Abstract
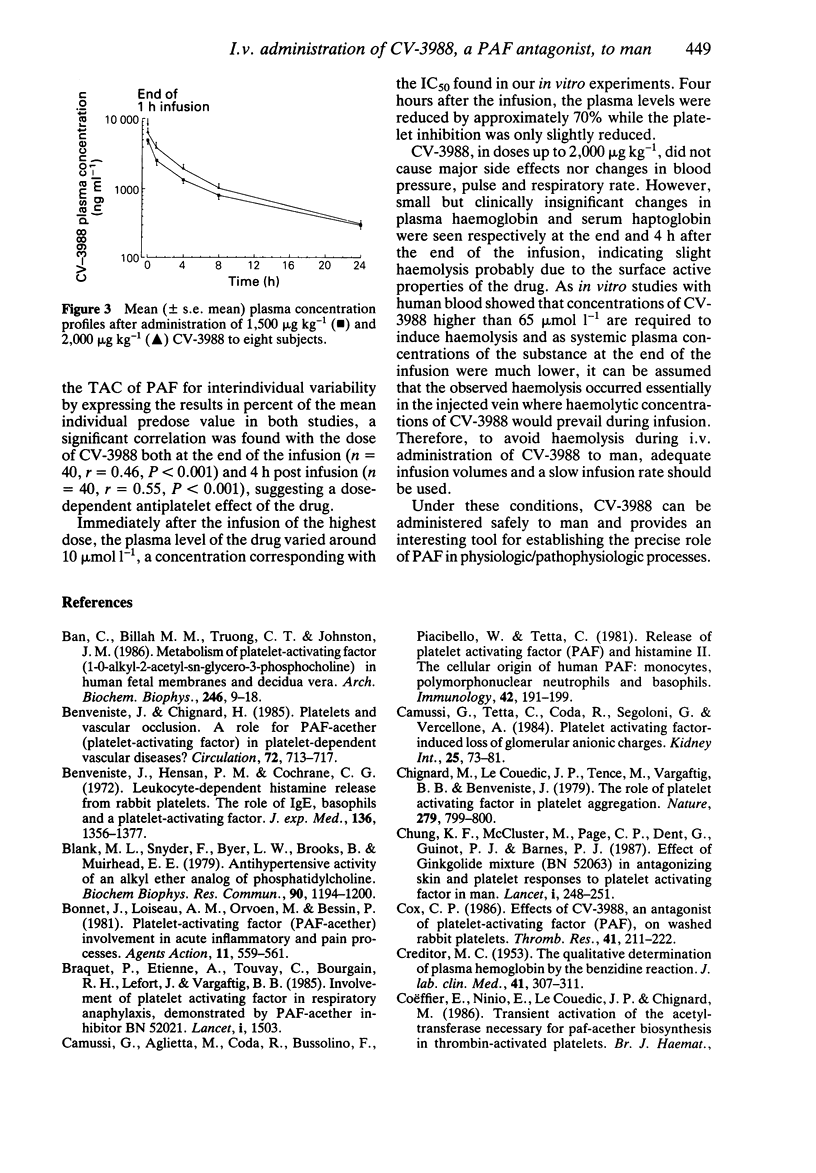
1. The efficacy and tolerability of CV-3988, a selective PAF antagonist with structural analogies with PAF, were studied after intravenous infusion in man. 2. The compound, in doses from 750 to 2,000 micrograms kg-1, significantly reduced platelet sensitivity for PAF. The threshold aggregating concentration (TAC) of PAF, expressed in % of the mean predosing value, increased in a dose dependent manner reaching 356 +/- 162% of the basal TAC at the end and 266 +/- 123% of the basal TAC 4 h after infusion of the highest dose. The TAC of PAF returned to the basal value within 24 h after the end of the infusion. 3. CV-3988 did not cause major side effects nor changes in blood pressure, pulse and respiratory rate. However, small but clinically insignificant changes in plasma haemoglobin and serum haptoglobin were seen at the end and 4 h after the end of the infusion, indicating a slight haemolysis. 4. Our results indicate that, when adequate infusion volumes and infusion rates are used, CV-3988 can safely be administered to man and should be useful in elucidating the role of PAF in disease.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ban C., Billah M. M., Truong C. T., Johnston J. M. Metabolism of platelet-activating factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) in human fetal membranes and decidua vera. Arch Biochem Biophys. 1986 Apr;246(1):9–18. doi: 10.1016/0003-9861(86)90444-3. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Chignard M. A role for PAF-acether (platelet-activating factor) in platelet-dependent vascular diseases? Circulation. 1985 Oct;72(4):713–717. doi: 10.1161/01.cir.72.4.713. [DOI] [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank M. L., Snyder F., Byers L. W., Brooks B., Muirhead E. E. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1194–1200. doi: 10.1016/0006-291x(79)91163-x. [DOI] [PubMed] [Google Scholar]
- Bonnet J., Loiseau A. M., Orvoen M., Bessin P. Platelet-activating factor acether (PAF-acether) involvement in acute inflammatory and pain processes. Agents Actions. 1981 Dec;11(6-7):559–562. doi: 10.1007/BF01978740. [DOI] [PubMed] [Google Scholar]
- CREDITOR M. C. The quantitative determination of plasma hemoglobin by the benzidine reaction. J Lab Clin Med. 1953 Feb;41(2):307–311. [PubMed] [Google Scholar]
- Camussi G., Aglietta M., Coda R., Bussolino F., Piacibello W., Tetta C. Release of platelet-activating factor (PAF) and histamine. II. The cellular origin of human PAF: monocytes, polymorphonuclear neutrophils and basophils. Immunology. 1981 Feb;42(2):191–199. [PMC free article] [PubMed] [Google Scholar]
- Camussi G., Tetta C., Coda R., Segoloni G. P., Vercellone A. Platelet-activating factor-induced loss of glomerular anionic charges. Kidney Int. 1984 Jan;25(1):73–81. doi: 10.1038/ki.1984.10. [DOI] [PubMed] [Google Scholar]
- Chignard M., Le Couedic J. P., Tence M., Vargaftig B. B., Benveniste J. The role of platelet-activating factor in platelet aggregation. Nature. 1979 Jun 28;279(5716):799–800. doi: 10.1038/279799a0. [DOI] [PubMed] [Google Scholar]
- Chung K. F., Dent G., McCusker M., Guinot P., Page C. P., Barnes P. J. Effect of a ginkgolide mixture (BN 52063) in antagonising skin and platelet responses to platelet activating factor in man. Lancet. 1987 Jan 31;1(8527):248–251. doi: 10.1016/s0140-6736(87)90066-3. [DOI] [PubMed] [Google Scholar]
- Cox C. P. Effects of CV-3988, an antagonist of platelet-activating factor (PAF), on washed rabbit platelets. Thromb Res. 1986 Jan 15;41(2):211–222. doi: 10.1016/0049-3848(86)90230-6. [DOI] [PubMed] [Google Scholar]
- Guinot P., Braquet P., Duchier J., Cournot A. Inhibition of PAF-acether induced weal and flare reaction in man by a specific PAF antagonist. Prostaglandins. 1986 Jul;32(1):160–163. doi: 10.1016/0090-6980(86)90161-9. [DOI] [PubMed] [Google Scholar]
- Henson P. M., Pinckard R. N. Basophil-derived platelet-activating factor (PAF) as an in vivo mediator of acute allergic reactions: demonstration of specific desensitization of platelets to PAF during IgE-induced anaphylaxis in the rabbit. J Immunol. 1977 Dec;119(6):2179–2184. [PubMed] [Google Scholar]
- Humphrey D. M., Hanahan D. J., Pinckard R. N. Induction of leukocytic infiltrates in rabbit skin by acetyl glyceryl ether phosphorylcholine. Lab Invest. 1982 Sep;47(3):227–234. [PubMed] [Google Scholar]
- Humphrey D. M., McManus L. M., Hanahan D. J., Pinckard R. N. Morphologic basis of increased vascular permeability induced by acetyl glyceryl ether phosphorylcholine. Lab Invest. 1984 Jan;50(1):16–25. [PubMed] [Google Scholar]
- Hwang S. B., Lam M. H., Biftu T., Beattie T. R., Shen T. Y. trans-2,5-Bis-(3,4,5-trimethoxyphenyl)tetrahydrofuran. An orally active specific and competitive receptor antagonist of platelet activating factor. J Biol Chem. 1985 Dec 15;260(29):15639–15645. [PubMed] [Google Scholar]
- Imura Y., Terashita Z., Nishikawa K. Possible role of platelet activating factor (PAF) in disseminated intravascular coagulation (DIC), evidenced by use of a PAF antagonist, CV-3988. Life Sci. 1986 Jul 14;39(2):111–117. doi: 10.1016/0024-3205(86)90444-3. [DOI] [PubMed] [Google Scholar]
- Kloprogge E., Akkerman J. W. Binding kinetics of PAF-acether (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) to intact human platelets. Biochem J. 1984 Nov 1;223(3):901–909. doi: 10.1042/bj2230901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornecki E., Ehrlich Y. H., Lenox R. H. Platelet-activating factor-induced aggregation of human platelets specifically inhibited by triazolobenzodiazepines. Science. 1984 Dec 21;226(4681):1454–1456. doi: 10.1126/science.6150550. [DOI] [PubMed] [Google Scholar]
- Mallet A. I., Cunningham F. M. Structural identification of platelet activating factor in psoriatic scale. Biochem Biophys Res Commun. 1985 Jan 16;126(1):192–198. doi: 10.1016/0006-291x(85)90590-x. [DOI] [PubMed] [Google Scholar]
- McManus L. M., Hanahan D. J., Pinckard R. N. Human platelet stimulation by acetyl glyceryl ether phosphorylcholine. J Clin Invest. 1981 Mar;67(3):903–906. doi: 10.1172/JCI110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus L. M., Pinckard R. N., Fitzpatrick F. A., O'Rourke R. A., Crawford M. H., Hanahan D. J. Acetyl glyceryl ether phosphorylcholine. Intravascular alterations following intravenous infusion into the baboon. Lab Invest. 1981 Oct;45(4):303–307. [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Benveniste J. Platelet-activating factor and macrophages. I. Evidence for the release from rat and mouse peritoneal macrophages and not from mastocytes. Eur J Immunol. 1979 May;9(5):409–415. doi: 10.1002/eji.1830090512. [DOI] [PubMed] [Google Scholar]
- Page C. P., Archer C. B., Guerreiro D., Morley J. Properties of PAF-acether appropriate to a mediator of the inflammatory aspects of asthma. Int J Tissue React. 1985;7(5):351–354. [PubMed] [Google Scholar]
- Pirotzky E., Bidault J., Burtin C., Gubler M. C., Benveniste J. Release of platelet-activating factor, slow-reacting substance, and vasoactive amines from isolated rat kidneys. Kidney Int. 1984 Feb;25(2):404–410. doi: 10.1038/ki.1984.31. [DOI] [PubMed] [Google Scholar]
- Prescott S. M., Zimmerman G. A., McIntyre T. M. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringdén O., Wilczek H., Lönnqvist B., Gahrton G., Wahren B., Lernestedt J. O. Foscarnet for cytomegalovirus infections. Lancet. 1985 Jun 29;1(8444):1503–1504. doi: 10.1016/s0140-6736(85)92272-x. [DOI] [PubMed] [Google Scholar]
- Robertson D. N., Smith G. M. CV3988 inhibits in vivo platelet aggregation induced by PAF-acether and collagen. Eur J Pharmacol. 1986 Apr 9;123(1):91–97. doi: 10.1016/0014-2999(86)90692-8. [DOI] [PubMed] [Google Scholar]
- Rosam A. C., Wallace J. L., Whittle B. J. Potent ulcerogenic actions of platelet-activating factor on the stomach. Nature. 1986 Jan 2;319(6048):54–56. doi: 10.1038/319054a0. [DOI] [PubMed] [Google Scholar]
- Shaw J. O., Pinckard R. N., Ferrigni K. S., McManus L. M., Hanahan D. J. Activation of human neutrophils with 1-O-hexadecyl/octadecyl-2-acetyl-sn-glycerol-3-phosphorylcholine (platelet activating factor). J Immunol. 1981 Sep;127(3):1250–1255. [PubMed] [Google Scholar]
- Shen T. Y., Hwang S. B., Chang M. N., Doebber T. W., Lam M. H., Wu M. S., Wang X., Han G. Q., Li R. Z. Characterization of a platelet-activating factor receptor antagonist isolated from haifenteng (Piper futokadsura): specific inhibition of in vitro and in vivo platelet-activating factor-induced effects. Proc Natl Acad Sci U S A. 1985 Feb;82(3):672–676. doi: 10.1073/pnas.82.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M., Landolfi R., Motola N. C., Turcotte J. G. Biological activity of platelet activating factor-amidophosphonate (PAF-AP), a novel phosphonolipid selective inhibitor of platelet activating factor (PAF). Biochem Biophys Res Commun. 1985 Dec 31;133(3):851–855. doi: 10.1016/0006-291x(85)91212-4. [DOI] [PubMed] [Google Scholar]
- Tarukoski P. H. Quantitative spectrophotometric determination of haptoglobin. Scand J Clin Lab Invest. 1966;18(1):80–86. doi: 10.3109/00365516609065610. [DOI] [PubMed] [Google Scholar]
- Terashita Z., Imura Y., Nishikawa K., Sumida S. Is platelet activating factor (PAF) a mediator of endotoxin shock? Eur J Pharmacol. 1985 Feb 26;109(2):257–261. doi: 10.1016/0014-2999(85)90427-3. [DOI] [PubMed] [Google Scholar]
- Terashita Z., Tsushima S., Yoshioka Y., Nomura H., Inada Y., Nishikawa K. CV-3988 - a specific antagonist of platelet activating factor (PAF). Life Sci. 1983 Apr 25;32(17):1975–1982. doi: 10.1016/0024-3205(83)90049-8. [DOI] [PubMed] [Google Scholar]
- Valone F. H. Inhibition of binding of the platelet-activating factor AGEPC to platelets by the AGEPC analog rac-3-(N-n-octadecylcarbamoyloxy)-2-methoxypropyl 2-thiazolioethyl phosphate (CV-3988). Biochem Biophys Res Commun. 1985 Jan 16;126(1):502–508. doi: 10.1016/0006-291x(85)90634-5. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Chignard M., Benveniste J. Platelet-activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur J Pharmacol. 1980 Jul 25;65(2-3):185–192. doi: 10.1016/0014-2999(80)90391-x. [DOI] [PubMed] [Google Scholar]
- Woodman D. D. Detection of blood in faeces. Clin Chim Acta. 1970 Aug;29(2):249–252. doi: 10.1016/0009-8981(70)90044-6. [DOI] [PubMed] [Google Scholar]
- Yasaka T., Boxer L. A., Baehner R. L. Monocyte aggregation and superoxide anion release in response to formyl-methionyl-leucyl-phenylalanine (FMLP) and platelet-activating factor (PAF). J Immunol. 1982 May;128(5):1939–1944. [PubMed] [Google Scholar]


