy to endocytosis). Alternatively, the SCAMPs may be in transit to sites at which they serve these roles. Interestingly, SCAMPs appear as foci distributed throughout much of the cell surface (Figures 2–5). Because the foci include multiple gold particles as viewed by immunoelectron microscopy (Figure 2), we believe that they represent microdomains in which the SCAMPs are concentrated in the form of homomultimeric or heteromultimeric complexes. Indeed, there is mounting evidence that SCAMPs multimerize (Brand and Castle, 1993; Wu and Castle, 1997, 1998; our unpublished observations).
We believe that our experiments have achieved one of the initial goals of the study, which was to relate the inhibitory effects of E peptide on exocytosis to the role of SCAMP2. The sequence is critical to its ability to block exocytosis at a very late step as a free oligopeptide (Figure 1) (Guo et al., 2002) and to support exocytosis in the full-length protein (Figure 6). It seems especially striking that two different single amino acid changes create versions of full-length SCAMP2 that serve as dominant secretory inhibitors when expressed at levels below those at which nonmutated SCAMP2 has no effect. Moreover, dose-dependent inhibition by mutants A and B suggests a possible competition with endogenous SCAMP2 in which mutant B is especially effective at blocking endogenous function. Apparently, even subtle structural changes in the short linker between the second and third transmembrane spans are sufficient to compromise the function of SCAMP2 or key interactions that are critical to other secretory machinery at the membrane interface. Although the dose-dependent inhibition is quite striking, it is curious, first, that the effective inhibitory dose differs so greatly for mutants A and B and second, that maximal inhibition achieved for both mutants is ∼70%. If SCAMP2 functions in a multimeric or complexed state, as suspected, it is possible that the W→A mutation has a much greater effect on the assembled SCAMP unit than the C→A mutation does and that the latter requires incorporation in greater copy number to be disruptive. The incomplete inhibition can be explained in large part by the presence of cells that express hGH but not exogenous SCAMP2: ∼20% of the hGH-positive cells by immunofluorescence. The remaining 10% is not explainable at this time. As shown in Figure 6C, residual secretion observed in the presence of mutant SCAMP proceeds at the same rate as in the absence of mutant. This finding suggests that overexpression has not rendered an earlier step in stimulus–secretion coupling as rate limiting. Furthermore, it is distinguished from that observed in the SCAMP1 knockout, in which the rate was decreased because of a slowdown in formation of stable fusion pores (Fernandez-Chacon et al., 1999). We attempted to increase the extent of inhibition by depolarizing PC12 cells before transfection in case the turnover of secretory machinery might increase equilibration of exogenous SCAMP into the endogenous pool. However, this strategy had no effect (our unpublished observations). Perhaps exogenous and endogenous SCAMP2 may not be fully interchangeable even if their distributions are very similar, or there may be partial compensation for defective SCAMP2 by other SCAMP isoforms, including SCAMP1.
What do our findings indicate about the role of SCAMP2 in the secretory event? The localization at docking/fusion sites for large dense-core vesicles and correlation between the inhibitory effects of synthetic E peptide and overexpressed mutant protein lead us to favor a direct role of SCAMP2 in exocytosis. Furthermore, the observation that exogenous LPC immediately relieves the inhibition imposed by the point mutants simultaneously supports our focus on the E peptide segment, the late role of SCAMP2 in membrane fusion, and a plasmalemmal site of action. However, because our study focuses on the dominant inhibitory effects of a membrane protein that is both overexpressed and concentrated primarily in intracellular membranes, we have considered alternative and less direct explanations for the perturbations that we have observed on secretion. In particular, we have addressed the possibilities that mutated but not wild-type SCAMP2 might interfere with calcium influx across the plasma membrane that is essential to depolarization-induced exocytosis or might cause cumulative defects in recirculation of exocytotic machinery (especially SNARE proteins) within endosomes during tet-regulated expression. In both cases, the less direct actions do not explain the inhibitory effects we have observed. Overexpressed mutant B blocks secretion of hGH when calcium ionophore and phorbol ester are combined as an exocytotic stimulus (Figure 6E), whereas modest interference with endocytic trafficking is a common effect of overexpressing mutant B or wild-type SCAMP2 (Figure 7). Although mutant B appears to reduce transferrin uptake somewhat more strongly than wild-type SCAMP2, in neither case are the distributions of plasmalemmal SNARE proteins noticeably altered (Figure 8).
Despite the potential caveats raised by the effects of SCAMP2 overexpression on endocytosis and the possible more broadly ranging actions of LPC, we believe that our data generally support a direct role of SCAMP2 in exocytosis involving its E peptide segment. If this is indeed the case, we speculate that SCAMP2 may contribute to forming the final fusion pore between the granule interior and the extracellular space. The highly conserved E peptide segment may serve as a binding site at the cytoplasmic interface within the broadly conserved membrane core of the full-length protein (Figure 2A), which is the functional unit. The propensity of SCAMPs to multimerize in vitro and in situ and their accumulation in membrane foci (Figure 2E) suggest further that any contributions (direct or indirect) to exocytosis are likely to involve the membrane cores of multiple SCAMPs, all bearing E peptide segments.
Interestingly, the function of SCAMP in expediting fusion at the cytoplasmic surface may be shared by other tetraspan proteins (Kitani et al., 1991; Fleming et al., 1997) and related proteins that facilitate exoplasmic fusion (Heiman and Walter, 2000; for review, see White and Rose, 2001) and by structurally similar proteins that function in cytoplasmic fusion events in yeast: Got1p and Sft2p in endoplasmic reticulum–Golgi and Golgi-endosome transport, respectively (Conchon et al., 1999) and V0 proteolipid oligomers in homotypic vacuole fusion (Peters et al., 2001). Missing at present, however, are the identities of SCAMP interaction partners, especially among the established exocytotic machinery. To date, there are no reports of direct binding of SCAMPs to SNAREs or other proteins, such as synaptotagmin, Munc18, and complexin, that have been implied to function in the final stages of exocytosis (Carr et al., 1999; Fisher et al., 2001; Reim et al., 2001; Tokumaru et al., 2001; Voets et al., 2001; Wang et al., 2001), although it was recently possible to detect coimmunoprecipitation of SNAP23 and SCAMPs 1 and 2 in mast cells (Guo et al., 2002). More direct examination of the function and binding partners of SCAMP is the subject of ongoing studies.
ACKNOWLEDGMENTS
We are grateful to Drs. Jim Casanova, Sam Green, and Judy White for advice; members of the Castle, Green, and Casanova laboratories for discussions; and Judy White for insightful comments on the manuscript. We appreciate the assistance of Kristin Hriniak in preparing plasma membrane sheets from PC12 cells, and we thank Caitlin Engelhard for contributing to studies of the effects of exogenous SCAMPs on endocytosis. We gratefully acknowledge the National Hormone and Peptide Program of National Institute of Diabetes and Digestive and Kidney Diseases and Dr. A. F. Parlow for providing anti-hGH antibody. We also thank the University of Virginia Biomolecular Research Facility for peptide synthesis and characterization and Jan Reddick and Bonnie Sheppard of the Central Electron Microscope Facility for electron microscopic specimen preparation. Our studies were supported by National Institutes of Health grant DE-09655.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E02–03–0136. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.E02–03–0136.
REFERENCES
- Betz A, Thakur P, Junge HJ, Ashery U, Rhee J-S, Scheuss V, Rosenmund C, Rettig J, Brose N. Functional interaction of the active zone proteins Munc13–1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30:183–196. doi: 10.1016/s0896-6273(01)00272-0. [DOI] [PubMed] [Google Scholar]
- Brand SH, Castle JD. SCAMP 37, a new marker within the general cell surface recycling system. EMBO J. 1993;12:3753–3761. doi: 10.1002/j.1460-2075.1993.tb06053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand SH, Laurie SM, Mixon MB, Castle JD. Secretory carrier membrane proteins 31–35 define a common protein composition among secretory carrier membranes. J Biol Chem. 1991;266:18949–18957. [PubMed] [Google Scholar]
- Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to S.N.A.R.E. complexes and concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Scales SJ, Patel SM, Doung YC, Scheller RH. SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Frolov VA, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Vogel SS, Sokoloff A, Onaran HO, Leikina EA, Zimmerberg J. Lysolipids reversibly inhibit Ca2+-, GTP- and pH-dependent fusion of biological membranes. FEBS Lett. 1993;318:71–76. doi: 10.1016/0014-5793(93)81330-3. [DOI] [PubMed] [Google Scholar]
- Chung SH, Joberty G, Gelino EA, Macara IG, Holz RW. Comparison of the effects on secretion in chromaffin and PC12 cells of Rab3 family members and mutants: evidence that inhibitory effects are independent of direct interaction with rabphilin3. J Biol Chem. 1999;274:18113–18120. doi: 10.1074/jbc.274.25.18113. [DOI] [PubMed] [Google Scholar]
- Conchon S, Cao X, Barlowe C, Pelham HR. Got1p and Sft2p: membrane proteins involved in traffic to the Golgi complex. EMBO J. 1999;18:3934–3946. doi: 10.1093/emboj/18.14.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Achiriloaie M, Janz R, Albanesi JP, Sudhof TC. SCAMP1 function in endocytosis. J Biol Chem. 2000;275:12752–12756. doi: 10.1074/jbc.275.17.12752. [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Sudhof TC. Novel SCAMPs lacking NPF repeats: ubiquitous and synaptic vesicle-specific forms implicate SCAMPs in multiple membrane-trafficking functions. J Neurosci. 2000;20:7941–7950. doi: 10.1523/JNEUROSCI.20-21-07941.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Toledo GA, Hammer RE, Sudhof TC. Analysis of SCAMP1 function in secretory vesicle exocytosis by means of gene targeting in mice. J Biol Chem. 1999;274:32551–32554. doi: 10.1074/jbc.274.46.32551. [DOI] [PubMed] [Google Scholar]
- Fisher RJ, Pevsner J, Burgoyne RD. Control of fusion pore dynamics during exocytosis by Munc18. Science. 2001;291:875–878. doi: 10.1126/science.291.5505.875. [DOI] [PubMed] [Google Scholar]
- Fleming TJ, Donnadieu E, Song CH, Laethem FV, Galli SJ, Kinet JP. Negative regulation of Fc epsilon RI-mediated degranulation by CD81. J Exp Med. 1997;186:1307–1314. doi: 10.1084/jem.186.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Baba M, Ohsumi Y, Novick PJ. Geranylgeranylated SNAREs are dominant inhibitors of membrane fusion. J Cell Biol. 2000;151:453–466. doi: 10.1083/jcb.151.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Liu L, Cafiso D, Castle D. Perturbation of a very late step of regulated granule exocytosis by a secretory carrier membrane protein (SCAMP2)-derived peptide. J Biol Chem. 2002;277:35357–35363. doi: 10.1074/jbc.M202259200. [DOI] [PubMed] [Google Scholar]
- Guo Z, Turner C, Castle D. Relocation of the t-SNARE SNAP-23 from lamellipodia-like cell surface projections regulates compound exocytosis in mast cells. Cell. 1998;94:537–548. doi: 10.1016/s0092-8674(00)81594-9. [DOI] [PubMed] [Google Scholar]
- Heiman MG, Walter P. Prm1p, a pheromone-regulated multispanning membrane protein, facilitates plasma membrane fusion during yeast mating. J Cell Biol. 2000;151:719–730. doi: 10.1083/jcb.151.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Hubbard C, Singleton D, Rauch M, Jayasinghe S, Cafiso D, Castle D. The secretory carrier membrane protein family: structure and membrane topology. Mol Biol Cell. 2000;11:2933–2947. doi: 10.1091/mbc.11.9.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain NK, Yamabhai M, Ramjaun AR, Guy AM, Baranes D, O'Bryan JP, Der CJ, Kay BK, McPherson PS. Splice variants of intersectin are components of the endocytic machinery in neurons and nonneuronal cells. J Biol Chem. 1999;274:15671–15677. doi: 10.1074/jbc.274.22.15671. [DOI] [PubMed] [Google Scholar]
- Kitani S, Berenstein E, Mergenhagen S, Tempst P, Siraganian RP. A cell surface glycoprotein of rat basophilic leukemia cells close to the high affinity IgE receptor (Fc epsilon RI): similarity to human melanoma differentiation antigen ME491. J Biol Chem. 1991;266:1903–1909. [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R. SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J. 2001;20:2202–2213. doi: 10.1093/emboj/20.9.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie SM, Cain CC, Lienhard GE, Castle JD. The glucose transporter GluT4 and secretory carrier membrane proteins (SCAMPs) colocalize in rat adipocytes and partially segregate during insulin stimulation. J Biol Chem. 1993;268:19110–19117. [PubMed] [Google Scholar]
- Littleton JT, Bai J, Vyas B, Desai R, Baltus AE, Garment MB, Carlson SD, Ganetzky B, Chapman ER. Synaptotagmin mutants reveal essential functions for the C2B domain in Ca2+-triggered fusion and recycling of synaptic vesicles in vivo. J Neurosci. 2001;21:1421–1433. doi: 10.1523/JNEUROSCI.21-05-01421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollner TH, Rothman JE. Compartmental specificity of cellular membrane fusion encoded in SNARE proteins. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- Muller O, Bayer MJ, Peters C, Andersen JS, Mann M, Mayer A. The Vtc proteins in vacuole fusion: coupling NSF activity to Vo trans-complex formation. EMBO J. 2002;21:259–269. doi: 10.1093/emboj/21.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabst S, Hazzard JW, Antonin W, Sudhof TC, Jahn R, Rizo J, Fasshauer D. Selective interaction of complexin with the neuronal SNARE complex: determination of the binding regions. J Biol Chem. 2000;275:19808–19818. doi: 10.1074/jbc.M002571200. [DOI] [PubMed] [Google Scholar]
- Parlati F, Weber T, McNew JA, Westermann B, Sollner TH, Rothman JE. Rapid and efficient fusion of phospholipid vesicles by the alpha-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc Natl Acad Sci USA. 1999;96:12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Bayer MJ, Buhler S, Andersen JS, Mann M, Mayer A. Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature. 2001;409:581–588. doi: 10.1038/35054500. [DOI] [PubMed] [Google Scholar]
- Reim K, Mansour M, Varoqueaux F, McMahon HT, Sudhof TC, Brose N, Rosenmund C. Complexins regulate a late step in Ca2+-dependent neurotransmitter release. Cell. 2001;104:71–81. doi: 10.1016/s0092-8674(01)00192-1. [DOI] [PubMed] [Google Scholar]
- Sanan DA, Anderson RG. Simultaneous visualization of LDL receptor distribution and clathrin lattices on membranes torn from the upper surface of cultured cells. J Histochem Cytochem. 1991;39:1017–1024. doi: 10.1177/39.8.1906908. [DOI] [PubMed] [Google Scholar]
- Sato K, Wickner W. Functional reconstitution of Ypt7p GTPase and a purified vacuole SNARE complex. Science. 1998;281:700–702. doi: 10.1126/science.281.5377.700. [DOI] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Singleton DR, Wu TT, Castle JD. Three mammalian SCAMPs (secretory carrier membrane proteins) are highly related products of distinct genes having similar subcellular distributions. J Cell Sci. 1997;110:2099–2107. doi: 10.1242/jcs.110.17.2099. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Huttner WB. In: Purification of Secretory Granules from PC12 Cells in Cell Biology, A Laboratory Handbook. Celis JE, editor. I. San Diego: Academic Press; 1994. pp. 557–566. [Google Scholar]
- Taylor SC, Peers C. Store-operated influx and voltage-gated calcium channels coupled to exocytosis in pheochromocytoma (PC12) cells. J Neurochem. 1999;73:874–880. doi: 10.1046/j.1471-4159.1999.0730874.x. [DOI] [PubMed] [Google Scholar]
- Tokumaru H, Umayahara K, Pelligrini LL, Ishizuka T, Saisu H, Betz H, Augustine GJ, Abe T. SNARE complex oligomerization by synaphin/complexin is essential for synaptic vesicle exocytosis. Cell. 2001;104:421–432. doi: 10.1016/s0092-8674(01)00229-x. [DOI] [PubMed] [Google Scholar]
- Voets T, Toonen RF, Brian EC, de Wit H, Moser T, Rettig J, Sudhof TC, Neher E, Verhage M. Munc18–1 promotes large dense-core vesicle docking. Neuron. 2001;31:581–591. doi: 10.1016/s0896-6273(01)00391-9. [DOI] [PubMed] [Google Scholar]
- Wang CT, Grishanin R, Earles CA, Chang PY, Martin TF, Chapman ER, Jackson MB. Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science. 2001;294:1111–1115. doi: 10.1126/science.1064002. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachi M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- White JM, Rose MD. Yeast mating: getting close to membrane merger. Curr Biol. 2001;11:R16–R20. doi: 10.1016/s0960-9822(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Pfeiffer JR, Oliver JM. Observing FcepsilonRI signaling from the inside of the mast cell membrane. J Cell Biol. 2000;149:1131–1142. doi: 10.1083/jcb.149.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TT, Castle JD. Evidence for colocalization and interaction between 37 and 39 kDa isoforms of secretory carrier membrane proteins (SCAMPs) J Cell Sci. 1997;110:1533–1541. doi: 10.1242/jcs.110.13.1533. [DOI] [PubMed] [Google Scholar]
- Wu TT, Castle JD. Tyrosine phosphorylation of selected secretory carrier membrane proteins, SCAMP1 and SCAMP3, and association with the EGF receptor. Mol Biol Cell. 1998;9:1661–1674. doi: 10.1091/mbc.9.7.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]