Abstract
Oligonucleotide and cDNA microarrays have been used to analyse the mRNA levels of 8,000 genes in Arabidopsis thaliana throughout the day/night cycle. Genes involved in signal transduction and in various metabolic pathways were found to be coordinately regulated by circadian rhythms and/or by light.
Text
Plants coordinate many biochemical and physiological processes with the Earth's 24 hour rotation, presumably improving their fitness by anticipating the predictable cycles of light and temperature in the environment [1]. Two recent papers have examined biological rhythms in plants using genome-wide transcript-analysis techniques. These approaches have uncovered candidate regulatory genes and have both confirmed and extended the range of physiological processes known to be under rhythmic control.
The analysis of biological rhythms is complicated by the fact that many rhythmic processes are directly influenced by light (Figure 1). In the natural day/night cycle, light regulation can lead to rhythmic changes that are referred to as diurnal responses. Additionally, many physiological changes anticipate the coming dawn or dusk and continue to show an oscillation of roughly 24 hours even in constant conditions; these are termed circadian rhythms. The many overt morphological rhythms include leaf movements [2], the opening of stomatal pores [3] and the photoperiodic induction of flowering [4]. In addition, biological rhythms have been found to regulate the expression of genes encoding enzymes and regulatory and structural proteins [5]. Daily cycling of these gene products appears to coordinate physiological processes in the plant with the ambient environment [1]. Together, these developmental and enzymatic rhythms are thought to optimize photon capture and avoid concurrent stresses, thus providing energy for both nitrogen and carbon uptake.
Figure 1.
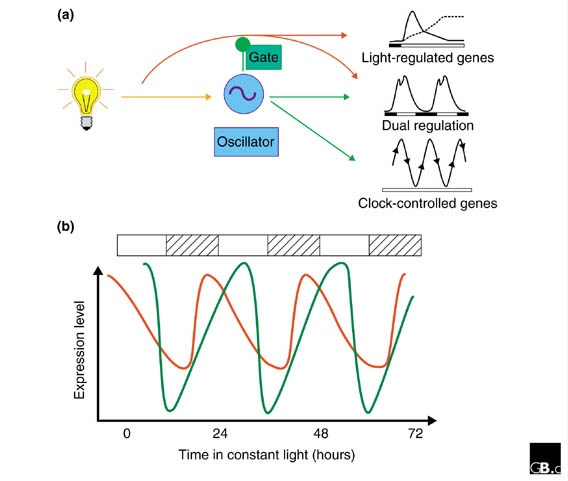
Circadian rhythms in plant gene expression. (a) Simplified regulatory circuit for the circadian system. Light (represented by a bulb) regulates some genes directly, leading to diurnal rhythms under cycles of light and dark. Light also resets the circadian oscillator, which in turn regulates the expression of clock-controlled genes. Some of the latter are also directly light-regulated. The circadian 'gate' rhythmically modulates light signaling. (b) Circadian-clock-controlled genes exhibit peak expression levels at several phases with respect to the light/dark cycle (represented by hatched and white bars above the graph). Examples of clock-regulated gene sets are given, with peak expression before subjective dawn (for example, the phenylpropanoid synthesis pathway; red) and early in the subjective day (for example, photosynthesis-related genes; green).
There are two main technologies for array-based global gene-expression analysis; both are based on nucleic-acid hybridization but one relies on cDNAs (usually attached to glass) while the other uses synthetic oligonucleotides. In two recent papers [6,7], each of these technologies was used to undertake the global analysis of steady-state mRNA levels in Arabidopsis thaliana plants maintained under diurnal and/or circadian growth conditions. This is the first time global transcript analysis has been used to uncover the networks of rhythmic genes in any organism. Schaffer et al. [7] analyzed gene expression using cDNA arrays generated by the Arabidopsis Functional Genomics Consortium (a consortium, funded by the US National Science Foundation, that includes Stanford, Wisconsin and Michigan State Universities). The Arabidopsis cDNA array, which represents approximately 7,800 unique genes or around 30% of expressed sequences, was used to identify diurnal expression changes in samples harvested under a 12 hour light/12 hour dark cycle, and circadian regulation in plants transferred to constant light or darkness. Schaffer et al. [7] analysed 13 slides hybridized to RNAs isolated after diurnal or circadian treatment. The Arabidopsis cDNA array results were quite reproducible, with fewer than 2% of transcripts measured as having a greater than two-fold difference in abundance in independent, replicate RNA samples. Approximately 11% of genes showed differential expression at one or more of four phases tested during the light/dark cycle (data available at [8]). About 25% of genes that showed diurnal regulation were also circadian-regulated, as revealed by differential transcript abundance after 12 hours versus 24 hours under constant conditions. In total, around 2% of the genes represented on the array were scored as circadian-regulated.
The same Arabidopsis cDNA array has been used to analyze transcripts obtained under a wide range of physiological conditions, including auxin treatment, viral challenge and acclimation to light [9]. Schaffer et al. [7] could therefore perform cluster analysis using available published and unpublished data, in order to identify 33 genes regulated principally by the circadian clock. Some of these genes will probably prove to be informative markers for future circadian studies; they might also represent a functionally significant group. As the publicly accessible expression databases grow, such cluster analysis should prove its utility in unraveling gene regulatory networks in plants.
Harmer et al. [6] used an oligonucleotide-based array (GeneChip, pioneered by Affymetrix; Santa Clara, CA) that contains information on approximately 8,200 genes. The RNA samples analyzed were isolated from plants grown in a light/dark cycle but harvested following transfer to continuous light, so this experiment specifically tested circadian regulation. The steady-state mRNA levels were measured in replicate hybridizations of 12 samples harvested every 4 hours, covering two circadian cycles in constant light. A dedicated biomathematical software tool was developed to detect rhythmic patterns in these data. Here, around 6% of genes were found to be under circadian control. Interestingly, all phases of expression were well represented, suggesting that the clock regulates gene expression throughout the day/night cycle, rather than clock-controlled genes being expressed only at a limited number of preferred phases.
Several authors have noted that genes with functions in photosynthesis are often maximally expressed in the early morning, presumably optimizing photosynthetic function during the daylight hours. Harmer et al. [6] propose several striking new examples in which rhythmic regulation appears orchestrated such that genes that act coordinately are expressed simultaneously. Some of these gene sets function in concert with the daily environmental cycle, in so-called external coordination. For example, 23 genes involved in the synthesis of photoprotective phenylpropanoids are maximally expressed before dawn, including CHALCONE SYNTHASE [10]. Other gene sets might be expressed in a fixed temporal relationship to key metabolic processes (internal coordination). Nine genes involved in the energetically demanding process of nitrogen assimilation were expressed early in the day, for example, when cellular energy levels are increased by light harvesting. The detection of such functional clustering is highly valuable, shedding new light on the potential importance of biological rhythms for vegetative plant growth. In such large data sets, however, this analysis currently requires either encyclopedic knowledge of plant biochemistry or an extensive literature review.
For many metabolic processes, diurnal regulation might allow sufficient coordination with the environment, so circadian anticipation of dawn/dusk might add little advantage [11]. It might not be surprising, therefore, that Schaffer et al. [7] found many more genes under direct diurnal control than regulated by the circadian system. Is it significant that genes conferring metabolic function were the largest identifiable class of diurnal- and circadian-controlled genes? Harmer et al. [6] suggest that this finding reflects the better understanding of enzymatic genes than those of other functions. For instance, about 25% of circadian-regulated genes encode proteins of unknown function and another 25% are uncharacterized genes containing limited sequence homology to known genes [6,7]. Many of these latter genes have sequence homology to kinases, phosphatases and transcription factors. A substantial number of rhythmic regulatory factors are therefore probably waiting to be discovered. Some of these presumably underlie the observed circadian 'gating' of signal transduction, which fine tunes biological responses over the day/night cycle [12].
Patterns of coordinate gene expression might reflect regulation by common transcription factor(s) binding to similar promoter element(s). Indeed, some transcripts encoding transcription factors were found to oscillate in phase with transcripts of their known target genes. Harmer et al. [6] identified a conserved sequence upstream of 31 genes that were expressed at the end of the subjective day. They confirmed in transgenic plants that this 'evening element' was required for robust circadian transcription from one such promoter. These results are profound, because bioinformatic approaches alone accurately predicted a gene-regulatory sequence. Correlations between coordinate gene expression and promoter sequences should be increasingly useful in uncovering regulatory mechanisms for organisms with sequenced genomes.
The simultaneous application of the two microarray methods allows some tentative technical comparisons that might highlight the importance of using both array technologies as well as of the choice of genes to analyze. The overall number of circadian-controlled genes observed in constant light was quite different: about 6% of genes for the oligonucleotide array; about 0.5% for the cDNA array, which identified fewer cycling genes in the light than in darkness. Setting aside differences in the numbers of RNA samples tested from circadian conditions and in the analytical methods, the identity of the genes assayed might also have contributed. Genes on the oligonucleotide-based chip were a relatively random sample created using genome sequence from the Arabidopsis Genome Initiative [13]. Genes on the cDNA array are not a random sample, as these genes were isolated as part of the Arabidopsis Expressed Sequence Tag collection [14], which contains virtually all highly expressed Arabidopsis genes and lacks many rare transcripts. Global expression profiling of the entire genome will be required to test the suggestion that a smaller percentage of abundant transcripts cycle, but this will be possible now that the complete Arabidopsis genome sequence is publicly available [13].
Global transcript analysis can potentially return four results: identifying new markers for research, revealing cis-acting elements and trans-acting factors that cluster with a pattern of regulation, identifying novel patterns of regulation, and finding clusters of co-regulated genes with a functional connection that suggests the physiological importance of their regulation. The two Arabidopsis circadian microarray papers were successful by all these criteria. The current challenge is to follow up some of these data with higher-resolution methods, before microarrays designed to sample the entire transcriptome of Arabidopsis uncover the complete complement of clock-controlled genes. Then, the work will really begin.
References
- Somers DE. The physiology and molecular bases of the plant circadian clock. Plant Physiol. 1999;121:9–19. doi: 10.1104/pp.121.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann W, Johnsson A. Rhythms in organ movement. Biological Rhythms and Photoperiodism in Plants. Edited by Lumsden PJ, Millar AJ. Oxford: BIOS Scientific; 1998.
- Webb AAR. Stomatal rhythms. Biological Rhythms and Photoperiodism in Plants. Edited by Lumsden PJ, Millar AJ. Oxford: BIOS Scientific; 1998.
- Samach A, Coupland G. Time measurement and the control of flowering in plants. BioEssays. 2000;22:38–47. doi: 10.1002/(SICI)1521-1878(200001)22:1<38::AID-BIES8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Kreps JA, Kay SA. Coordination of plant metabolism and development by the circadian clock. Plant Cell. 1997;9:1235–1244. doi: 10.1105/tpc.9.7.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer SL, Hogenesch LB, Straume M, Chang HS, Han B, Zhu T, Wang X, Kreps JA, Kay SA. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science. 2000;290:2110–2113. doi: 10.1126/science.290.5499.2110. [DOI] [PubMed] [Google Scholar]
- Schaffer R, Landgraf J, Monica A, Simon B, Larson M, Wisman E. Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis. Plant Cell. 2001;13:113–123. doi: 10.1105/tpc.13.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downloadable files of differentially expressed genes showing diurnal or circadian rhythm http://www.prl.msu.edu/circl
- Arabidopsis Functional Genetics Consortium (Stanford University) http://AFGC.Stanford.EDU/afgc_html/site2.htm
- Deikman J, Hammer PE. Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiol. 1995;108:47–57. doi: 10.1104/pp.108.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Millar AJ, Kay SA. Integration of circadian and phototransduction pathways in the network controlling CAB gene transcription in Arabidopsis. Proc Natl Acad Sci USA. 1996;93:15491–15496. doi: 10.1073/pnas.93.26.15491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Newman T, De Bruijn FJ, Green P, Keegstra K, Kende H, McIntosh L, Ohlrogge J, Raikhel N, Somerville S, Thomashow M, et al. Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 1994;106:1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]


