Abstract
The RKKEE cluster of charged residues located within the cytoplasmic helix of the bacterial mechanosensitive channel, MscL, is essential for the channel function. The structure of MscL determined by x-ray crystallography and electron paramagnetic resonance spectroscopy has revealed discrepancies toward the C-terminus suggesting that the structure of the C-terminal helical bundle differs depending on the pH of the cytoplasm. In this study we examined the effect of pH as well as charge reversal and residue substitution within the RKKEE cluster on the mechanosensitivity of Escherichia coli MscL reconstituted into liposomes using the patch-clamp technique. Protonation of either positively or negatively charged residues within the cluster, achieved by changing the experimental pH or residue substitution within the RKKEE cluster, significantly increased the free energy of activation for the MscL channel due to an increase in activation pressure. Our data suggest that the orientation of the C-terminal helices relative to the aqueous medium is pH dependent, indicating that the RKKEE cluster functions as a proton sensor by adjusting the channel sensitivity to membrane tension in a pH-dependent fashion. A possible implication of our results for the physiology of bacterial cells is briefly discussed.
INTRODUCTION
Mechanosensation is a physiological process during which mechanical stimuli are converted into intracellular electrochemical signals. In prokaryotes this function is carried out by mechanosensitive (MS) channels of small (MscS) and large (MscL) conductance (1–10). When challenged by a hypo-osmotic shock upon exposure to media of low osmolarity, bacterial cells rapidly expel the cytoplasmic contents (11–13). Although the exact size of the cytoplasmic contents being expelled during a hypo-osmotic shock has been debated (14–16), they include osmoprotectants such as potassium glutamate, trehalose, and glycine betaine. Such osmotically induced effluxes occur through MscS and MscL, which function as emergency valves in the bacterial cytoplasmic membrane by preventing excessive cellular turgor pressures that can cause lysis of bacterial cells (17). The MscL function as an emergency valve is facilitated by a large aqueous pore of the open channel allowing passage of large organic cations of up to 35 Å in size (18).
The alignment of the primary amino acid sequence of the MscL homologs revealed a highly conserved charged cluster within the C-terminus of the MscL channel family (19). Whereas deletion of C-terminal residues 110–136 in Escherichia coli MscL had limited effect on the gating properties of the mutant channel, the MscL channel activity was abolished in a mutant with 33 C-terminal residues deleted, which included the charged cluster RKKEE (20,21). Together with the results of a study which showed that the proteolytic digestion of the C-terminal portion of MscL led to an increase in the channel pressure sensitivity (22), this confirms that the charged cluster is essential for the channel function. In addition, it has been proposed that the structure of the C-terminal charged cluster aids in stabilizing the closed configuration of the MscL channel (23,24).
A similar cluster of charged residues can be identified in the C-terminal domains of various prokaryotic and eukaryotic MS channels (Table 1). A conserved sequence of charged residues in two members of the tandem-pore K+ channel family, TREK1 and TREK2, is nearly identical. Both channels are activated by acidic conditions in addition to membrane stretch or unsaturated fatty acids. In contrast, the C-terminal charged cluster, although present, is less well preserved in a third member of this family, TRAAK, which has been shown to be an acid-insensitive but alkali-sensitive MS channel (25–31). A sequence of chemically similar charged residues can also be found within the C-terminal regions of several types of TRP (transient receptor potential) ion channels (Table 1), which are activated by osmotic, chemical, or mechanical stimuli (32–34). To date, the functional role of the sequences of charged residues has not yet been demonstrated in these ion channel proteins. Although the molecular mechanism of the channel gating may differ between prokaryotic and eukaryotic MS channels, the conservation of similar charged clusters in their C-terminal domains further indicates the importance of the preservation of the charged structural motifs in the evolution of MS channels.
TABLE 1.
Conservation of clusters of charged residues in prokaryotic and eukaryotic MS channels
| Channel | Residue number | Charged cluster | Phylogenetic origin | References |
|---|---|---|---|---|
| MscL | 104–108 | RKKEE | Bacteria | (18) |
| MscS | 255–260 | ERIKRE | Bacteria | (19) |
| MscK | 972–978 | DFDRKE | Bacteria | (19) |
| MscMJ | 325–329 | KIKEE | Archaea | (6) |
| MscMJLR | 336–340 | KIKEE | Archea | (7) |
| MscTA | 110–116 | KKEKKE | Archaea | (8) |
| TRPY1 (YVC1) | 642–649 | EDKDEVKE | Eukarya | (54) |
| TREK1 | 301–306 | KKTKEE | Eukarya | (25) |
| TREK2 | 327–332 | KKTKEE | Eukarya | (55) |
| TRAAK | 262–267 | RRTRAE | Eukarya | (28,56) |
| TRPC1 | 690–695 | KQKRDE | Eukarya | (57) |
| LTRPC2* | 1400–1405 | RKLKR | Eukarya | (58) |
| TRPA1 | 1089–1094 | DRFKKE | Eukarya | (59) |
| TRPV4 | 816–821 | RLRRDR | Eukarya | (60) |
| Osm-9 | 834–840 | EERSESK | Eukarya | (61) |
Putative MS channel.
The crystal structure of MscL from Mycobacterium tuberculosis was obtained at acidic pH showing the channel in a closed state (35). It confirmed the topology of the two transmembrane domains, TM1 and TM2, and the intracellularly located C-terminus of the MscL homopentamer (Fig. 1, B and C). In the crystal structure the residues comprising the charged cluster RKKEE are facing each other inside the C-terminal helical bundle. However, an independent study of the channel closed structure of MscL from E. coli using cysteine scanning mutagenesis and electronparamagnetic resonance (EPR) spectroscopy (23) showed that at neutral pH these residues are outwardly oriented facing the aqueous medium. This suggests that the orientation of the C-terminal helices relative to the aqueous medium is pH dependent. Thus, it is possible that the RKKEE cluster functions as a pH sensor. To test this hypothesis, we examined the effects of pH as well as of the charge reversal and residue substitution within the RKKEE cluster on the mechanosensitivity of E. coli MscL reconstituted into liposomes using the patch-clamp technique.
FIGURE 1.
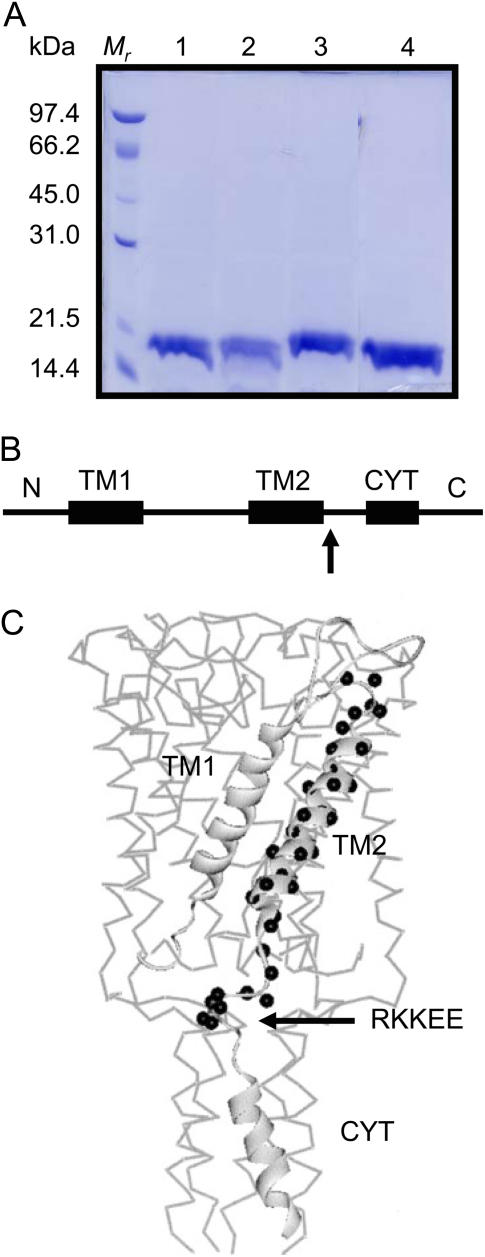
The molecular structure of MscL. (A) SDS-PAGE of the Ni-NTA purified His6 recombinant MscL proteins expressed in E. coli. Lane 1, EEKKR mutant MscL; lane 2, QQQEE mutant MscL; lane 3, RKKQQ mutant MscL; lane 4, wild-type MscL. The size and the gel pattern of the molecular weight markers are shown on the left-hand side. (B) Membrane topology showing the transmembrane domains TM1 and TM2 and the cytoplasmic helix (CYT). The position of the charged cluster is indicated by the arrow. (C) The MscL monomer showing the position of TM2 residues and adjacent cluster of charged residues (black spheres) superimposed onto the three-dimensional crystal structure of the pentameric channel. (Modified from Perozo et al. (23)).
MATERIALS AND METHODS
MscL constructs, bacterial strains, and culture conditions
RKKEE mutants were generated using a site-directed mutagenesis kit “Transformer” (Clontech Laboratories, Mountain View, CA). The pQE32-MscL construct containing 6xHis epitope at the N-terminus was used as a template for mutagenesis reactions. Mutants were generated using the following primers: GAATCGGAAAAAACAACAACCAGCAGCCGCAC for the RKKQQ mutant, CAACAAACTGAATCAGCAACAAGAAGAACCAGC for the QQQEE mutant, and CAAACTGAATGAGGAAAAAAAACGACCAGCAGCCGCAC for the EEKKR mutant. E. coli cells M15 (pREP4∷kan) (Qiagen, Clifton Hill, Australia) harboring the recombinant MscL were cultured in Luria-Bertani broth containing 10 g/L Bacto-tryptone, 5 g/L yeast extract, 5 g/L NaCl supplemented with 100 μg/ml ampicillin, and 25 μg/ml kanamycin.
Purification of 6xHis tagged recombinant MscL
Bacterial cells harboring recombinant MscL plasmids were cultured overnight on antibiotics (100 μg/ml ampicillin and 25 μg/ml kanamycin), subcultured until the absorbance A600 was 0.6, and induced with 1 mM IPTG for 3 h. Pelleted cells were resuspended in a breaking buffer (2 mM MgSO4, 5% sucrose, 100 mM NaCl, 20 μg/ml DNase, and 50 mM NaH2PO4, pH 7.5) and disrupted twice at 8000 psi by the French Press. The membrane fractions were isolated by differential centrifugation: first at 10,000 rpm for 15 min to eliminate cell debris, then for 30 min at 90,000 rpm. The membrane pellet was solubilized in 4 ml of extraction buffer (50 mM NaH2PO4, 200 mM NaCl, 10 mM imidazole, 1 mM PMSF, and 1.5% of octylglucoside, pH 8.0). The extract was mixed with 1 ml of 50% Ni-NTA agarose resin and gently rotated for 1 h. The agarose resin was then packed into a column and washed with 16 × bed volume of wash buffer (extraction buffer, 20 mM imidazole) according to the manufacturer's instructions (Qiagen). Three fractions of 0.5 ml were collected, the protein content was determined by Dc protein assay (Bio-Rad, Regents Park, Australia), and the purity of the recombinant protein was examined using 12% SDS-PAGE mini gel. The recombinant proteins were desalted on a PD-10 desalting column equilibrated with the required buffer containing 1.5% octylglucoside and used for further experiments.
Electrophysiological recordings
Channel currents from the isolated liposome patches were recorded by using the improved patch-clamp techniques (36). Pipettes were made from borosilicate glass using a flaming Brown Micropipette puller (P-87, Sutter Instrument, Novato, CA) and tested by measuring the pipette bubble number, which was ∼3.4–4.2. This parameter corresponded to an average resistance of 4–5 MΩ in a recording solution containing 200 mM KCl, 40 mM MgCl2, 5 mM Hepes-KOH.
A small aliquot of rehydrated liposomes was placed in a patch-clamp chamber filled with the recording solution of the required pH. Pipettes were back-filled with the symmetric recording solution of the same pH, and giga-ohm seals were formed either on contact between the pipette and a blister or by a brief application of suction. Channel currents were induced by negative pressure applied to the back of the pipette. Single channel currents were filtered at 2 kHz, digitized at 5 kHz, and analyzed using pCLAMP6 data acquisition and analysis software (Axon Instruments, Union City, CA). Current recordings were viewed by the Axoscope for Windows program (Axon Instruments). Current amplitudes were determined by measuring the difference between the cursor aligned at the peak and baseline currents. Suction applied to the patch-clamp pipette was measured by the piezoelectric pressure transducer (Omega Engineering, Shelton, CT).
Analysis of Data
The open probability of the MscL channels was plotted against the negative pressure (suction) applied to the patch pipette and fitted to a Boltzmann distribution function of the form NPo = NPmax [1 + exp α (p1/2 – p)]−1, where N is the unknown number of channels in the patch, Po is the open probability, Pmax is the maximum open probability, p is the negative pressure applied to the patch pipette, p1/2 is the pressure at which the open probability is 0.5, and α is the channel sensitivity to pressure. The single channel open probability was estimated from the total current divided by the single channel current giving NPo and divided by the maximum number of channels observed in the patch. By using a two-state Boltzmann model with the change of area t·ΔA being the dominant energy term, it can be shown that α·p1/2 = ΔG0/kT where ΔG0 is the free energy difference between the closed and open conformation of the MS channel in the absence of the externally applied membrane tension (24).
RESULTS
The wild-type and mutated MscL were expressed in E. coli as His6-tagged recombinant proteins followed by a single step purification of detergent solubilized proteins on a Ni-NTA column. SDS-PAGE analysis showed that the recombinant wild-type and mutated MscL run as 17 kDa bands, which correspond to their predicted molecular size (Fig. 1 A). Upon reconstitution into liposomes, the recombinant MscL mutants responded to the application of negative pressure to the patch pipette similar to the wild-type MscL.
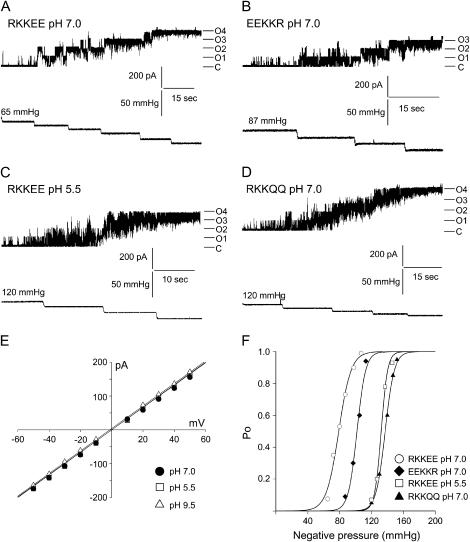
We examined first the effect of pH on MscL single channel conductance and found that changes in pH did not affect the conductance of the channel (Fig. 2 E). Next, we examined the effect of mutations within the RKKEE charged cluster and pH changes on the MS properties of MscL (Figs. 2 and 3). Open probability of MscL channels was plotted as a function of negative pipette pressure and fitted to a Boltzmann distribution function. Since the product ΓMSC = αp1/2 = ΔG0/kT (24), we used the Boltzmann parameters to estimate the free energy ΔG0 required for the channel activation (Table 2). Statistical analysis (ANOVA) showed that there are significant differences in p1/ 2 and ΔG0 between the wild-type MscL examined at physiological pH and the mutant MscL channels having negative charges neutralized (Table 2; Fig. 2). The charge reversal mutation EEKKR did not affect the free energy of activation or the activation pressure of the channel: ΔG0 = 15.8 kT, p1/2 = 76.3 mm Hg and ΔG0 = 16.4 kT, p1/2 = 84.2 mm Hg for the RKKEE wild type and the EEKKR mutant, respectively. However, protonation of E107 and E108 residues, achieved by decreasing the experimental pH or replacement of negative charges by glutamine, shifted the Boltzmann curves toward higher activation pressure without change in the pressure sensitivity α. This result indicated that there was a significant increase in the free energy of activation for the wild-type MscL channel examined at pH 5.5 (ΔG0 = 26.9 kT, p1/2 = 120.2 mm Hg) and the RKKQQ mutant channel examined at pH 7.0 (ΔG0 = 26.1 kT, p1/2 = 130.3 mm Hg).
FIGURE 2.
The effect of negative charges neutralization of C-terminal RKKEE cluster on gating properties of MscL. A, B, C, and D show representative current traces and the corresponding pressure traces of the liposome reconstituted E. coli MscL activated by suction applied to the patch pipette at a pipette potential of +30 mV. (A) Wild-type MscL at physiological pH; (B) MscL harboring the charge reversal mutations; (C) MscL with protonated glutamine residues; (D) MscL with glutamine substituted negative charges. C marks the closed state of channels, whereas On marks open levels of n number of channels. (E) Current-voltage relationship for MscL at physiological, acidic, and alkaline pH (n = 4). (F) Boltzmann distribution curves for MscL channels showing rightward shift in the pressure axis in channels where negative charges were neutralized. Boltzmann distribution function of the form: Po = Pmax/[1 + exp α (p1/2 − p)] was fitted to the data shown above. Po and Pmax are the open and maximal open probabilities; p is the applied negative pressure (in mm Hg), p1/2 is the pressure at which the channels are open half the time, and α is the slope factor describing the sensitivity to negative pressure of the channels.
FIGURE 3.
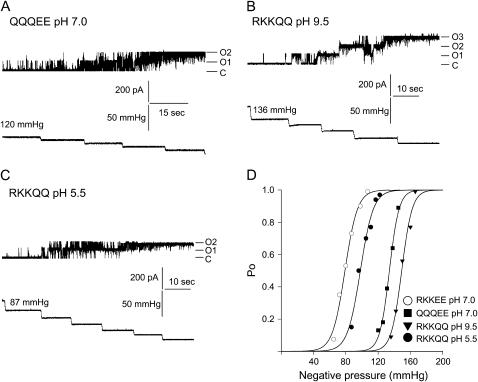
The effect of neutralizing positive charges of the RKKEE cluster on gating properties of MscL. A, B, and C show representative current traces and the corresponding pressure traces of MscL mutants where positive charges were neutralized (A and B) or protonated (C). (D) Boltzmann distribution fits calculated from the displayed traces showing rightward shift in the pressure axis in MscL mutants where positive charges were neutralized. See legend of Fig. 2 for details.
TABLE 2.
Summary of Boltzmann parameters of the wild-type MscL and the MscL channels harboring mutations within the charged cluster
| Mutation | pH | p1/2(mm Hg) | 1/α(mm Hg−1) | ΔG0(kT) | n |
|---|---|---|---|---|---|
| RKKEE | 7.0 | 76.3 ± 4.3 | 5.0 ± 0.6 | 15.8 ± 1.4 | 4 |
| EEKKR | 7.0 | 84.2 ± 5.5 | 5.1 ± 0.2 | 16.4 ± 1.2 | 4 |
| RKKEE | 5.5 | 120.2 ± 8.2 | 4.4 ± 0.2 | 26.9 ±1.3 | 4 |
| RKKQQ | 7.0 | 130.3 ± 8.8 | 5.0 ± 0.4 | 26.1 ± 1.2 | 4 |
| QQQEE | 7.0 | 118.6 ± 7.3 | 5.1 ± 0.4 | 23.8 ± 1.7 | 5 |
| RKKQQ | 9.5 | 124.0 ± 11.3 | 4.9 ± 0.6 | 25.7 ± 2.2 | 4 |
| RKKQQ | 5.5 | 89.0 ± 7.7 | 7.0 ± 0.3 | 13.0 ± 1.4 | 4 |
Statistical analysis (ANOVA) of Boltzmann parameters shows that there are statistically significant differences (p < 0.05) between p1/2 and ΔG0 of RKKEE MscL examined at pH 7.0 versus RKKEE MscL examined at pH 5.5, as well as RKKQQ mutant channels examined at pH 7.0 and 9.5 and QQQEE mutant channels examined at pH 7.0.
A similar increase in ΔG0 due to increase in activation pressure was observed when positive charges were substituted by glutamine residues in the QQQEE mutant (ΔG0 = 23.8 kT, p1/2 = 118.6 mm Hg at pH 7.0) or the overall charge of the cluster was neutralized by increasing the experimental pH of the RKKQQ mutant (ΔG0 =25.7 kT, p1/2 =124.0 at pH 9.5) (Fig. 3; Table 2). In contrast, protonation of the positively charged residues of the RKKQQ mutant by lowering the experimental pH to 5.5 resulted in a decrease of activation pressure p1/2 (89.0 mm Hg) and ΔG0 (13.0 kT) comparable to the wild-type MscL at physiological pH of 7.0.
DISCUSSION
Currently there are two models that describe gating of MS channels by mechanical force: the bilayer and tethered models (24). Since prokaryotic cells lack a cytoskeleton, it is the lipid bilayer that is a tension-bearing element transmitting the mechanical force to the MS channels (9,37). Recent studies demonstrated that changes in the transbilayer tension profile, which gate bacterial MS channels, may be caused either by protein-lipid bilayer hydrophobic mismatch and/or membrane curvature (38,39). In this study we employed site-directed mutagenesis and patch-clamp recording from liposome reconstituted recombinant MscL channels to show that bilayer tension dependence of the E. coli MscL gating could be modulated by changes in pH. This is indeed indicated by our experimental results showing that ionization states of the RKKEE charged cluster and/or the lipid headgroups brought about by changes in pH affected the channel activity.
Protonation of E107 and E108 residues, achieved by decreasing experimental pH or their replacement by glutamine, significantly increased ΔG0 due to an increase in activation pressure p1/2. A similar increase in ΔG0 was observed when positive charges were either substituted by glutamine or the overall charge of the cluster was neutralized by increasing experimental pH. Furthermore, increasing the overall charge of the RKKQQ mutant by lowering the experimental pH and thus protonating positively charged residues resulted in p1/2 and ΔG0 comparable to the wild-type MscL at physiological pH of 7.0. The pK of the side chain of acidic glutamic acid is ∼4.1, whereas that of arginine and lysine is ∼12.5 and 10.5, respectively. Thus, at physiological pH the glutamate residues will loose all protons and the charged cluster will contain a balanced ratio of three positive and two negative charges, which assures smooth operation of the channel. It appears that any disturbance to this balance affects the channel gating properties since neutralization of either positive or negative charges leads to a similar increase in half activation pressure and consequently increases the free energy of activation of the channel. This is in agreement with the results of this study showing that a charge reversal mutation does not affect the energetics of the MscL channel.
A decrease in pH will cause protonation of basic and acidic chains of residues within the RKKEE charged cluster and consequently may prevent the channel from opening. Although the exact molecular mechanism remains to be determined, it is possible that pH induces a conformational change of the MscL protein similar to the pH-dependent structural rearrangements observed in influenza hemagglutinin, which involves refolding of the secondary and tertiary structure of the protein facilitating fusion of the host cell and viral membranes (40). In the case of MscL such conformational changes may create protein-lipid bilayer hydrophobic mismatch and subsequently increase the activation energy of the channel. Indeed gating of the MscL channel involves a significant reorientation of transmembrane helices during the channel gating (38,41–43). Furthermore, hydrophobic mismatch was found to stabilize the structurally distinct closed state intermediate of the channel (39). Taken together, the RKKEE charged cluster may undergo pH-dependent modification, which stabilizes different conformations of the channel during its transition from closed to open states.
Alternatively, it is possible that changes in pH alter the electrostatics and/or other physical properties of the phospholipid headgroups, which may specifically interact with the residues within the charged cluster. The pH-dependent ability of lipids to form a nonbilayer structure is well documented (44). Low pH reduces the fraction of anionic amphiphiles and triggers instability of lipid vesicles (45). In addition molecular dynamic (MD) simulations (46) and experimental data (38) demonstrated that changing lipid composition modulates the MscL channel gating. Since the membrane curvature influences the activity of bacterial and eukaryotic MS channels (25,28,47), pH-dependent ionization states of the lipid headgroups may induce changes in the geometry of the lipid bilayer, which then could modulate the MscL channel gating.
A study by Anishkin et al. (48) proposed that the C-terminus of MscL may serve as a size-exclusion filter at the cytoplasmic side of the MscL pore, preventing loss of essential metabolites. According to this model COOH-terminal domain is stably associated in both closed and open conformations of the channel. Our study, however, shows that the stability of the domain is pH dependent. Thus, in addition to the gating model proposed by Anishkin et al. (48), this study shows that the cytoplasmic helix may not only function as a size-exclusion filter, but may also substantially influence channel gating in a pH-dependent manner. Here, we provided experimental evidence that the charged cluster RKKEE within the proximal part of the MscL TM2 helix could function as a proton sensor similar to what has been found for the TREK-1 potassium channel (26). Lipid interactions with the RKKEE cluster indicated by this study were noted in the MD simulations investigating lipid composition effects on the MscL channel (46). As already suggested by the MD simulations, these interactions may play a general role in influencing the structure and the functional role of the MscL C-terminal domain. The charged cluster could thus be involved in the channel response to membrane tension by creating pH-dependent hydrophobic mismatch between the protein and the lipid bilayer and/or changing the membrane curvature.
For TREK1, K+ channel cytosolic acidosis increases the open probability for the channel leading to channel opening at atmospheric pressure (26,49) that is opposite to what we found in MscL. The C-terminal glutamate E306 of TREK1 is suggested to act as an intracellular proton sensor (26). In the channel gating model, low intracellular pH is proposed to protonate the E306 residue of TREK1 and thus affect electrostatic coupling of the charged cluster of the channel with the anionic phospholipids of the inner leaflet of the bilayer, which in turn affects the channel gating properties (49). In contrast, the corresponding C-terminal region and a homologous glutamic acid residue of alkali-sensing TRAAK channel are not involved in the channel regulation by pressure, pH, or fatty acids (26,30). For TREK2, channel deletion of the KKTKEE charged cluster abolished sensitivity of the channel to fatty acids and low pH although its substitution with uncharged residues produced little change (31). Such pH-dependent differences in molecular gating between different types of MS channels are most likely related to different physiological requirements these molecules have evolved to serve.
Homeostasis of intracellular pHi is essential to the physiology of a bacterial cell. In neutrophilic bacteria, such as E. coli, the internal pHi ranges between 7.5 and 8.0 (50). E. coli grows optimally in media having pHo >6.0 and <8.0. Since changes in external pHo always have some effect on pHi they are expected to affect the cell growth. For example at pHo 6.0, E. coli grows poorly (51). It is possible that the RKKEE cluster of bacterial MscL also functions as a pH sensor by keeping the MscL channel closed when the external pHo drops below the optimal values, since MscL forms a pathway for influx of protons (52). Therefore, it is advantageous to keep the channel closed once pHo starts falling below the optimum range.
In conclusion, this study provides further support for the bilayer model of MS channel gating (37–39,47,53) and emphasizes the functional importance of clusters of charged residues found in C-terminal domains of MS channels of prokaryotes and eukaryotes (6,25). Further characterization of the role of sequences of charged residues in pH-dependent gating of MS channels will help to identify their structural and physiological significance.
Acknowledgments
This work was supported by the Australian Research Council.
References
- 1.Martinac, B., M. Buechner, A. H. Delcour, J. Adler, and C. Kung. 1987. Pressure-sensitive ion channels in Escherichia coli. Proc. Natl. Acad. Sci. USA. 84:2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinac, B., A. H. Delcour, M. Buechner, J. Adler, and C. Kung. 1992. Mechanosensitive ion channels in bacteria. In Comparative Aspects of Mechanoreceptor Systems. F. Ito, editor. Springer Verlag, Berlin, Germany. 3–18.
- 3.Berrier, C., M. Besnard, B. Ajouz, A. Coulombe, and A. Ghazi. 1996. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J. Membr. Biol. 151:175–187. [DOI] [PubMed] [Google Scholar]
- 4.Sukharev, S. I., P. Blount, B. Martinac, and C. Kung. 1997. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu. Rev. Physiol. 59:633–657. [DOI] [PubMed] [Google Scholar]
- 5.Le Dain, A. C., N. Saint, A. Kloda, A. Ghazi, and B. Martinac. 1998. Mechanosensitive ion channels of the archaeon Haloferax volcanii. J. Biol. Chem. 273:12116–12119. [DOI] [PubMed] [Google Scholar]
- 6.Kloda, A., and B. Martinac. 2001a. Molecular cloning of a mechanosensitive ion channel in Archaea. Biophys. J. 80:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kloda, A., and B. Martinac. 2001b. Functional and structural differences between two homologous MS channels of M. jannaschii. EMBO J. 20:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kloda, A., and B. Martinac. 2001. c. Mechanosensitive ion channel in Thermoplasma, the cell wall-less Archaea: cloning and molecular characterization. Cell Biochem. Biophys. 34:321–347. [DOI] [PubMed] [Google Scholar]
- 9.Martinac, B. 2004. Mechanosensitive ion channels: molecules of mechanotransduction. J. Cell Sci. 117:2449–2460. [DOI] [PubMed] [Google Scholar]
- 10.Sukharev, S. I., P. Blount, B. Martinac, F. R. Blattner, and C. Kung. 1994. A large mechanosensitive channel in E. coli encoded by mscL alone. Nature. 368:265–268. [DOI] [PubMed] [Google Scholar]
- 11.Britten, R. J., and F. T. McClure. 1962. The amino acid pool of Escherichia coli. Bacteriol. Rev. 26:292–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koo, S. P., S. F. Higgins, and I. R. Booth. 1991. Regulation of compatible solute accumulation in Salmonella taphimurium: evidence for glycine betaine efflux system. J. Gen. Microbiol. 137:2617–2625. [DOI] [PubMed] [Google Scholar]
- 13.Schleyer, M., R. Schmid, and E. P. Bakker. 1993. Transient, specific and extremely rapid release of osmolytes from growing cells of Escherichia coli. Arch. Microbiol. 160:424–431. [DOI] [PubMed] [Google Scholar]
- 14.Ajouz, B., C. Berrier, A. Garrigues, M. Besnard, and A. Ghazi. 1998. Release of thioredoxin via mechanosensitive channel MscL during osmotic downshock of Escherichia coli cells. J. Biol. Chem. 273:26670–26674. [DOI] [PubMed] [Google Scholar]
- 15.Berrier, C., A. Garrigues, G. Richarme, and A. Ghazi. 2000. Elongation factor Tu and DnaK are transferred from the cytoplasm to the periplasm of Escherichia coli during osmotic downshock presumably via the mechanosensitive channel MscL. J. Bacteriol. 182:248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasquez-Laslop, N., H. Lee, R. Hu, and A. A. Neyfakh. 2001. Molecular sieve mechanism of selective release of cytoplasmic proteins by osmotically shocked Escherichia coli. J. Bacteriol. 183:2399–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levina, N., S. Totemeyer, N. R. Stokes, P. Louis, M. A. Jones, and I. R. Booth. 1999. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruickshank, C., R. Minchin, A. LeDain, and B. Martinac. 1997. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys. J. 73:1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moe, P. C., P. Blount, and C. Kung. 1998. Functional and structural conservation in the mechanosensitive channel MscL implicate elements crucial for mechanosensation. Mol. Microbiol. 28:583–592. [DOI] [PubMed] [Google Scholar]
- 20.Häse, C. C., A. C. LeDain, and B. Martinac. 1997. Molecular dissection of the large mechanosensitive ion channel (MscL) of Escherichia coli: mutants with altered channel gating and pressure sensitivity. J. Membr. Biol. 157:17–25. [DOI] [PubMed] [Google Scholar]
- 21.Blount, P., S. I. Sukharev, M. J. Schroeder, S. K. Nagle, and C. Kung. 1996. Single residue substitutions that change gating properties of a mechanosensitive channel in Escherichia coli. Proc. Natl. Acad. Sci. USA. 93:11652–11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ajouz, B., C. Berrier, M. Besnard, B. Martinac, and A. Ghazi. 2000. Contributions of the different extramembranous domains of the mechanosensitive channel MscL to its response to membrane tension. J. Biol. Chem. 275:1015–1022. [DOI] [PubMed] [Google Scholar]
- 23.Perozo, E., A. Kloda, D. M. Cortes, and B. Martinac. 2001. Site-directed spin-labelling analysis of reconstituted MscL in the closed state. J. Gen. Physiol. 118:193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamill, O. P., and B. Martinac. 2001. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 81:685–740. [DOI] [PubMed] [Google Scholar]
- 25.Patel, A. J., E. Honoré, F. Maingret, F. Lesage, M. Fink, F. Duprat, and M. Lazdunski. 1998. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 17:4283–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honoré, E., F. Maingret, M. Lazdunski, and A. J. Patel. 2002. An intracellular proton sensor commands lipid- and mechano-gating of the K(+) channel TREK-1. EMBO J. 21:2968–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maingret, F., A. J. Patel, F. Lesage, M. Lazdunski, and E. Honoré. 1999a. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J. Biol. Chem. 274:26691–26696. [DOI] [PubMed] [Google Scholar]
- 28.Maingret, F., M. Fosset, F. Lesage, M. Lazdunski, and E. Honoré. 1999b. TRAAK is a mammalian neuronal mechano-gated K+ channel. J. Biol. Chem. 274:1381–1387. [DOI] [PubMed] [Google Scholar]
- 29.Bang, H., Y. Kim, and D. Kim. 2000. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J. Biol. Chem. 275:17412–17419. [DOI] [PubMed] [Google Scholar]
- 30.Kim, Y., H. Bang, and C. Gnatenco. 2001a. Synergistic interaction and the role of C-terminus in the activation of TRAAK K+ channels by pressure, free fatty acids and alkali. Pflugers Arch. 442:64–72. [DOI] [PubMed] [Google Scholar]
- 31.Kim, Y., C. Gnatenco, H. Bang, and D. Kim. 2001b. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflugers Arch. 442:952–960. [DOI] [PubMed] [Google Scholar]
- 32.Zhou, X. L., A. F. Batiza, S. H. Loukin, C. P. Palmer, C. Kung, and Y. Saimi. 2003. The transient receptor potential channel on the yeast vacuole is mechanosensitive. Proc. Natl. Acad. Sci. USA. 100:7105–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clapham, D. E. 2003. TRP channels as cellular sensors. Nature. 426:517–534. [DOI] [PubMed] [Google Scholar]
- 34.Barritt, G., and G. Rychkov. 2005. TRPs as mechanosensitive channels. Nat. Cell Biol. 7:105–107. [DOI] [PubMed] [Google Scholar]
- 35.Chang, G., R. Spencer, A. Lee, M. Barclay, and C. Rees. 1998. Structure of the MscL homologue from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 282:2220–2226. [DOI] [PubMed] [Google Scholar]
- 36.Hamill, O. P., A. Marty, E. Neher, B. Sakmann, and F. J. Sigworth. 1981. Improved patch clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- 37.Kung, C. 2005. A possible unifying principle for mechanosensation. Nature. 436:647–654. [DOI] [PubMed] [Google Scholar]
- 38.Perozo, E., D. M. Cortes, P. Sompornpisut, A. Kloda, and B. Martinac. 2002a. Structure of MscL in the open state and the molecular mechanism of gating in mechanosensitive channels. Nature. 418:942–948. [DOI] [PubMed] [Google Scholar]
- 39.Perozo, E., A. Kloda, D. M. Cortes, and B. Martinac. 2002b. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 9:696–703. [DOI] [PubMed] [Google Scholar]
- 40.Bullough, P., F. Hugson, J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 371:37–43. [DOI] [PubMed] [Google Scholar]
- 41.Betanzos, M., C. S. Chiang, H. R. Guy, and S. I. Sukharev. 2002. A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. Nat. Struct. Biol. 9:704–710. [DOI] [PubMed] [Google Scholar]
- 42.Gullingsrud, J., D. Kosztin, and K. Schulten. 2001. Structural determinants of MscL gating studied by molecular dynamics simulations. Biophys. J. 80:2074–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sukharev, S. I., M. Betanzos, C. S. Chiang, and R. Guy. 2001. The gating mechanism of the large mechanosensitive channel MscL. Nature. 409:720–724. [DOI] [PubMed] [Google Scholar]
- 44.Hafez, I. M., S. Ansell, and P. R. Cullis. 2000. Tunable pH-sensitive liposomes composed of mixture of cationic and anionic lipids. Biophys. J. 79:1438–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, X., and M. Schick. 2001. Theory of tunable pH-sensitive vesicles of anionic and cationic lipids or anionic and neutral lipids. Biophys. J. 80:1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elmore, D. E., and D. A. Dougherty. 2003. Investigating lipid composition effects on the mechanosensitive channel of large conductance (MscL) using molecular dynamics simulations. Biophys. J. 85:1512–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinac, B., J. Adler, and C. Kung. 1990. Mechanosensitive ion channels of E. coli activated by amphipaths. Nature. 348:261–263. [DOI] [PubMed] [Google Scholar]
- 48.Anishkin, A., V. Gendel, N. A. Sharifi, C. S. Chiang, L. Shirinian, H. R. Guy, and S. I. Sukharev. 2003. On the conformation of the COOH-terminal domain of the large mechanosensitive channel MscL. J. Gen. Physiol. 121:227–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chemin, J., A. J. Patel, F. Duprat, I. Lauritzen, M. Lazdunski, and E. Honoré. 2005. A phospholipid sensor controls mechanogating of the K+ channel TREK1. EMBO J. 24:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ingraham, J. 1987. Effect of temperature, pH, water activity, and pressure on growth. In Escherichia coli and Salmonella: Cellular and Molecular Biology. F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. Brooks Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger, editors. ASM Press, Washington, DC. 1543–1554.
- 51.Krulwich, T. A., R. Agus, M. Schneier, and A. A. Guffanti. 1985. Buffering capacity of bacilli that grow at different pH ranges. J. Bacteriol. 162:768–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Buurman, E. T., D. McLaggan, J. Naprstek, and W. Epstein. 2004. Multiple paths for nonphysiological transport of K+ in Escherichia coli. J. Bacteriol. 186:4238–4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinac, B. 1993. Mechanosensitive ion channels: biophysics and physiology. In Thermodynamics of Membrane Receptors and Channels. M. B. Jackson, editor. CRC Press, Boca Raton, FL. 327–351.
- 54.Palmer, C. P., X. L. Zhou, J. Lin, S. H. Loukin, C. Kung, and Y. Saimi. 2001. A TRP homolog in Saccharomyces cerevisiae forms an intracellular Ca2+-permeable channel in the yeast vacuolar membrane. Proc. Natl. Acad. Sci. USA. 98:7801–7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lesage, F., C. Terrenoire, G. Romey, and M. Lazdunski. 2000. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J. Biol. Chem. 275:28398–28405. [DOI] [PubMed] [Google Scholar]
- 56.Lesage, F., F. Maingret, and M. Lazdunski. 2000. Cloning and expression of human TRAAK, a polyunsaturated fatty acids-activated and mechano-sensitive K+ channel. FEBS Lett. 471:137–140. [DOI] [PubMed] [Google Scholar]
- 57.Wes, P. D., J. Chevesich, A. Jeromin, C. Rosenberg, G. Stetten, and C. Montell. 1995. TRPC1, a human homolog of a Drosophila store-operated channel. Proc. Natl. Acad. Sci. USA. 92:9652–9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nagamine, K., J. Kudoh, S. Minoshima, K. Kawasaki, S. Asakawa, F. Ito, and N. Shimizu. 1998. Molecular cloning of a novel putative Ca2+ channel protein (TRPC7) highly expressed in brain. Genomics. 54:124–131. [DOI] [PubMed] [Google Scholar]
- 59.Jaquemar, D., T. Schenker, and B. Trueb. 1999. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J. Biol. Chem. 274:7325–7333. [DOI] [PubMed] [Google Scholar]
- 60.Liedtke, W., Y. Choe, M. A. Marti-Renom, A. M. Bell, C. S. Denis, A. Sali, A. J. Hudspeth, J. M. Friedman, and S. Heller. 2000. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 103:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Colbert, H. A., T. L. Smith, and C. I. Bargmann. 1997. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 17:8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]