Abstract
Clathrin-coated vesicles mediate the transport of the soluble vacuolar protein CPY from the TGN to the endosomal/prevacuolar compartment. Surprisingly, CPY sorting is not affected in clathrin deletion mutant cells. Here, we have investigated the clathrin-independent pathway that allows CPY transport to the vacuole. We find that CPY transport is mediated by the endosome and requires normal trafficking of its sorting receptor, Vps10p, the steady state distribution of which is not altered in chc1 cells. In contrast, Vps10p accumulates at the cell surface in a chc1/end3 double mutant, suggesting that Vps10p is rerouted to the cell surface in the absence of clathrin. We used a chimeric protein containing the first 50 amino acids of CPY fused to a green fluorescent protein (CPY-GFP) to mimic CPY transport in chc1. In the absence of clathrin, CPY-GFP resides in the lumen of the vacuole as in wild-type cells. However, in chc1/sec6 double mutants, CPY-GFP is present in internal structures, possibly endosomal membranes, that do not colocalize with the vacuole. We propose that Vps10p must be transported to and retrieved from the plasma membrane to mediate CPY sorting to the vacuole in the absence of clathrin-coated vesicles. In this circumstance, precursor CPY may be captured by retrieved Vps10p in an early or late endosome, rather than as it normally is in the trans-Golgi, and delivered to the vacuole by the normal VPS gene-dependent process. Once relieved of cargo protein, Vps10p would be recycled to the trans-Golgi and then to the cell surface for further rounds of sorting.
INTRODUCTION
Vesicles mediate protein and lipid trafficking between different compartments within cells. Formation of vesicles is driven by coat proteins, which are recruited from the cytosol onto a particular membrane. Coat proteins are responsible for selecting cargo vesicles and for deforming the lipid bilayer to drive budding (for review, see Springer et al., 1999). In Saccharomyces cerevisiae cells, three types of vesicles have been identified with different functions and coat composition (for review, see Schekman and Orci, 1996). COPI and COPII vesicles are involved in early transport steps between the ER and the Golgi, whereas clathrin-coated vesicles (CCVs) are involved in the late secretory pathway between the TGN and the plasma membrane. Clathrin proteins are either recruited onto the plasma membrane to mediate endocytosis or onto the TGN for protein transport to the prevacuolar/endosomal compartment (for review, see Schmid, 1997). In this regard, yeast cells deficient in clathrin heavy or light chains display a delay in endocytosis and mislocalize Golgi proteins (Payne and Schekman, 1989; Tan et al., 1993; Chu et al., 1996). It has been proposed that sorting of trans-Golgi proteins occurs in the endosome (Redding et al., 1996; Deloche et al., 2001). In this model Golgi proteins are transported in CCVs from the TGN to the endosome, possibly by a bulk flow process, but they are efficiently recycled back to the TGN due to a retrieval signal. Indeed, Golgi proteins contain aromatic residues at the carboxy-terminus that allow their retrieval to the TGN mediated by a retrograde vesicular transport complex called retromer (Wilcox et al., 1992; Nothwehr et al., 1993; Seaman et al., 1997; Seaman et al., 1998).
Clathrin also participates in vacuolar protein transport. Carboxypeptidase Y (CPY), a soluble vacuolar hydrolase, is diverted from the secretory pathway to the TGN in a manner analogous to soluble lysosomal proteins, which are transported to the late endosome by the mannose 6-phosphate receptor (M6PR) in mammalian cells (Kornfeld, 1992). In the TGN, the vacuolar sorting receptor Vps10p interacts with CPY (Marcusson et al., 1994; Cooper and Stevens, 1996). The receptor/ligand complex is then packaged into vesicles and transported to the endosome en route to the vacuole. Originally, evidence showing that CCVs are involved in the vacuolar pathway came from chc1-521 and chc1-5, two clathrin mutants that display a CPY transport defect (Seeger and Payne, 1992; Chen and Graham, 1998). Recently, the Golgi form of CPY (p2CPY) and Vps10p were detected in purified and immunoisolated CCVs, respectively, confirming the role of CCVs in the anterograde transport of vacuolar enzymes from the TGN to the endosome (Pishvaee et al., 2000; Deloche et al., 2001). However, an alternative pathway has been proposed to compensate for the clathrin defect and transport CPY to the vacuole. The alternative path appears to take over in cells that are deprived of clathrin during a long period of inactivation of a clathrin ts mutant (chc1-521) or in deletion mutant strains missing the clathrin heavy or light chain (Seeger and Payne, 1992; Chu et al., 1996).
In this study, we investigated Vps10p transport in the absence of clathrin. We show that Vps10p distribution is not significantly affected in chc1 mutants. However, we find that Vps10p is rerouted to the cell surface in clathrin chc1 and vps1 (dynamin homolog) mutants. Dynamin is a protein that is thought to interact with clathrin in the formation of CCVs at the TGN. Our results suggest that in clathrin mutants, Vps10p is first diverted to the plasma membrane before being internalized to an endosome and at which point p2CPY is recovered and transported to the vacuole.
MATERIALS AND METHODS
Strains, Growth Conditions, and Reagents
Yeast strains used in this study are listed in Table 1 and their construction is described below. Mating, sporulation, and tetrad dissection were performed using standard techniques (Guthrie and Fink, 1991). Yeast cells were grown in synthetic complete (SC) or in rich (YPD) media (Guthrie and Fink, 1991) at the permissive temperature (24°C) or at the indicated temperature. Yeast transformation was accomplished using standard methods (Ausubel et al., 1987–1995)
Table 1.
Yeast strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| LSY93-2A | MATa leu2-3,112 ura3-52 his3-Δ200 trpl-Δ901 suc2-Δ9 | Silveira et al., (1990) |
| GPY409 | MATa leu2-3,112 ura3-52 his4 and/or his6 trp1-289 chc1-521(ts) sst1-3 pep::LEU2 | Rad et al., (1995) |
| EHY202 | MATα chc1-521(ts) ura3-52 | Deloche et al., (2001) |
| ODY107 | MATα ura3-52 leu2-3,112 trp1 chc1-521(ts) | Deloche (unpublished data) |
| LSY6-2A | MATα ura3-52 leu2-3,112 his3-Δ200 trp1-901 pho8::ste13-pho8 vps35Δ::HIS3 | Nothwehr et al., (1999) |
| RSY1306 | MATα ura3-52 leu2,3-113 his3-Δ200 trpl-Δ901 lys2-801 pep12Δ::HIS3 | Becherer et al., (1996) |
| EHY62 | MATα sec6-4 vps10Δ::TRP1 his3-Δ200 leu2-3,112 lys2-801 trp1-1 ura3-52 | Harsay et al., (2002) |
| EHY361 | MATa trp his3Δ200 leu2-3 ura3-52 vps1::LEU2 | Harsay (unpublished data) |
| EHY227 | MATa sec6-4 TP1::SUC2::TRP1 his3-Δ200 leu2-3,112 trp1-1 ura3-52 | Harsay et al., (2002) |
| EHY242 | MATα sec6-4 chc1-521(ts) TPI::SUC2::URA3 his3-Δ200 ura3-52 | Harsay et al., (2002) |
| EHY225 | MATα sec6-4 vps1::LEU2 TP1::SUC2::HIS3 his3-Δ200 leu2-3,112 lys2-801 trp1-1 ura3-52 | Harsay et al., (2002) |
| ODY95 | MATα sec6-4 chc1-521(ts) TP1::SUC2::ura3 his3-Δ200 ura3-52 | This study |
| RSY1733 | MATa bar1 end3-1 leu2 ura3 his4 | R.S. collection |
| ODY76 | MATa ura3-52 leu2-3 trp1 his chc1-521(ts), pep12Δ::HIS3 | This study |
| ODY65 | MATa ura3-52 leu2-3 his3-Δ200 trp1 vps1Δ::LEU2, pep12Δ::HIS3 | This study |
| ODY133 | MATa ura3 leu2 end3-1 chc1-521(ts) | This study |
| ODY94 | MATa ura3-52 leu2-3,112 his trpl chc1-521(ts) vps35Δ::HIS3 | This study |
| ODY199 | EHY202::vps10Δ::URA3 | This study |
| ODY200 | ODY95::pep4Δ::LEU2 | This study |
| ODY50 | LSY93-2A::VPS10HA::URA3 | Deloche et al. (2001) |
| ODY221 | EHY227::VPS10HA::URA3 | This study |
| ODY222 | RSY1733::VPS10HA::URA3 | This study |
| ODY129 | EHY202::VPS10HA::URA3 | Deloche et al. (2001) |
| ODY102 | ODY95::VPS10HA::URA3 | This study |
| ODY119 | EHY225::VPS10HA::URA3 | This study |
| ODY138 | ODY133::VPS10HA::URA3 | This study |
The chcl-521, sec6-4, and end3-1 mutations are temperature-sensitive mutant alleles and throughout the text will be referred to as chcI-ts, sec6-ts, and end3-ts.
Enzymes for the manipulation of DNA were purchased from New England Biolabs (Beverly, MA) or Boehringer Mannheim Biochemicals (Indianapolis, IN). Antisera against Vps10p and CPY have been described (Feldheim et al., 1993; Cooper and Stevens, 1996). Mouse12CA5 antihemagglutinin (HA) antibodies and Cy3 (indocarbocyamine)-conjugated goat anti-mouse secondary antibodies were purchased from Berkeley Antibody Co. (Richmond, CA) and Jackson ImmunoResearch Laboratories (West Grove, PA), respectively. Donkey anti-rabbit and sheep anti-mouse secondary antibodies coupled to horseradish peroxidase were obtained from Amersham Corp. (Arlington Heights, IL). Zymolyase 100T and oxalyticase were obtained from United States Biological (Swampscott, MA) and Enzogenetics (Corvallis, OR), respectively. 35S-Promix was purchased from Amersham Corp. The lipophylic styryl dye N-(3-triethylammoniumpropyl)-4-(p-diethylaminophenylhexatrienyl) pyridinium dibromide FM 4–64 was purchased from Molecular Probes Inc. (Eugene, OR). Other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO), unless indicated.
Plasmid and Strain Construction
The pRS306vps10::URA3 plasmid was constructed by inserting a URA3 cassette into the HindIII site of the VPS10 open reading frame of pRS306VPS10 (Deloche et al., 2001). The open reading frame of CPY(1–50)-GFP containing the first 50 amino acids of CPY fused to green fluorescent protein (GFP) was subcloned from pYWG1CPY(1–50)-GFP (Humair et al., 2001) into the pGAL39ΔBglII vector (P. Linder plasmid collection), leading to the pGAL1CPY-GFP plasmid. The pTS17 plasmid containing pep4::LEU2 (R.S. plasmid collection) and the pRS306VPS10-HA and pRS416vps10G1423stop-HA plasmids have been described previously (Nothwehr et al., 1995; Deloche et al., 2001).
The haploid ODY76, ODY65, ODY94, and ODY133 strains were obtained after sporulation of crosses RSY1306/GPY409, RSY1306/EHY361, LSY6–2A/GPY409, and RSY1733/ODY107 diploid strains, respectively. Spores containing disrupted genes were selected by testing the presence of the corresponding auxotrophic markers. Authentic chc1-521 strains were identified based on the instability of Chc1p at the restrictive temperature. EHY242 was selected for Ura+ loss on medium containing 5-fluoroorotic acid to give rise to ODY95. The VPS10 gene of EHY202 was disrupted to give rise to ODY199 by transforming pRS306 vps10::URA3 linearized with BglII and SphI and selecting for Ura+ colonies. The PEP4 gene of ODY95 was disrupted to give rise to ODY200 by transforming pTS17 linearized with BamHI and selecting for Leu+ colonies. Both disruptions were confirmed by PCR. Constructions of strains containing a single copy of VPS10-HA were performed as described previously (Deloche et al., 2001). pRS306VPS10-HA was digested by AflII and transformed into EHY227, RSY1733, ODY95, EHY225, and ODY133, resulting in ODY221, ODY222, ODY102, ODY119, and ODY138, respectively.
Radiolabeling, Immunoprecipitation, and Immunoblot Analysis
Vps10p, CPY, and HA-tagged proteins were immunoprecipitated under denaturing conditions from extracts of radiolabeled cells using a procedure described previously (Nothwehr et al., 1995; Bryant et al., 1998; Deloche et al., 2001), with the appropriate polyclonal or monoclonal antibodies.
Fluorescence and Indirect Immunofluorescence Microscopy
Cells were grown to 0.8 OD/ml in rich media (YPD) at the permissive temperature and shifted to 36°C for 60 min unless indicated, before fixation. Preparation of cells for immunofluorescence was essentially as described (Chuang and Schekman, 1996). Vps10p-HA and Kex2p-HA were detected using Mouse12CA5 antihemagglutinin (HA) antibodies at a 1:400 dilution. Cy3 (indocarbocyamine)-conjugated goat anti-mouse secondary antibodies were used at 1:400 dilution.
For expression of CPY-GFP, cells containing the pGAL1CPY-GFP construct were grown to early exponential phase (OD600 of 0.4) at the permissive temperature in synthetic complete SC medium containing 2% raffinose. Cultures were then incubated for 30 min in the presence of 16 μM FM 4–64 and rinsed for 45 min in SC medium/2% raffinose in order to stain specifically the vacuolar membrane at the permissive temperature. Galactose was added to 3%, and cells were shifted immediately to the restrictive temperature (36°C) for 3 h. Cells were washed two times in PBS buffer, resuspended in SC medium (20 ODs), and plated onto glass coverslips that had been precoated with concanavalin A (Sigma) as described (Vida and Emr, 1995). Images were obtained using a Zeiss microscope (Thornwood, NY).
Cellular Fractionation
For sucrose equilibrium density gradient centrifugation, cells were grown in 500 ml of YPD to OD600 = 0.6 at the permissive temperature and shifted to 36°C for 60 min. Cells were harvested, converted to spheroplasts, and lysed as described (Harsay and Bretscher, 1995). The cell extract was clarified at 500 × g for 5 min, centrifuged at 20,000 × g for 20 min (SS34; Beckman Instruments, Inc., Berkeley, CA) and subsequently centrifuged at 100,000 × g for 1 h (SW41; Beckman Instruments, Inc.). The resulting P100 fraction was layered onto a 30–55% (wt/wt) sucrose/TEA gradient, followed by centrifugation (SW41; Beckman Instruments, Inc.) at 150,000 × g for 20 h as described (Chuang and Schekman, 1996). Fractions (0.4 ml) were collected from the top and 10-μl aliquots of every two fractions were subjected to SDS-PAGE and immunoblotting.
Susceptibility to External Proteases
Protein degradation at the cell surface was performed as described (Davis et al., 1993). Briefly, cells were grown at the nonpermissive temperature (36°C) for 60 min or as indicated. For each reaction, 3 × 107 cells were collected and resuspended in an ice-cold poisoned solution (20 mM potassium fluoride and 20 mM sodium azide) for 20 min. Cells were resuspended in 400 μl of digestion buffer (DB; 1.4 M sorbitol, 25 mM Tris-HCl, pH 7.5, 10 mM sodium azide, 10 mM potassium fluoride, 2 mM MgCl2) with 0.5% β-mercaptoethanol and incubated at 37°C for 30 min. One half of the sample was incubated in 200 μl of DB containing Pronase (360 U/ml) for 60 min at 37°C. The other half was processed identically in parallel without protease. Protease was removed by two washes of the centrifuged cells with 400 μl of ice-cold DB containing 1 mM PMSF. To prepare protein extracts for immunoblotting, we incubated cells in 400 μl of DB with 0.5% β-mercaptoethanol and oxalyticase (1 mg/ml) for 30 min at 30°C. Spheroplasts were centrifuged, resuspended in 40 μl of lysis buffer (8 M urea, 2% SDS, 0.1 mM EDTA, 20 mM Tris-HCl, pH 7.5, 1% β-mercaptoethanol and 0.5 mM PMSF), and heated 5 min at 65°C. Two volumes of loading buffer 1× (60 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, and 0.005% bromophenol blue) were added, and 15 μl of each reaction was subjected to SDS-PAGE and immunoblotting. The accessibility of pronase to the ribosomal Rpl3p (TCM) was assessed as a control. TCM was shown to be sensitive to pronase when cells are incubated with 0.2% Triton X-100.
Incubation of spheroplasts with pronase and the subsequent immunoprecipitation of CPY were performed as follows: Cells were grown in YPD at the permissive temperature and 1 OD600 U of cells was collected for each time point. Cells were first incubated in 0.5 ml of 50 mM Tris-HCl, pH 8.0, 1% β-mercaptoethanol for 10 min at 30°C and then converted to spheroplasts by treatment with zymolyase-100T (10 μg/OD600) in 0.5 ml of 1.2 M sorbitol, 50 mM potassium phosphate, pH 7.5, 1 mM magnesium chloride for 30 min at 30°C. Spheroplasts were washed twice and grown in synthetic complete (SC)/1.2 M sorbitol buffer (SC medium containing 1.2 M sorbitol, 2 mM MgCl2 and buffered with 20 mM Tris-HCl, pH 7.5) for 30 min at 36°C. Spheroplasts were divided in two microcentrifuge tubes. One half was incubated in 0.4 ml of SC/1.2 M sorbitol buffer containing pronase (360 U/ml) for 90 min at 36°C. The second half was incubated without pronase as a control. Pronase was removed by two washes of the centrifuged cells with 0.4 ml of SC/1.2 M sorbitol buffer. Spheroplast labeling and immunoprecipitation of CPY were described above except that 35S metabolic labeling of proteins was performed in the presence of 1.2 M sorbitol. Samples treated with pronase were exposed longer (10 d) because of a weak 35S-protein incorporation.
RESULTS
Distribution of Vps10p Is Not Affected in the chc1-ts Mutant
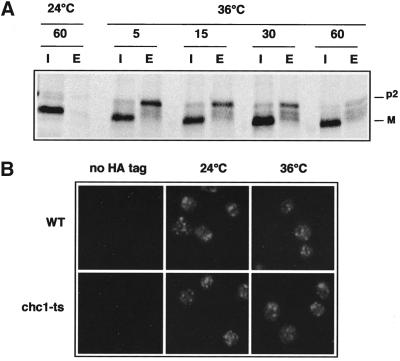
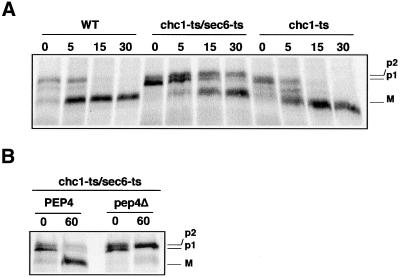
To determine the clathrin-independent pathway of CPY transport to the vacuole, we reexamined the compensatory mechanism in the chc1-ts temperature sensitive (ts) mutant as described by Seeger et al. (Seeger and Payne, 1992). CPY en route to the vacuole undergoes a series of characteristic modifications that can be used to monitor transport (Stevens et al., 1982). Newly synthesized CPY is translocated into the ER, where cleavage of the signal sequence and N-linked core glycosylation occur, leading to the p1 form (67 kDa). In the Golgi, addition of mannose residues takes place, producing the 69-kDa Golgi form (p2CPY). Finally, p2CPY is proteolytically processed in the vacuole to give the mature 61-kDa form (mCPY). We measured the time required for cells to restore CPY transport to the vacuole in the absence of functional clathrin. As shown by Seeger et al. (Seeger and Payne, 1992), we confirmed that a 5-min inactivation of chc1-ts at 36°C followed by a 10-min 35S-labeling and a chase of 40 min resulted in defective CPY maturation, with 40% of the unprocessed p2CPY form secreted into the medium (Figure 1A). However, after a 60-min incubation at 36°C before protein labeling, cells adapted to the clathrin deficiency, and CPY maturation was restored to normal (Figure 1A).
Figure 1.
CPY maturation and Vps10p distribution in chc1-ts. (A) EHY202 (chc1-ts) cells were grown at 24 or 36°C for 5, 15, 30, and 60 min, respectively, before a 10-min protein labeling with [35S]methionine and cysteine and a 40-min chase with excess unlabeled amino acids. An aliquot from each sample was harvested, and CPY was immunoprecipitated from intracellular (I) and extracellular (E) fractions. Immunoprecipitates were subjected to SDS-PAGE and autoradiography. The Golgi and mature forms of CPY are labeled p2 and M, respectively. (B) LSY93–2A (WT), EHY202 (chc1-ts), ODY50 (WT::VPS10-HA), and ODY129 (chc1-ts:: VPS10-HA) were grown at 24°C or shifted to 36°C for 60 min as indicated. Cells were labeled with anti-HA antibodies followed by Cy3-conjugated secondary antibodies.
Clathrin-coated vesicles mediate the transport of CPY/Vps10p from TGN to the endosome. Previously, we showed that Vps10p stability is somewhat compromised in a clathrin mutant (Deloche et al., 2001). Therefore, we examined the extent to which a clathrin defect affected Vps10p localization in cells. We used a HA-tagged Vps10p construct to compare the localization of Vps10p in WT and chc1-ts cells at 24 and 36°C by indirect immunofluorescence microscopy. We found that Vps10p-HA localization in the chc1-ts mutant was not grossly affected after a 60-min incubation at 36°C. A punctate distribution of Vps10p-HA was observed, similar to that reported for a number of other late Golgi proteins (Redding et al., 1991; Roberts et al., 1992; Nothwehr et al., 1993) (Figure 1B). This suggests that Vps10p remains distributed between the trans-Golgi and the endosome after inactivation of clathrin.
Vps10p Is Recycled from the Late Endosome to the TGN in the chc1-ts Mutant
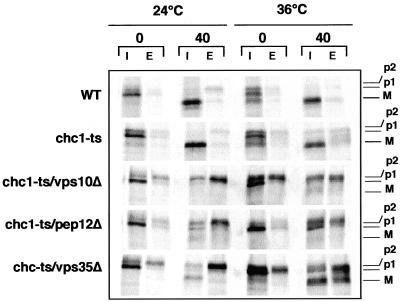
To determine whether Vps10p is also responsible for the sorting of vacuolar CPY in the absence of clathrin, we constructed a double chc1-ts/vps10Δ mutant and tested the fidelity of CPY transport to the vacuole by pulse chase and immunoprecipitation analysis. In this procedure, cells were incubated at the permissive temperature (24°C) or shifted to the nonpermissive temperature (36°C) for 60 min before protein labeling for 10 min. At this point, an aliquot of cells was harvested (time 0), and the remaining cells were chased by addition of an excess of nonradioactive amino acids for 40 min. Each sample was separated into intracellular (I) and extracellular (E) fractions. As shown in Figure 2, the chc1-ts/vps10Δ cells exhibited a similar sorting defect at 24 and 36°C, indicating that Vps10p is essential for CPY sorting either in the presence or absence of functional clathrin. Because the steady state distribution of Vps10p did not depend on clathrin, we next examined the possibility that Vps10p traverses the endosome compartment in a clathrin mutant, as in WT cells. Pep12p is a t-SNARE component required for the fusion of vesicles with the late endosome (Becherer et al., 1996; Burd et al., 1997). In a pep12 mutant, Vps10p transport to the late endosome is blocked, leading to the secretion of the p2CPY form. At 36°C, chc1-ts/pep12Δ cells display an obvious CPY sorting defect, indicating the requirement of the endosome for the proper delivery of CPY to the vacuole in the chc1-ts mutant (Figure 2). CPY is produced at >20-fold the rate of Vps10p synthesis (Cooper and Stevens, 1996). Because the stoichiometry of CPY binding to Vps10p in cells is 1:1, Vps10p must be recycled back from the endosome to the TGN for multiple rounds of CPY sorting. Previous reports showed that Vps35p likely interacts with Vps10p and is required for its retrieval to the TGN (Seaman et al., 1997; Nothwehr et al., 1999; Nothwehr et al., 2000). Thus, we examined CPY maturation in a chc1-ts/vps35Δ double mutant. Figure 2 shows that CPY was also missorted in chc1-ts/vps35Δ cells at the nonpermissive temperature, supporting the model in which Vps10p traverses the late endosome in the absence of clathrin and must be recycled back to the TGN for efficient CPY delivery to the vacuole. The alternative pathway that bypasses the endosome to selectively transport alkaline phosphatase (ALP) to the vacuole (Piper et al., 1997) is not responsible for the transport of CPY in the chc1 mutant. Although transport of ALP from the TGN to the vacuole requires the AP-3 clathrin adaptor complex, the maturation of CPY is not affected in a chc1/ap-3 double mutant (G. Payne, personal communication).
Figure 2.
CPY maturation is affected in chc1-ts/vps10Δ, chc1-ts/pep12Δ and chc1-ts/vps35Δ double mutants. LSY93–2A (WT), EHY202 (chc1-ts), ODY199 (chc1-ts/vps10Δ), ODY76 (chc1-ts/pep12Δ), and ODY94 (chc1-ts/vps35Δ) were grown at 24°C or shifted to 36°C for 1 h before protein 35S-labeling for 10 min and initiation of the chase. For each culture, one half of the cells was immediately harvested (0 chase), and the remaining cells were incubated for an additional 40 min at the indicated temperature. CPY was immunoprecipitated and analyzed as described in the legend to Figure 1. The ER, Golgi, and mature forms of CPY are labeled p1, p2, and M, respectively.
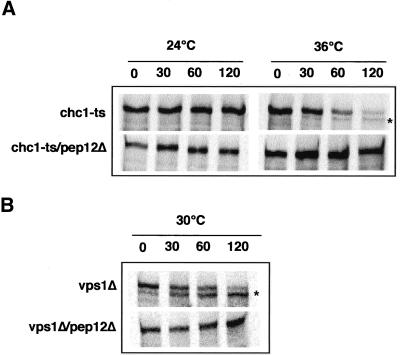
Recently, we showed that a PEP4-dependent cleavage of Vps10CtΔp-HA, a HA-tagged VPS10 mutant lacking its C-terminal cytoplasmic domain is blocked in a pep12 mutant (Deloche et al., 2001). Therefore, we examined the ability of a pep12 mutation to block the degradation of Vps10p in a chc1-ts/pep12Δ mutant. In the experiment shown in Figure 3A, chc1-ts and chc1-ts/pep12Δ cells were preincubated at either 24°C or 36°C, labeled for 10 min with 35S-methionine, and chased for the indicated times. Although Vps10p was slowly degraded in chc1-ts cells at the restrictive temperature, degradation was blocked in the chc1-ts/pep12Δ double mutant.
Figure 3.
Stability of Vps10p in chc1-ts/pep12Δ and vps1Δ/pep12Δ. (A) EHY202 (chc1-ts) and ODY76 (chc1-ts/pep12Δ) strains were incubated at 24°C or shifted to 36°C for 30 min as indicated. (B) EHY361 (vps1Δ) and ODY65 (vps1Δ/pep12Δ) strains were incubated at 30°C. Cells were then labeled for 10 min and chased for the indicated times. Vps10p was immunoprecipitated and analyzed as described previously (Deloche et al., 2001). The PEP4-dependent cleavage product is indicated (*).
Vps1p is a dynamin-like protein that is peripherally associated with Golgi membranes (Rothman et al., 1990). Dynamin may interact with clathrin proteins to form CCVs at the TGN. A mutation in the VPS1 gene results in the secretion of CPY (Vater et al., 1992), indicating a direct role of Vps1p in CPY sorting. In vps1Δ mutant cells, the half-time of turnover of Vps10p was greatly reduced, but this degradation was blocked after 2 h of chase at 30°C in a vps1Δ/pep12Δ mutant (Figure 3B). These results are consistent with a model in which Vps10p continues to cycle between the TGN and the late endosome in the absence of clathrin.
Vps10p Travels to the Cell Surface in chc1-ts and vps1Δ Mutants
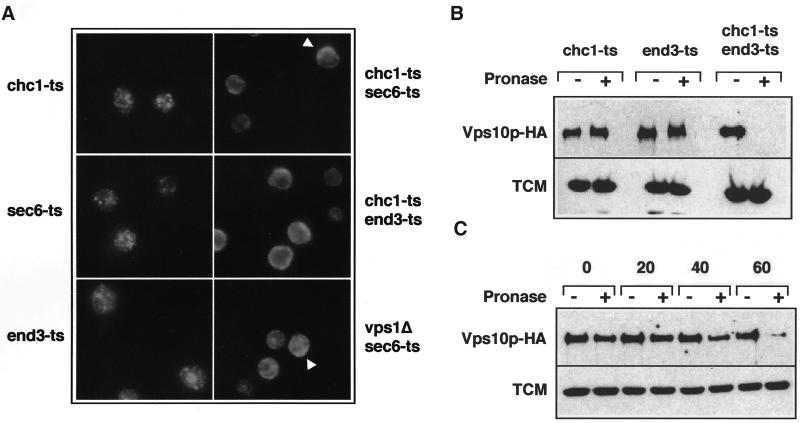
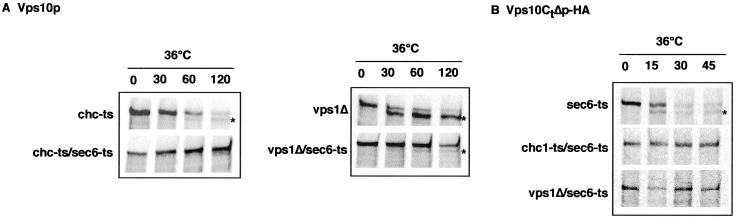
Several lines of evidence indicate that Vps10p may be transported to the plasma membrane before reaching the endosome via the endocytic pathway in chc1 cells. First, trans-Golgi proteins (Kex2p and DPAP A) that are normally transported to the endosome in WT cells are rerouted to the plasma membrane in chc1 and vps1 mutants (Seeger and Payne, 1992; Nothwehr et al., 1995). Second, Pep12p is also implicated in membrane/protein trafficking from the plasma membrane to the late endosome (Holthuis et al., 1998b). Therefore, we considered the possibility that Vps10p trafficking is blocked in the endocytic pathway after being mislocalized to the plasma membrane in chc1-ts/pep12Δor vps1Δ/pep12Δdouble mutants. To test this possibility, we used sec6-ts and end3-ts, temperature-sensitive mutations that block fusion of secretory vesicles to the plasma membrane and endocytosis, respectively, but do not affect protein transport to the vacuole. However, if Vps10p travels to the cell surface in the absence of CCVs, then chc1-ts/s6-ts or chc1-ts/end3-ts double mutants should affect the normal distribution of Vps10p at the nonpermissive temperature. As described in Figure 1B, the localization of Vps10p-HA was visualized by indirect immunofluorescence microscopy. We first tested the distribution of Vps10p in sec6-ts and end3-ts mutants. In both cases, we observed a punctate pattern of Vps10p similar to that observed in WT cells (Figure 1B), suggesting normal Vps10p localization and CPY transport to the vacuole. Correspondingly, we found that the maturation of CPY was not affected in sec6-ts and end3-ts cells (our unpublished results). In contrast, in the chc1-ts/sec6-ts mutant, Vps10p-HA was more dispersed, likely representing vesicular structures. Occasionally, the diffuse staining was observed close to the cell surface (Figure 4A; see arrowheads), suggesting that proteins were trapped in secretory vesicles close to or attached to the plasma membrane. In chc1-ts/end3-ts cells, Vps10p-HA appeared to accumulate at the plasma membrane at the restrictive temperature.
Figure 4.
Accumulation of Vps10p-HA at the cell surface in the chc1-ts/end3-ts mutant. (A) Immunofluorescence localization of Vps10p-HA. ODY129 (chc1-ts:: VPS10-HA), ODY221 (sec6-ts:: VPS10-HA), ODY222 (end3-ts:: VPS10-HA), ODY102 (chc1-ts/sec6-ts:: VPS10-HA), ODY138 (chc1-ts/end3-ts:: VPS10-HA), and ODY119 (vps1Δ/sec6-ts:: VPS10-HA) strains were grown to midlog phase at 24°C and then incubated for 60 min at 36°C. Cells were labeled with monoclonal anti-HA antibodies followed by Cy3-conjugated secondary antibodies. Arrows indicate the accumulation of Vps10p-HA at the cell surface. (B) Trafficking of Vps10p-HA in the chc1-ts/end3-ts mutant. ODY129 (chc1-ts:: VPS10-HA), ODY222 (end3-ts:: VPS10-HA), and ODY138 (chc1-ts/end3-ts:: VPS10-HA) strains were grown to midlog phase at 24°C and then incubated for 60 min at 36°C. Cells were then incubated for 1 h at 36°C in the presence of pronase as indicated. Proteolysis of Vps10p-HA was determined by Western analysis. (C) ODY138 (chc1-ts/end3-ts:: VPS10-HA) strain was grown to midlog phase at 24°C and then incubated at 36°C. Samples were taken 0, 20, 40, and 60 min after the temperature shift. Cells were digested with pronase and the stability of Vps10p-HA was determined as described in B. As a control, accessibility of pronase on a cytoplasmic protein TCM (the ribosomal Rpl3p) was assessed.
The endocytic pathway is essential in a vps1Δ background in which vacuolar membrane traffic is diverted to the cell surface (Nothwehr et al., 1995). Thus it was not possible to construct a double mutant of vps1Δand end3-ts. Instead, we examined the distribution of Vps10p-HA in a vps1Δ/sec6-ts strain. As expected, the induction of a sec6 block in vps1Δcells showed a dispersed signal with a slight accumulation at the plasma membrane, similar to that observed in chc1-ts/sec6-ts cells (Figure 4A; see arrowheads).
As an independent test of the transport of Vps10p to the plasma membrane in the chc1 mutant, we examined the susceptibility of Vps10p to digestion by a protease added to intact chc1-ts/end3-ts cells. Cells were grown for 60 min at 36°C to induce the accumulation of Vps10p at the cell surface, poisoned by the addition of sodium azide, and treated for 60 min with exogenous protease (Davis et al., 1993). As seen in Figure 4B, Vps10p was resistant to proteolysis in chc1-ts or end3-ts single mutants but was degraded in the chc1-ts/end3-ts double mutant. We next examined the time required for Vps10p to reach the plasma membrane after inactivation of clathrin. The chc1-ts/end3-ts mutant was incubated for different periods of time at 36°C and then treated with protease as described above. As shown in Figure 4C, Vps10p began to be degraded after 40 min incubation at 36°C, suggesting a slow transport of Vps10p to the cell surface.
Redding et al. (1996) showed that the PEP4-dependent degradation of Kex2p in chc1-ts cells is blocked by a sec1 lesion, which blocks secretory vesicle fusion with the plasma membrane, suggesting that the transport of Kex2p to the plasma membrane is affected in this cell background. In a similar experiment, we examined the stability of Vps10p in chc1-ts/sec6-ts and vps1Δ/sec6-ts mutant strains. In a pulse chase and immunoprecipitation analysis, we showed that Vps10p degradation was blocked in chc1-ts/sec6-ts and reduced in vps1Δ/sec6-ts cells, respectively, after inducing the sec6 block (Figure 5A). Interestingly, we found that after a longer inactivation of chc1-ts/sec6-ts at 36°C, Vps10p was degraded by a vacuolar protease-independent pathway (our unpublished results) as was reported for Kex2p (Redding et al., 1996). Finally, we tested the transport of Vps10CtΔp-HA, which is transported by CCVs to the endosome before being rapidly degraded in the vacuole (Deloche et al., 2001). Previously, we showed that the degradation of Vps10CtΔp-HA lacking its localization motifs is blocked by specific vps mutants (vps45 and vps34) involved in anterograde protein transport from the TGN to the endosome (Bryant et al., 1998; Deloche et al., 2001). As shown in Figure 5B, degradation of Vps10CtΔp-HA was not affected in the sec6 mutant but was blocked in both chc1-ts/sec6-ts and vps1Δ/sec6-ts mutants, indicating that Vps10CtΔp-HA, like the full length Vps10p, is rerouted to the plasma membrane in the absence of Chc1p or Vps1p. Our results suggest that mutants that prevent formation of CCVs at the TGN lead to a mislocalization of different forms of Vps10p to the cell surface, as is true of other Golgi membrane proteins.
Figure 5.
Stability of Vps10p and Vps10CtΔ-HA in chc1-ts/sec6-ts and vps1Δ/sec6-ts mutants. (A) EHY202 (chc1-ts), ODY95 (chc1-ts/sec6-ts), EHY361(vps1Δ), and EHY225 (vps1Δ/sec6-ts) strains were grown to midlog phase at 24°C and then incubated for 45 min at 36°C before labeling at 36°C. After the indicated chase time, aliquots of cells were removed and subjected to immunoprecipitation with antibodies to Vps10p. (B) EHY227 (sec6-ts), EHY242 (chc1-ts/sec6-ts), and EHY225 (vps1Δ/sec6-ts) containing plasmid encoding Vps10CtΔp-HA were subjected to pulse chase immunoprecipitation and analyzed as described in A, except that monoclonal anti-HA antibody was used to immunoprecipitate Vps10CtΔp-HA from cell extracts. The PEP4-dependent cleavage product is indicated (*).
CPY Maturation Occurs in the Late Secretory Pathway in the chc1-ts/sec6-ts Mutant
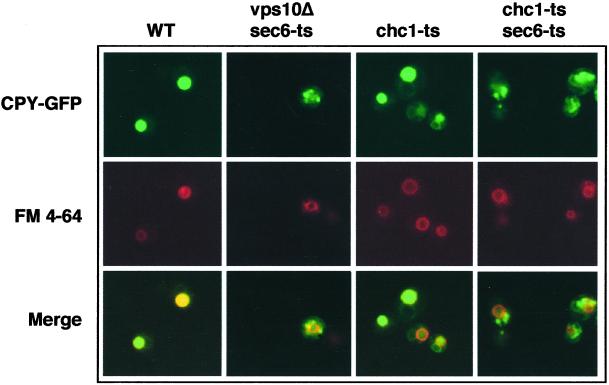
Seeger et al. (Seeger and Payne, 1992) showed that CPY is normally processed in a chc1Δ/sec1mutant. Because this appeared inconsistent with our results, we investigated CPY maturation in the chc1-ts/sec6-ts mutant. We found that CPY maturation in the chc1-ts/sec6-ts mutant occurs more slowly than in WT and chc1-ts cells (Figure 6A) but was still dependent on the proteinase A (Pep4p), a soluble vacuolar protease (Figure 6B). Pep4p and CPY share the same requirement for Vps10p and clathrin in transport (Seeger and Payne, 1992; Cooper and Stevens, 1996). Thus, we considered the possibility that CPY and Pep4p, together are diverted to the secretory pathway where the maturation of CPY occurs in the chc1-ts/sec6-ts mutant. To confirm this hypothesis, we used a protein fusion where the first 50 amino acids of CPY was fused to a GFP (CPY-GFP). A previous study established that CPY-GFP resides in the vacuole and that its localization is dependent on Vps10p (Humair et al., 2001). To follow the localization of newly synthesized CPY-GFP, we used an inducible GAL1 promoter on a centromeric vector. In the experiment shown in Figure 7, CPY-GFP synthesis was induced just before shifting cells to the restrictive temperature. As expected, newly synthesized CPY-GFP accumulated in the lumen of the vacuole in WT cells, as judged by staining with the tracer dye FM4–64. In contrast, CPY-GFP was trapped in dispersed intracellular structures in the vps10Δ/sec6-ts mutant at 36°C. In chc1-ts cells, CPY-GFP was visualized in vacuoles similar to WT cells, indicating that CPY-GFP reached the vacuole in the absence of clathrin, confirming previous results in which CPY was localized by immunoelectron microscopy in the vacuole of chc1Δcells (Payne et al., 1988). Finally, in chc1-ts/sec6-ts cells, CPY-GFP failed to reach the vacuole and appeared within intracellular structures (Figure 7). This result suggests that CPY is trapped en route to the cell surface in the sec6-ts/chc1-ts double mutant.
Figure 6.
CPY maturation is delayed in the chc1-ts/sec6-ts mutant. (A) LSY93–2A (WT), EHY202 (chc1-ts), and EHY242 (chc1-ts/sec6-ts) strains were grown to midlog phase at 24°C and then incubated for 60 min at 36°C before a 10-min protein labeling with [35S]methionine and cysteine at 36°C. After the indicated chase time, aliquots of cells were removed and subjected to immunoprecipitation with antibodies to CPY. (B) EHY242 (chc1-ts/sec6) and ODY200 (chc1-ts/sec6-ts::pep4−) cells were radiolabeled as in A. Aliquots of cells were removed immediately (0 chase) or 60 min after initiation of the chase and subjected to immunoprecipitation with antibodies to CPY. The ER, Golgi, and mature forms of CPY are labeled p1, p2, and M, respectively.
Figure 7.
CPY-GFP does not reach the vacuole in chc1-ts/sec6-ts mutants. The production of CPY-GFP was under the control of the galactose-inducible GAL1 promoter in all strains. LSY93–2A (WT), EHY62 (vps10Δ/sec6-ts), EHY202 (chc1-ts), and EHY242 (chc1-ts/sec6) strains were grown to midlog phase at 24°C. Galactose was added and the cells were immediately incubated for 3 h at 36°C. Vacuolar membranes were stained with FM4–64 dye. The cells were viewed under a fluorescence microscope with filters for CPY-GFP or FM 4–64.
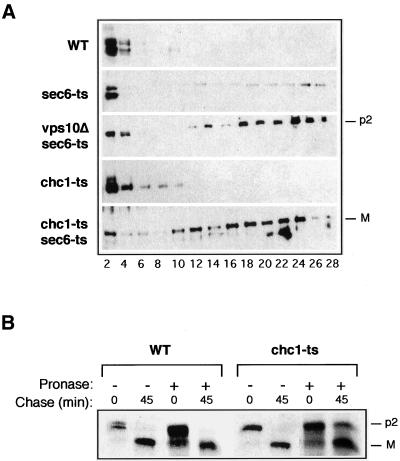
In a more direct test of the localization of CPY, we isolated Golgi-derived constitutive secretory vesicles accumulated in sec6-ts/chc1-ts cells. In this experiment, temperature-shifted cells were lysed by osmotic shock and organelles were fractionated by differential centrifugation (Harsay and Bretscher, 1995). The slowly sedimenting membrane fraction isolated in the high-speed pellet (100,000 × g) was fractionated on a sucrose (30–55%) equilibrium density gradient. Because CPY is not normally transported to the vacuole via late secretory vesicles, little material was detected in sucrose density gradient fractions corresponding to dense vesicles (Figure 8A). Furthermore, little CPY was detected in dense vesicles isolated from chc1-ts cells (Figure 8A). However, the precursor form of CPY (p2) that is diverted to the secretory pathway was readily apparent in dense vesicle fractions isolated from the vps10Δ/sec6-ts mutant. The mature form of CPY in vesicles displayed over a broad density range on a sucrose gradient of membranes from chc1-ts/sec6-ts cells. These results suggest that CPY traffic is interrupted when a late event in the secretory pathway is blocked in cells deficient in clathrin.
Figure 8.
Accumulation of the mature form of CPY in the late secretory pathway in the chc1-ts/sec6-ts mutant. (A) Sucrose equilibrium density gradient fractionation of membrane from LSY93–2A (WT), EHY227 (sec6-ts), EHY62 (vps10Δ/sec6-ts), EHY202 (chc1-ts), and EHY242 (chc1-ts/sec6-ts) cells. A fraction containing small membranes (P100) was loaded onto the top of a 30–55% sucrose gradient and centrifuged at 150,000 × g for 20 h. Fractions were collected from the top and analyzed by immunobloting with antisera against CPY. (B) CPY trafficking in pronase-treated LSY93–2A (WT) and EHY202 (chc1-ts) mutants. Spheroplasts were grown for 90 min at 36°C in the presence of pronase as indicated. Subsequently, spheroplasts were radiolabeled, and aliquots were removed immediately after the pulse (0 chase) or 45 min after initiation of the chase and subjected to immunoprecipitation with antibodies to CPY as described in the legend of Figure 1A. The Golgi and mature forms of CPY are labeled p2 and M, respectively.
We considered the possibility that Vps10p exported to the cell surface in a chc1-ts mutant must be recycled to retrieve p2 CPY for transport to the vacuole. If so, externally exposed Vps10p would be susceptible to pronase and not available for routing of CPY. We first incubated spheroplasts of chc1-ts cells at 36°C in the presence of pronase for 90 min and then labeled the cells in a pulse (time 0) and chase (45 min) regimen. As shown in Figure 8B, the maturation of CPY was not affected by the incubation of wild-type spheroplasts with pronase. In contrast, we found a CPY maturation defect (≈10–20%) in the chc1-ts mutant after 45 min of chase. Thus at least some of the Vps10p cycling via the cell surface is required for the transport of CPY in the chc1-ts mutant. The limited extent of this maturation defect may arise from the Vps10p-independent proteolysis of CPY as seen in the sec6-ts/chc1-ts mutant (Figure 6A).
DISCUSSION
Vps10p Travels from the TGN to the Plasma Membrane in the chc1-ts Mutant
Yeast cells require 1 h of incubation after inactivation of clathrin-dependent transport to restore normal CPY sorting to the vacuole. This indicates that cells are able to adapt to a clathrin block and set up a clathrin-independent pathway to transport CPY to the vacuole. Here, we show that the full-length vacuolar CPY sorting receptor, Vps10p, and a Vps10p mutant, lacking its C-terminal cytoplasmic domain (Vps10CtΔp-HA), are diverted to the cell surface in chc1 or vps1 mutants (Figure 9). Similar behavior has been described for other Golgi membrane proteins (Seeger and Payne, 1992; Nothwehr et al., 1995).
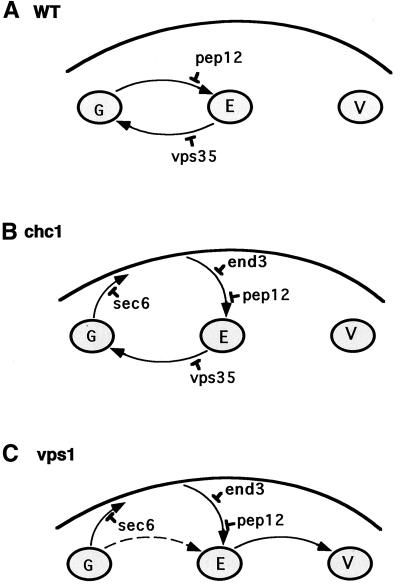
Figure 9.
Model for Vps10p trafficking in chc1 and vps1 mutants. TGN, endosome, vacuole, and plasma membrane are represented by the letters G, E, V, and PM, respectively. The specific blocks in protein transport in pep12, vps35, sec6, and end3 mutants are displayed. (A) In WT cells, Vps10p travels to the endosome (Pep12 compartment) and is recycled back to the TGN via the retromer-dependent pathway for another round of CPY sorting. (B) In chc1 cells, Vps10p transport from TGN to endosome is blocked, so Vps10p is diverted to the plasma membrane before being endocytosed to the endosome and recycled back to the TGN. (C) In vps1 cells, Vps10p reaches the endosome as in B. However, because Vps1p is likely involved in the retrieval pathway (E-G), Vps10p is transported and degraded in the vacuole. The dashed arrow refers to the pathway by which a small amount of Vps10p might be delivered to the vacuole without traversing the PM (see text).
Our results suggest that sorting of CPY depends on the retrieval of Vps10p from the cell surface in the chc1-ts mutant. We found that the mature form of CPY is missorted and accumulates in the secretory pathway when secretion is blocked in a chc1-ts/sec6-ts mutant strain. It has been reported that soluble proteins that cannot be transported to the endosome are diverted to the secretory pathway, possibly by default. Therefore, the accumulation of newly synthesized CPY in the secretory pathway is likely due to a block in Vps10p transport in the chc1-ts/sec6-ts mutant. However, the vesicles in which CPY accumulate in the chc1-ts/sec6-ts cells equilibrate at a lower buoyant density than the secretory vesicles that accumulate p2 CPY in a vps10Δ/sec6-ts mutant strain (see Figure 8A). One possibility is that p2 CPY is delayed in a late Golgi or endosomal compartment from which it can be recovered when Vps10p is recycled from the cell surface. The images in Figures 4A and 7 suggest that CPY-GFP and Vps10p are in distinct cellular compartments in chc1-ts/sec6-ts cells. CPY-GFP is present in few larger intermediate compartments (bright spots), whereas Vps10p is more dispersed within the cells. Furthermore, no PEP4-dependent degradation of Vps10p was detected in the chc1-ts/sec6-ts mutant, quite in contrast to the PEP4-dependent maturation of CPY in this strain. We speculate that Vps10p and p2CPY somehow are segregated in a chc1-ts/sec6-ts strain with Vps10p in authentic secretory vesicles and p2 CPY in a Golgi or endosomal compartment in which active Pep4p accumulates. In this compartment, premature proteolytic maturation of CPY removes the sorting signal required for Vps10p-dependent traffic to the vacuole.
It has been proposed that Pep12p is selectively transported by CCVs from the TGN to the late endosome (Black and Pelham, 2000). This transport requires a sorting signal containing the FSDSPEF motif located on the cytoplasmic domain of Pep12p and is dependent on the clathrin-associated GGA proteins. In contrast to Vps10p, Pep12p does not seem to be rerouted to the plasma membrane in gga or chc1 mutants. This is likely due to the nature of its transmembrane domain (TMD), which segregates Pep12p from the secretory pathway (Lewis et al., 2000). However, a Pep12-Sso1 chimera (the TMD of Pep12p was replaced with that of the plasma membrane syntaxin Sso1p) that contains mutations in the FSDSPEF Golgi-endosome transport motif accumulates at the cell surface in an end4 mutant (Black and Pelham, 2000). In addition, the expression of a similar Pep12-Sso1 chimera (PNTS; Pep12p NH2 terminus fused to the Sso1p TMD) is partially missorted to the plasma membrane in a clc1 or chc1 cell. Together, these data indicate that cells have a tendency to divert proteins traveling to the late or early endosomes to the cell surface as a consequence of a clathrin defect.
Vps10p Is Recycled from the Plasma Membrane to the TGN in the chc1-ts Mutant
Protein transport to the plasma membrane and constitutive endocytosis in the yeast cell can be extremely rapid. Indeed, the Ste3p receptor, which is responsible for the internalization of a-factor, is synthesized, transported to the cell surface, and delivered to the vacuole for degradation with a half-life of 15 min at 30°C (Roth et al., 1998). In contrast, we found that Vps10p reaches the plasma membrane 40 min after inactivation of clathrin. This long transit time may reflect an inherently inefficient traffic of Vps10p or the sum of a period of adaptation followed by rapid mislocalization of Vps10p to the plasma membrane.
The role of CCVs in endocytosis in yeast is unclear and possibly limited. In the chc1Δ mutant, the internalization of the α-factor pheromone and the Ste3p receptor is only reduced to 30–50% of WT rates (Payne et al., 1988). Therefore, the rate of Vps10p internalization may not be affected in the chc1 mutant.
We considered the possibility that p2CPY accompanies Vps10p to the cell surface followed by recapture of the complex to the endosome and vacuole. The accumulation of mature CPY in nonvacuolar vesicles within chc1-ts/sec6-ts mutant cells suggested that CPY precursor may engage in a secretion recapture pathway that when interrupted by a block in secretion causes premature maturation of CPY (Figures 7 and 8A). However, we found no evidence for a cell surface bound fraction of p2 or mature CPY in the chc1 ts strain. Incubation of cells at low pH, conditions expected to liberate p2CPY from cell surface Vps10p, failed to delay transport and maturation of CPY in the chc1-ts strain (our unpublished results). Thus, the inactivation of clathrin may lead to the accumulation of p2 CPY in an intracellular compartment (early endosome?) to which internalized Vps10p has access en route to the late endosome and vacuole (Figure 9B). After releasing p2CPY in the late endosome, Vps10p would experience the normal path of retrieval to the trans-Golgi and then escape to the cell surface once again.
We recently reported that p2CPY transits an endosome en route to the cell surface in cells lacking Vps10p (Harsay and Schekman, 2002). However, the role of clathrin in this missorting has not been evaluated.
It is likely that different pathways are necessary to deliver all endocytosed proteins to the appropriate target membranes within the cells (Holthuis et al., 1998b). At least one pathway transports proteins to the late endosomal compartment where proteins are either delivered to the vacuole or recycled back to the plasma membrane, whereas a second pathway recycles proteins back to the TGN in order to sustain the secretory pathway. In this regard, the exocytic SNARE Snc1p was shown to return to the Golgi before being recycled to the plasma membrane. This retrieval pathway is independent of Pep12p, indicating that one endocytic pathway can bypass the late endosome to reach the Golgi (Lewis et al., 2000). Our data show that CPY is missorted in both chc1-ts/pep12Δ and chc1-ts/vps35Δ strains. Thus, we propose that Vps10p recycles from the plasma membrane through the late endosome and requires the components of the retromer coat such as Vps35p to reach the TGN (Figure 9B). However, it should be noted that the distinction between the late and early endosomes, based on the protein and lipid composition of these two organelles, may be eliminated in clathrin mutant cells (Black and Pelham, 2000).
Surprisingly, in contrast to Vps10p, Kex2p is not properly recycled to the TGN in chc1 strains. Alpha-factor precursor is made and remains unprocessed under conditions where cells have adapted to a clathrin block to transport of CPY to the vacuole (Payne and Schekman, 1989). It is possible that Kex2p is not efficiently endocytosed, therefore depleting the amount of Kex2p in the TGN. Another interpretation is that Kex2p is mislocalized after its internalization from the plasma membrane and is rapidly degraded in the vacuole. Both Kex2p and Vps10p require a tyrosine motif within their cytosolic domain to be recycled from the endosome to the TGN in WT cells. Interestingly, in a chc1-ts strain, WT Kex2p and a Kex2p mutant with a complete deletion of the cytosolic tail are degraded in the vacuole at similar rates (Redding et al., 1996). This observation suggests that the cytosolic sorting signal of Kex2p is inefficient in chc1 mutants. Thus, Vps10p may contain an additional sorting motif, allowing its return to the TGN from the endocytic pathway. Indeed, we cannot exclude the possibility that Vps10p and Kex2p travel in two distinct pathways and that Vps10p is retrieved from a specific intermediate compartment. In fact, although Vps10p and Kex2p traffic between the TGN and the endosome, they likely do not travel in the same pathway because only the transport of Kex2p requires the two syntaxins, Tlg1p and Tlg2p (t-SNARE affecting a late Golgi compartment; Holthuis et al., 1998a). This suggests that Kex2p, but not Vps10p, travels mainly through the early endosome.
A previous study showed that Golgi and vacuolar proteins are diverted to the vacuole via the plasma membrane in vps1 cells (Nothwehr et al., 1995). Similarly, we found that Vps10p is degraded in the vacuole after being rerouted to the plasma membrane in a vps1 mutant. This degradation is blocked in the absence of Pep12p, demonstrating that Vps10p travels through the late endosome. It is possible that in contrast to Chc1p, Vps1p is required for protein retrieval from the endosome to the TGN. Therefore, instead of returning to the TGN, Vps10p may be delivered to the vacuole in vps1 cells (Figure 9C). In this context, previous work showed that dynamin is involved in the recycling of cation-independent mannose 6-phosphate receptor (CI-MPR) from endosomes to the TGN in a clathrin-independent pathway (Draper et al., 1990; Goda and Pfeffer, 1991; Nicoziani et al., 2000).
In conclusion, we propose a model in which Vps10p travels to the cell surface before being retrieved to an endosome to mediate the transport of p2 CPY to the vacuole in chc1 cells. The roughly 30–60-min period of adaptation of a chc1 ts strain, during which time cells accommodate to the absence of clathrin, may be required to transport and retrieve Vps10p from the cell surface. This suggests that mislocalized protein at the plasma membrane can be efficiently recycled to the TGN in the absence of CCVs.
ACKNOWLEDGMENTS
We are grateful to Tom H. Stevens and D. Kressler for anti-Vps10p and TCM antibodies, respectively, and J. M. Neuhaus for plasmid containing CPY-GFP. We also thank Edina Harsay for providing unpublished strains and results, G. Payne and Dan Baggott for helpful discussion, and Debbie Ang for reading the manuscript. During the initial stage of this work, O.D. was supported by a fellowship from the Swiss National Science Foundation. This work was supported by grants from the National Institutes of Health and the Howard Hughes Medical Institute to R.S and from the Swiss National Science Foundation (FN-31.47283.96) to C.G.
Abbreviations used:
- YPD
standard rich medium
- TGN
trans-Golgi network
- CCV
clathrin-coated vesicle
- CPY
carboxylpeptidase Y
- WT
wild-type
- t-SNARE
target-soluble NSF (N-ethylmaleimide sensitive factor) attachment protein receptor
- TMD
transmembrane domain
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–07–0105. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–07–0105.
REFERENCES
- Ausubel, F.M., Brent, R., Kingston, R.E., More, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. (1987–1995). Current Protocols in Molecular Biology. New York: John Wiley, and Sons, Inc.
- Becherer KA, Rieder SE, Emr SD, Jones EW. Novel syntaxin homologue, Pep12p, required for the sorting of lumenal hydrolases to the lysosome-like vacuole in yeast. Mol Biol Cell. 1996;7:579–594. doi: 10.1091/mbc.7.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MW, Pelham HR. A selective transport route from Golgi to late endosomes that requires the yeast GGA proteins. J Cell Biol. 2000;151:587–600. doi: 10.1083/jcb.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, Piper RC, Gerrard SR, Stevens TH. Traffic into the prevacuolar/endosomal compartment of Saccharomyces cerevisiae: a VPS45-dependent intracellular route and a VPS45- independent, endocytic route. Eur J Cell Biol. 1998;76:43–52. doi: 10.1016/S0171-9335(98)80016-2. [DOI] [PubMed] [Google Scholar]
- Burd CG, Peterson M, Cowles CR, Emr SD. A novel Sec18p/NSF-dependent complex required for Golgi-to-endosome transport in yeast. Mol Biol Cell. 1997;8:1089–1104. doi: 10.1091/mbc.8.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Graham TR. An arf1Delta synthetic lethal screen identifies a new clathrin heavy chain conditional allele that perturbs vacuolar protein transport in Saccharomyces cerevisiae. Genetics. 1998;150:577–589. doi: 10.1093/genetics/150.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu DS, Pishvaee B, Payne GS. The light chain subunit is required for clathrin function in Saccharomyces cerevisiae. J Biol Chem. 1996;271:33123–33130. doi: 10.1074/jbc.271.51.33123. [DOI] [PubMed] [Google Scholar]
- Chuang JS, Schekman RW. Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J Cell Biol. 1996;135:597–610. doi: 10.1083/jcb.135.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AA, Stevens TH. Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J Cell Biol. 1996;133:529–541. doi: 10.1083/jcb.133.3.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NG, Horecka JL, Sprague GF., Jr Cis- and trans-acting functions required for endocytosis of the yeast pheromone receptors. J Cell Biol. 1993;122:53–65. doi: 10.1083/jcb.122.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloche O, Yeung BG, Payne GS, Schekman R. Vps10p transport from the trans-Golgi network to the endosome is mediated by clathrin-coated vesicles. Mol Biol Cell. 2001;12:475–485. doi: 10.1091/mbc.12.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper RK, Goda Y, Brodsky FM, Pfeffer SR. Antibodies to clathrin inhibit endocytosis but not recycling to the trans Golgi network in vitro. Science. 1990;248:1539–1541. doi: 10.1126/science.2163108. [DOI] [PubMed] [Google Scholar]
- Feldheim D, Yoshimura K, Admon A, Schekman R. Structural and functional characterization of Sec66p, a new subunit of the polypeptide translocation apparatus in the yeast endoplasmic reticulum. Mol Biol Cell. 1993;4:931–939. doi: 10.1091/mbc.4.9.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y, Pfeffer SR. Identification of a novel, N-ethylmaleimide-sensitive cytosolic factor required for vesicular transport from endosomes to the trans-Golgi network in vitro. J Cell Biol. 1991;112:823–831. doi: 10.1083/jcb.112.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C, Fink GR. Guide to yeast genetics and molecular biology. Methods Enzymol. 1991;194:3–21. [PubMed] [Google Scholar]
- Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay E, Schekman R. A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J Cell Biol. 2002;156:271–285. doi: 10.1083/jcb.200109077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JC, Nichols BJ, Dhruvakumar S, Pelham HR. Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 1998a;17:113–126. doi: 10.1093/emboj/17.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthuis JC, Nichols BJ, Pelham HR. The syntaxin Tlg1p mediates trafficking of chitin synthase III to polarized growth sites in yeast. Mol Biol Cell. 1998b;9:3383–3397. doi: 10.1091/mbc.9.12.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humair D, Hernández Felipe D, Neuhaus J-M, Paris N. Demonstration in yeast of the function of BP-80, a putative plant vacuolar sorting receptor. Plant Cell. 2001;13:781–792. doi: 10.1105/tpc.13.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Nichols BJ, Prescianotto-Baschong C, Riezman H, Pelham HR. Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol Biol Cell. 2000;11:23–38. doi: 10.1091/mbc.11.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcusson EG, Horazdovsky BF, Cereghino JL, Gharakhanian E, Emr SD. The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell. 1994;77:579–586. doi: 10.1016/0092-8674(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Nicoziani P, Vilhardt F, Llorente A, Hilout L, Courtoy PJ, Sandvig K, van Deurs B. Role for dynamin in late endosome dynamics and trafficking of the cation-independent mannose 6-phosphate receptor. Mol Biol Cell. 2000;11:481–495. doi: 10.1091/mbc.11.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Bruinsma P, Strawn LA. Distinct domains within Vps35p mediate the retrieval of two different cargo proteins from the yeast prevacuolar/endosomal compartment. Mol Biol Cell. 1999;10:875–890. doi: 10.1091/mbc.10.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Conibear E, Stevens TH. Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J Cell Biol. 1995;129:35–46. doi: 10.1083/jcb.129.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Ha SA, Bruinsma P. Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J Cell Biol. 2000;151:297–310. doi: 10.1083/jcb.151.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr SF, Roberts CJ, Stevens TH. Membrane protein retention in the yeast Golgi apparatus: dipeptidyl aminopeptidase A is retained by a cytoplasmic signal containing aromatic residues. J Cell Biol. 1993;121:1197–1209. doi: 10.1083/jcb.121.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne G, Baker D, Van Tuinen E, Schekman R. Protein transport to the vacuole and receptor-mediated endocytosis by clathrin heavy chain-deficient yeast. J Cell Biol. 1988;106:1453–1461. doi: 10.1083/jcb.106.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne GS, Schekman R. Clathrin: a role in the intracellular retention of a Golgi membrane protein. Science. 1989;245:1358–1365. doi: 10.1126/science.2675311. [DOI] [PubMed] [Google Scholar]
- Piper R, Bryant N, Stevens T. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–546. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishvaee B, Costaguta G, Yeung BG, Ryazantsev S, Greener T, Greene LE, Eisenberg E, McCaffery JM, Payne GS. A yeast DNA J protein required for uncoating of clathrin-coated vesicles in vivo. Nat Cell Biol. 2000;2:958–963. doi: 10.1038/35046619. [DOI] [PubMed] [Google Scholar]
- Rad MR, Phan HL, Kirchrath L, Tan PK, Kirchhausen T, Hollenberg CP, Payne GS. Saccharomyces cerevisiae Apl2p, a homologue of the mammalian clathrin AP beta subunit, plays a role in clathrin-dependent Golgi functions. J Cell Sci. 1995;108:1605–1615. doi: 10.1242/jcs.108.4.1605. [DOI] [PubMed] [Google Scholar]
- Redding K, Holcomb C, Fuller RS. Immunolocalization of Kex2 protease identifies a putative late Golgi compartment in the yeast Saccharomyces cerevisiae. J Cell Biol. 1991;113:527–538. doi: 10.1083/jcb.113.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding K, Seeger M, Payne GS, Fuller RS. The effects of clathrin inactivation on localization of Kex2 protease are independent of the TGN localization signal in the cytosolic tail of Kex2p. Mol Biol Cell. 1996;7:1667–1677. doi: 10.1091/mbc.7.11.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CJ, Nothwehr SF, Stevens TH. Membrane protein sorting in the yeast secretory pathway: evidence that the vacuole may be the default compartment. J Cell Biol. 1992;119:69–83. doi: 10.1083/jcb.119.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth AF, Sullivan DM, Davis NG. A large PEST-like sequence directs the ubiquitination, endocytosis, and vacuolar degradation of the yeast a-factor receptor. J Cell Biol. 1998;142:949–961. doi: 10.1083/jcb.142.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JH, Raymond CK, Gilbert T, O'Hara PJ, Stevens TH. A putative GTP binding protein homologous to interferon-inducible Mx proteins performs an essential function in yeast protein sorting. Cell. 1990;61:1063–1074. doi: 10.1016/0092-8674(90)90070-u. [DOI] [PubMed] [Google Scholar]
- Schekman R, Orci L. Coat proteins and vesicle budding. Science. 1996;271:1526–1533. doi: 10.1126/science.271.5255.1526. [DOI] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: an integrated process. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Seaman MN, Marcusson EG, Cereghino JL, Emr SD. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol. 1997;137:79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger M, Payne GS. A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J. 1992;11:2811–2818. doi: 10.1002/j.1460-2075.1992.tb05348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira LA, Wong DH, Masiarz FR, Schekman R. Yeast clathrin has a distinctive light chain that is important for cell growth. J Cell Biol. 1990;111:1437–1449. doi: 10.1083/jcb.111.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Spang A, Schekman R. A primer on vesicle budding. Cell. 1999;97:145–148. doi: 10.1016/s0092-8674(00)80722-9. [DOI] [PubMed] [Google Scholar]
- Stevens T, Esmon B, Schekman R. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell. 1982;30:439–448. doi: 10.1016/0092-8674(82)90241-0. [DOI] [PubMed] [Google Scholar]
- Tan PK, Davis NG, Sprague GF, Payne GS. Clathrin facilitates the internalization of seven transmembrane segment receptors for mating pheromones in yeast. J Cell Biol. 1993;123:1707–1716. doi: 10.1083/jcb.123.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater CA, Raymond CK, Ekena K, Howald-Stevenson I, Stevens TH. The VPS1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with two functionally separable domains. J Cell Biol. 1992;119:773–786. doi: 10.1083/jcb.119.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CA, Redding K, Wright R, Fuller RS. Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol Biol Cell. 1992;3:1353–1371. doi: 10.1091/mbc.3.12.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]