Abstract
Hematopoiesis in most vertebrate species occurs in two distinct phases, primitive and definitive, which diverge from FLK1+VE-cadherin– mesoderm and FLK1+VE-cadherin+ endothelial cells (EC), respectively. This study aimed at determining the stage at which hematopoietic lineage fate is determined by manipulating the SCL/tal-1 expression that is known to be essential for the early development of the primitive and definitive hematopoietic systems. We established SCL-null ES cell lines in which SCL expression is rescued by tamoxifen-inducible Cre recombinase-loxP site-mediated recombination. While no hematopoietic cells (HPC) were detected in SCL-null ES cell differentiation cultures, SCL gene reactivation from day 2 to day 4 after initiation of differentiation could rescue both primitive and definitive hematopoiesis. SCL reactivation at later phases was ineffective. Moreover, generation of VE-cadherin+ EC that can give rise to definitive HPC required SCL reactivation prior to VE-cadherin expression. These results indicated that the competence to become HPC is acquired at the mesodermal stage by a SCL-dependent process that takes place independently of determination of endothelial fate.
Keywords: ES cell/FLK1/MerCreMer/SCL/tal-1/VE-cadherin
Introduction
In mouse embryos, the hematopoietic and vascular systems develop from the lateral mesoderm that exfoliates from the primitive streak between days 6.5 and 7.0 of gestation. Blood islands, the first hematopoietic tissue, develop from the undifferentiated mesodermal cell clusters in the yolk sac. Blood islands consist of an inner cluster of primitive erythroblasts that express embryonic hemoglobin and an external layer of endothelial cells. Following the first wave of hematopoiesis, which is restricted to producing primitive erythrocytes, the major site of hematopoiesis shifts to the fetal liver, where most definitive hematopoietic cell types found in the adult animals are produced. The hematopoietic site subsequently shifts from fetal liver to spleen and eventually settles in the bone marrow at day 15–16 of gestation.
While the primitive HPC lineage originates in extra-embryonic mesoderm in the yolk sac, the definitive HPC lineage has been proposed to develop in an intra-embryonic region. After a long quest for the origin of hematopoietic stem cells in the mouse embryo, it is almost established that the first hematopoietic stem cell capable to reconstitute intra-marrow hematopoiesis of irradiated adult mice becomes detectable in an intra-embryonic region called aorta-gonad-mesonephros (AGM) at 10.5 d.p.c. (Muller et al., 1994; Medvinsky and Dzierzak, 1996). Clusters of immature HPC develop along the endothelium of ventral aspect of the dorsal aorta in the AGM region at this stage. The intra-aortic clusters are equivalently detected in embryos of many vertebrate species and cell labeling experiments in vivo, using chick embryos, showed that these clusters are derived from the endothelium. Hematogenic potential of VE-cadherin+ endothelial cells (EC) sorted from the AGM region of mouse embryos was also demonstrated ex vivo (Nishikawa et al., 1998b). Recently, spatial position of hematopoietic stem cell emergence in the mouse embryo was pinpointed to the EC layer of the dorsal aorta (de Bruijn et al., 2002; North et al., 2002). Although it becomes most probable that the definitive HPC lineage originates in the endothelium, further dissection and manipulation of the process of HPC development are difficult to achieve using embryos as a source of cells.
A way to circumvent this problem is to use mouse embryonic stem (ES) cells as a model, as this overcomes the quantitative limitations, and various experimental systems that allow synchronous ES cell differentiation to each cell lineage are available. By using such systems, a contradictory debate for the diverging point of the primitive and definitive HPC lineages was held. Some studies proposed a common precursor for primitive and definitive HPC lineages (Kennedy et al., 1997), whereas others suggested that these two lineages are derived from distinct origins (Nakano, 1996). The contradiction seemed to stem from their retrospective detection of progenitor cells. By taking a prospective approach in which various precursor stages were isolated by flow cytometry, our recent studies suggested two diverging points of the HPC lineages (Nishikawa et al., 1998a; Fujimoto et al., 2001). The first is the FLK1+VE-cadherin– mesodermal stage, from which the primitive erythrocytes diverge, and the second is the FLK1+VE-cadherin+ endothelial stage, from which the definitive HPC diverge (Fujimoto et al., 2001). This scheme for the development of HPC lineages is consistent with the results obtained from in vivo studies described above, although other possibilities, such as that the VE-cadherin+ population is in fact the definitive hemangioblast (Lacaud et al., 2002) that has not committed to endothelial lineage, can not be ruled out.
Given that HPC diverge at two points, the next question is at which stage the fate of hematopoietic lineage is determined. Here we attempted to address this question by manipulating the expression of the SCL/tal-1 gene. Several observations indicated that the SCL/tal-1 gene (hereafter referred to as SCL), a member of the basic helix–loop–helix (bHLH) transcription factor family, plays an important role in commitment of mesoderm to HPC. Gene targeting studies show that SCL is essential for both primitive and definitive hematopoiesis, as well as for the proper remodeling of the primary capillary plexus in the yolk sac (Robb et al., 1995; Shivdasani et al., 1995; Visvader et al., 1998). Overexpression of SCL in zebrafish embryos leads to the expanded induction of marker genes specific to hematopoietic and endothelial lineages (Gering et al., 1998), and overexpression of SCL in zebrafish cloche mutant embryos rescues defects in the hematopoietic and endothelial lineages (Liao et al., 1998). Given that SCL plays an essential role for specification of HPC, use of an experimental system that allows manipulation of the timing of SCL expression might allow us to specify the stage of commitment to HPC.
In this study, we established SCL-null ES cell lines in which the expression of SCL can be rescued by tamoxifen-dependent Cre recombinase-loxP site-mediated recombin ation. We found that expression of SCL prior to day 4 of differentiation of the ES cells can rescue both primitive and definitive hematopoiesis. However, reactivation of SCL was unable to rescue not only primitive but also definitive hematopoiesis after day 4.5 of differentiation, when VE-cadherin+ EC had become detectable. These results suggest that the definitive HPC pathway has already initiated at the lateral mesoderm stage irrespective of the determination program to EC.
Results
Generation of tamoxifen-controlled inducible SCL ES cell lines
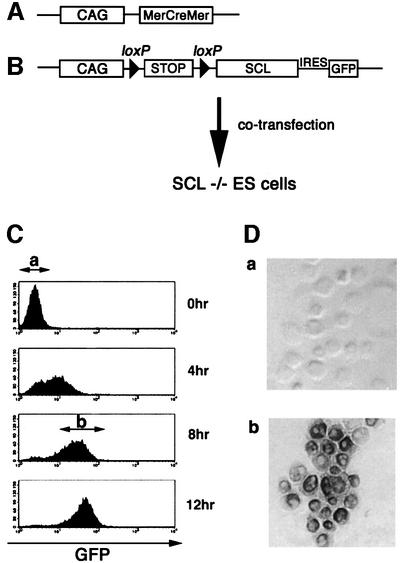
Verrou et al. (1999) previously reported a chimeric Cre recombinase construct carrying mutated estrogen receptor hormone-binding domains at both the N- and C-termini (MerCreMer). As they showed that tamoxifen can efficiently induce Cre-mediated recombination in ES cells expressing the chimeric protein, we employed this strategy for establishing ES cell lines in which the expression of a SCL transgene was induced by activation of Cre recombinases. SCL-null ES cells were transfected with two constructs; one carrying the MerCreMer coding sequence under control of the chicken β actin (CAG) promoter and the other containing a CAG promoter, floxed stop cassette and SCL-coding sequence fused to the IRES–green fluorescent protein (GFP; Figure 1A and B). Theoretically, ES cells bearing these two constructs could be induced to express both SCL and GFP upon addition of tamoxifen, which would recruit the MerCreMer into nuclei to delete the stop cassette. However, GFP was used as a marker to indicate SCL expression.
Fig. 1. Generation of SCL-inducible ES cells. (A) Structure of the expression vector carrying the MerCreMer-coding sequence under control of the chicken β actin (CAG) promoter. (B) Structure of the expression vector containing a CAG promoter, floxed stop cassette and SCL-coding sequence fused to the IRES–GFP. (C) FACS analysis of the time course of GFP expression in inducible SCL ES cells after the addition of 4′OHT. GFP– (a) and GFP+ (b) cells were sorted from 0 and 8 h cultures, respectively. (D) Expression of SCL protein in GFP– (a) and GFP+ (b) ES cells. Sorted cells were stained with anti-SCL polyclonal Ab (original magnification ×400).
In order to verify whether or not the transfected genes operate as we planned, tamoxifen-dependent Cre recombinase activity in the undifferentiated ES cells was measured by FACS analysis of GFP expression. As early as 4 h after addition of tamoxifen, significant expression of GFP was observed in most cells, and saturating levels were reached by 8–12 h (Figure 1C). Efficient GFP induction was also observed during ES cell differentiation on OP9 stromal cells (data not shown). We sorted GFP+ cells 8 h after tamoxifen addition and measured SCL expression by immunostaining with anti-SCL polyclonal antibody. While no SCL protein was detectable before tamoxifen induction, virtually all GFP+ cells expressed SCL protein (Figure 1D). These results indicated that induction of SCL expression in these ES cells occurs ∼8–12 h after addition of tamoxifen.
Kinetics of hematopoietic potential of ES cells differentiated on OP9 stromal cells
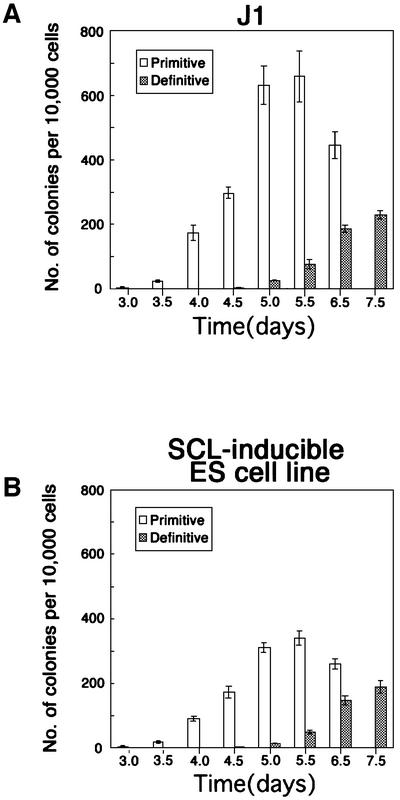
We investigated whether or not transfection of these constructs affected the potential of ES cells by comparing the time course of the generation of primitive and definitive hematopoietic progenitors between SCL-inducible and wild-type ES cells. Cells were cultured on OP9 stromal cells in the presence of tamoxifen. Differentiated cells were harvested every 12 h from day 3 of the culture and transferred to semi-solid medium containing methylcellulose and a cocktail of SCF, IL-3, Epo and IL-6. Primitive erythroid progenitors were first detected at day 3 of the culture, and reached peak levels at day 5.5 in both ES cell lines (Figure 2A and B). Definitive hematopoietic progenitors (CFU-M, CFU-G/GM, BFU-E/Mix) were first detected at day 5 of the culture and continued to increase in number until the latest point tested (day 7.5) in both ES cells (Figure 2A and B). These results indicated that the transfection of the transgenes did not affect the time course of ES cell differentiation to primitive and definitive HPC progenitors.
Fig. 2. Kinetics of hematopoietic potential of ES cells differentiated on OP9 stromal cells J1 (A) and inducible SCL (B) ES cells were induced to differentiation on OP9 stromal cell layer in the presence of 4′OHT. At the indicated day of differentiation, the cells were dissociated and sorted to remove the stromal cells. The sorted cells were replated in methylcellulose semi-solid culture in the presence of Epo, SCF, IL-3 and IL-6. The number of primitive erythroid colonies was scored at day 8 of total differentiation, and that of definitive hematopoietic cell colonies was scored at day 14 of total differentiation. Error bars indicate standard deviations for three independent determinations.
SCL specified both primitive and definitive HPC progenitors by day 4 of differentiation.
In agreement with studies by other groups, neither primitive nor definitive hematopoietic cells were generated from SCL(–/–) ES cells under our culture conditions. Using the tamoxifen-inducible experimental system, we attempted to specify the stage for completion of the SCL-dependent process for HPC differentiation.
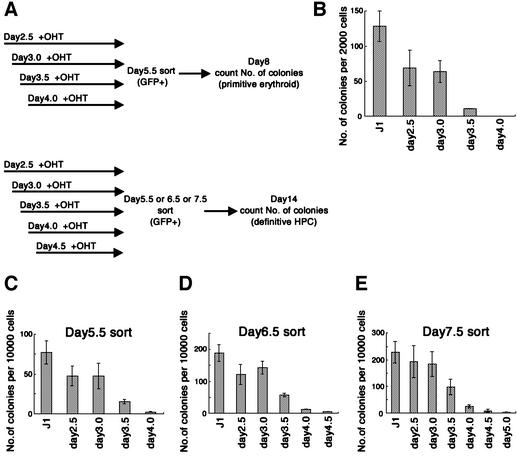
ES cells were cultured on OP9 stromal cells for 5.5–7.5 days. Tamoxifen was added once at different time points from day 2 of culture (Figure 3A). GFP+ cells were sorted to exclude OP9 stromal cells, and the number of hematopoietic progenitors was measured by colony assay in methylcellulose-containing media. The frequency of primitive and definitive HPC progenitors was measured by changing the incubation time of the colony assay; eight days of total differentiation for primitive and 14 days of total differentiation for definitive hematopoiesis. The ability of this method to distinguish primitive and definitive HPC was validated by cytological examination of cells present in the colonies.
Fig. 3. (A) Experimental design. Inducible SCL ES cells were induced to differentiation on OP9 stromal cells. On the indicated day, 4′OHT was added to the culture to induce expression of SCL. On day 5.5 (B and C), 6.5 (D) or 7.5 (E), differentiated ES cells were dissociated and GFP+ cells were sorted. The sorted cells were plated in methylcellulose cultures with Epo, SCF, IL-3 and IL-6. (B) The number of primitive erythroid colonies was counted at day 8 of total differentiation. (C–E) Number of definitive hematopoietic colonies was counted at day 14 of total differentiation. Error bars indicate standard deviations for three independent determinations.
The cells harvested at day 5.5 of the culture were used for measuring the progenitors of primitive hematopoiesis. Colonies induced under this condition were comprised mostly of nucleated primitive erythrocytes. Consistent with previous studies, progenitors of primitive erythrocytes were absent in the cultures of SCL null ES cells (data not shown). This defect was restored almost completely by addition of tamoxifen before day 3.0, and partially by the addition on day 3.5 (Figure 3B). On the other hand, tamoxifen induction of SCL at day 4 had almost no effect in restoring the response. As the cells harvested at day 6.5 after tamoxifen induction of SCL at day 4 hardly formed any primitive erythroid colonies either (data not shown), the activity of SCL was dependent on chronological period of expression, but not on total duration of expression. These results suggest that the SCL-dependent process for the differentiation of primitive erythrocytes occurs early, probably 3–4 days after induction of ES cell differentiation.
We next investigated the chronological timing of SCL requirement for differentiation of definitive hematopoiesis using the same experimental system. To our surprise, SCL was required at nearly the same time point as that for primitive hematopoiesis, despite the fact that the progenitors for definitive HPC appeared 1–2 days later. As summarized in Figure 3C–E, addition of tamoxifen later than day 4 of the culture could not restore definitive hematopoiesis, in contrast to the effectiveness of earlier addition. As the cells harvested on days 5.5–7.5 showed essentially the same requirement for SCL, the activity of SCL is clearly dependent on chronological period of expression, but not on total duration of expression. These results indicated that the SCL-dependent processes for differentiation of both primitive and definitive hematopoietic lineages occur by day 4 in culture.
SCL expression in VE-cadherin+ stage failed to induce HPC
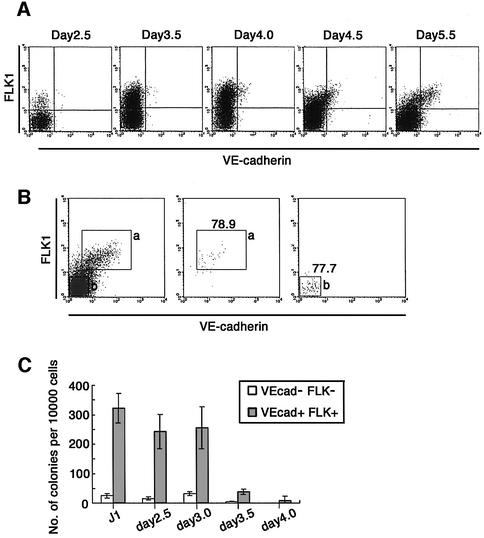
Previously, we have shown the existence of two successive stages from which HPC diverge from other cell lineages; FLK1+VE-cadherin– mesoderm (1st stage) and FLK1+VE-cadherin+ hemogenic EC (2nd stage) appearing at ∼3 and 5 days, respectively, of the culture (Nishikawa et al., 1998a; Fujimoto et al., 2001). The kinetics of EC development in the culture of SCL-null ES cells were comparable with those of wild-type ES cells (Figure 4A; data not shown). Therefore, results in the preceding section indicate that specification of both primitive and definitive HPC lineages by SCL takes place prior to expression of VE-cadherin. It is likely that the mesodermal cells complete the SCL-dependent process to acquire hemogenic competence before differentiation to VE-cadherin+ EC.
Fig. 4. (A) Time course of EC marker expression. Inducible SCL ES cells were induced to differentiation on OP9 stromal cells. On the indicated day, differentiated ES cells were dissociated and expression of FLK1 and VE-cadherin was analyzed by FACS. (B and C) Hematopoietic potential of VE-cadherin+ fraction. Inducible SCL ES cells were induced to differentiation on OP9 stromal cells. On the indicated day, 4′OHT was added to the culture. On day 5.5, differentiated ES cells were dissociated and VE-cadherin+FLK1+ (a) and VE-cadherin–FLK1– (b) cells were sorted (B, left panel). Re-analyzes of sorted cells were performed (B, middle and right panels). The sorted cells were plated in methylcellulose cultures with Epo, SCF, IL-3 and IL-6. On day 14, the number of CFU-C was counted (C). Error bars indicate standard deviations for three independent determinations.
In order to confirm this, we measured the frequency of primitive and definitive HPC progenitors in FLK1+VE-cadherin+ populations sorted on day 5.5 of the culture (Figure 4B). Expression of SCL was induced at 2.5, 3, 3.5 and 4 days of the culture. When SCL expression was induced earlier than day 3.5, one in 30–40 cells from the VE-cadherin+ fraction gave rise to definitive hematopoietic cell colonies, whereas only a few hematopoietic progenitors are found in the VE-cadherin– population (less than one in 250 cells; Figure 4C). On the other hand, SCL expression after day 4 of the culture could not induce VE-cadherin+ progenitors with the potential to generate any type of HPC. No progenitor activity was found in the VE-cadherin– fraction (less than one in 1000 cells; Figure 4C). These results demonstrated that the SCL-dependent process required for the specification of definitive HPC proginitors is completed before differentiation of VE-cadherin+ EC cells.
Effect of SCL overexpression on the generation of hematopoietic progenitors
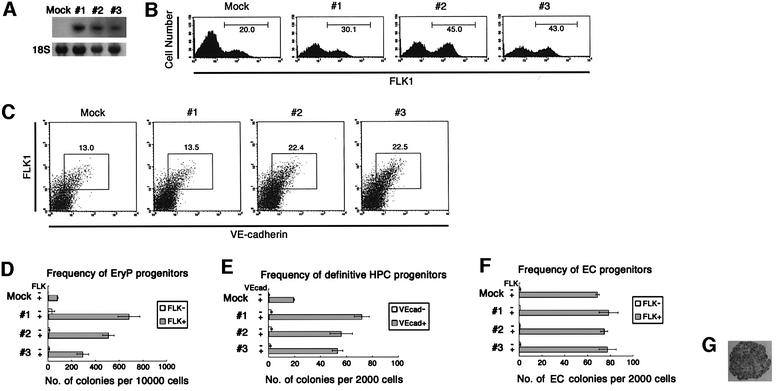
It was reported that ectopic expression of SCL in zebrafish embryos perturbs normal development, resulting in the overproduction of hematopoietic and endothelial progenitors, at the expense of other mesodermal cell lineages (Gering et al., 1998). To examine whether this is also the case in ES cell differentiation, we established ES cell lines that constitutively overexpress SCL under the chicken β actin (CAG) promoter and analyzed the effect of SCL overexpression on HPC and EC development using an in vitro differentiation system. Three independent lines were established (clones 1–3; Figure 5A). The lines were induced to differentiate on OP9 stromal cells. FACS analysis showed that FLK1+ cells were first detectable at day 3 of differentiation in both mock and SCL transfectants, indicating that the kinetics of differentiation were not affected by SCL overexpression (data not shown). Within 12 h following differentiation, more FLK1+ cells were generated in the SCL-transfected group (30–45%) than in the mock-transfected group (20%) (Figure 5B). While it was demonstrated that SCL overexpression in zebrafish suppressed paraxial mesoderm differentiation, neither decrease nor increase of the proportion of platelet-derived growth factor receptor-α (PDGFRα)+ paraxial mesodermal cells was induced by SCL overexpression (data not shown). While the kinetics of the differentiation of VE-cadherin+ cells were not altered by SCL overexpression, the proportion was slightly higher in the SCL-transfected group than the control group; 13–23% versus 13%, respectively (Figure 5C), which might reflect the increase of FLK1+ mesoderm cells.
Fig. 5. Effect of SCL overexpression on the generation of hematopoietic progenitors. (A) Northern blot analysis of SCL expression in mock-transfected and SCL-transfected ES clones (Mock, 1, 2 and 3, respectively). Twenty micrograms of total RNA from indicated ES clones was hybridized with [32P]dCTP-labeled SCL probes. Equal loading of total RNA in each lane was demonstrated with 18S ribosomal RNA. (B) Mock-transfected and SCL-transfected ES clones were allowed to differentiate on OP9 stromal cells. Cultured cells were harvested at day 3.5 and expression of FLK1 in those cells was analyzed by FACS. (C) Cultured cells were harvested at day 5.5 and expression of VE-cadherin was analyzed by FACS. (D) FLK1+ and FLK1– cells were sorted from differentiating ES cells at day 3.5 of differentiation and transferred to methylcellulose semisolid culture in the presence of SCF, IL-3, Epo and IL-6. The number of primitive erythroid colonies was scored at day 8 of total differentiation. (E) VE-cadherin+ FLK1+ and VE-cadherin– FLK1– cells were sorted from differentiating ES cells at day 5.5 of differentiation and transferred to methylcellulose semisolid culture in the presence of SCF, IL-3, Epo and IL-6. The number of CFU-C was scored at day 14 of total differentiation. (F and G) FLK1+ and FLK1– cells were sorted from differentiating ES cells at day 3.5 of differentiation and recultured on OP9 stromal cell layer for 4 days. The cultured cells were stained in situ with anti-VE-cadherin mAb (original magnification ×200; G). The frequency of cells capable of formation of endothelial cell colony in the indicated fractions was scored (F). Error bars indicate standard deviations for three independent determinations.
To measure the frequency of HPC progenitors in the FLK1+VE-cadherin– population, ES cell lines were cultured for 3.5 days, separated into FLK1+ and FLK1– fractions by cell sorting and hematopoietic progenitors were measured by colony forming assay. At this stage, VE-cadherin+ cells were not yet detectable (Figure 4A). Under these culture conditions, almost all colony-forming cells were present within the FLK1+ population and colonies consisted of primitive erythrocytes in both SCL-transfected and control groups. As shown in Figure 5D, SCL overexpression resulted in a 3- to 9-fold increase of colony-forming cells in the FLK1+ population. We next investigated the effect of SCL overexpression on generation of the hemogenic VE-cadherin+ population. As was the case with the FLK1+VE-cadherin– stage, SCL overexpression induced a 3- to 4-fold increase in the frequency of hematopoietic progenitors in the VE-cadherin+ population sorted from day 5.5 culture (Figure 5E). Most colonies detected by this protocol were of the definitive type containing enucleated erythrocytes, monocytes and polymorphonuclear cells. The proportion of each type of colony was comparable in both groups (data not shown).
In contrast to its effect on HPC differentiation, SCL overexpression had only a slight effect, if any, on the frequency of clonogenic progenitors in FLK1+ cells of day 3.5 culture that can give rise to EC clusters on the OP9 stromal cell layer (Figure 5F). The size of the EC clusters was also unaffected by SCL overexpression.
Discussion
Experimental systems that allow in vitro ES cell differentiation to HPC have provided a useful system for dissecting the process of HPC differentiation during early embryogenesis. An important contribution of studies using these systems is the identification of distinct intermediate stages that otherwise would have been difficult to define. We have focused on the identification of stage markers that are detectable by flow cytometry, and introduced mAbs and GFP markers for defining several distinct intermediate stages. Among these, FLK1+VE-cadherin– and FLK1+VE-cadherin+ populations comprised two important hub stages that appeared successively (Nishikawa et al., 1998a,b; Fujimoto et al., 2001). While these two stages do not express CD45, a specific marker for HPC, our previous studies demonstrated that HPCs are generated directly from these two populations. Based upon the spatio-temporal location of these markers, we proposed that the FLK1+VE-cadherin– and FLK1+VE- cadherin+ populations represent mesoderm and EC, respectively.
It has been widely accepted that a subset of mesodermal cells, such as FLK1+VE-cadherin–, represents the diverging point of HPC and EC, which have been designated as hemangioblasts. Whether or not this stage is fate-restricted only to EC and HPC is difficult to prove, but the presence of FLK1+ progenitors that can give rise to the two lineages has been demonstrated experimentally (Kabrun et al., 1997; Nishikawa et al., 1998a; Fujimoto et al., 2001). On the other hand, whether or not FLK1+VE-cadherin+ EC serves as the second diverging point of HPC from EC remains debatable. For instance, Lacaud et al. proposed a model of HPC differentiation in which HPC and EC lineages diverge only once at the hemangioblast stage (Kennedy et al., 1997; Lacaud et al., 2001). However, the notion that VE-cadherin+ EC serves as the second diverging point for HPC and EC, particularly the definitive HPC lineage, has gained strong support from recent studies analyzing the hemogenic potential of EC lining of the dorsal aorta (Jaffredo et al., 1998; de Bruijn et al., 2002). It was also reported recently that VE-cadherin+ cells sorted from the AGM region of mid-gestation mouse embryo are capable of reconstituting bone marrow hematopoiesis of irradiated adult mice (North et al., 2002). Accordingly, Lacaud’s proposal was also revised; the hemangioblast formerly considered as a uniform population was subclassified into primitive- and definitive-restricted subpopulations (Lacaud et al., 2002). Given that HPC and EC diverge at two stages, FLK1+VE-cadherin– and FLK1+VE-cadherin+, the next issue to be addressed would be at which stage the fate to HPC is determined; only once at the early stage or at each successive point of HPC divergence. The major aim of this study is to address this question.
Several studies have demonstrated that SCL is an essential molecule for both primitive and definitive hematopoiesis, and thus one can expect SCL to be a pivotal molecule for fate determination to HPC (Robb et al., 1995; Shivdasani et al., 1995; Elefanty et al., 1997; Gering et al., 1998; Mead et al., 1998). However, the continuous expression of SCL throughout the course of HPC differentiation from the early mesodermal stages, renders it difficult to specify the timing of SCL-dependent events for definitive HPC differentiation. To overcome this obstacle, we established SCL-null ES cell lines in which SCL gene expression is induced by Cre recom binase-loxP site-mediated recombination that is activated by the addition of tamoxifen. The constructs functioned efficiently in our ES cell differentiation system, giving rise to a sharp increase in SCL expression after tamoxifen addition, with relatively homogeneous expression levels (Figure 1C and D; data not shown). This result is consistent with the original study describing this method and confirmed its usefulness for inducing genes in ES cell differentiation culture systems (Verrou et al., 1999; Vallier et al., 2001).
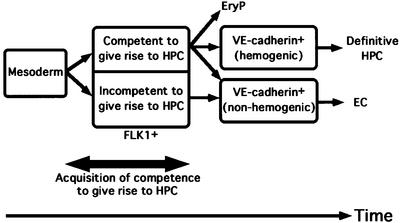
Our data clearly demonstrated that tamoxifen induction has to be commenced prior to day 4 of the ES cell differentiation culture for both primitive and definitive hematopoiesis to occur. As ∼8–12 h are required for the transgene expression to reach peak levels after tamoxifen addition (Figure 1C), SCL is likely required around day 3.5–4.5. Considering that the first FLK1+ cells and VE-cadherin+ cells appear at days 3.0 and 5.0, respectively, the most probable interpretation of this time course is that SCL is required during the FLK1+VE-cadherin– mesodermal stage. As this is the first study to examine re-activation of the SCL gene in SCL(–/–) ES differentiation cultures, direct comparison with previous studies may be difficult. But our results are consistent with the proposal by Robertson et al. that SCL is involved in the early process of HPC differentiation, which is derived from an observation that the blast colony stage that can give rise to primitive, definitive hematopoietic and endothelial cells is absent in SCL(–/–) ES cultures (Robertson et al., 2000). Our results further revealed that the induction of SCL later than day 4.0 could not restore the competence to differentiate to HPC. Hence, mesodermal cells that failed to express SCL at the right stage appear to be irreversibly excluded from HPC fate. This result could be interpreted to mean that ‘the right stage’ is the stage where HPC lineages do diverge. If one accepts the notion that definitive HPC are generated directly from EC, however, the SCL-dependent events in the mesoderm would not represent an exclusive fate-determination process for definitive HPC, as they first proceed to a VE-cadherin+ EC fate. In fact, we showed here that differentiation of VE-cadherin+ cells that can give rise to HPC requires the SCL-dependent process during mesoderm differentiation (Figure 4C). The process that is regulated by SCL during mesoderm differentiation may be more properly designated as an acquisition of competence, a latent potential to become HPC, rather than actual fate determination of HPC. Taking the two-diverging point model into consideration, our results indicate that: (i) one of the processes to acquire the competence to differentiate into HPC (HPC competence) occurs at the mesoderm stage; (ii) SCL is a requisite molecule for this process; and (iii) this is a process that is independent from the actual divergence of HPC and EC (Figure 6). These results seem consistent with a previous study demonstrating that SCL is required for development of blast colony precursors, but not for transitional colony precursors, in the sense that SCL-dependent processes are necessary at the mesodermal stage for development of primitive and definitive hemato poietic lineages, though we did not detect these cells in our culture conditions (data not shown; Robertson et al., 2000).
Fig. 6. Model depicting the stage at which the competence to give rise to HPC is acquired. A competence to give rise to HPC was acquired at FLK1+VE-cadherin– stage by a SCL-dependent process prior to differentiation of VE-cadherin+ EC.
We showed previously that FLK1+VE-cadherin– cells that express GATA-1 are strongly biased towards primitive erythrocytes (Fujimoto et al., 2001). In the case of primitive hematopoiesis, acquisition of competence and commitment to HPC may occur almost simultaneously; probably all necessary molecules for HPC differentiation including GATA-1 are assembled before the commencement of the EC program. Moreover, Mead et al. demonstrated that expression of SCL with LMO2 and GATA-1 in Xenopus animal pole explants or embryos strongly direct the differentiation toward primitive erythrocytes (Mead et al., 2001). Hence, GATA-1 expression co-incident with SCL and LMO2 expression may be required for generating primitive erythrocytes directly from mesoderm. Of note in this context is that none of the VE-cadherin+ ECs express GATA-1 irrespective of their HPC competence, suggesting that either the EC differentiation program excludes GATA-1 expression or GATA-1 expression excludes EC fate. As GATA-1+ mesodermal cells retain a capacity to differentiate to EC, the former possibility is more acceptable (Fujimoto et al., 2001). On the other hand, SCL, LMO2 and GATA2, which are essential for HPC differentiation, are also expressed in some ECs (Dorfman et al., 1992; Hwang et al., 1993; Kallianpur et al., 1994; Yamada et al., 2000). This pattern of gene expression may be useful to understand the molecular mechanisms of HPC development from EC.
What then is the function of SCL for the acquisition of HPC competence during the mesoderm stage? Ectopic expression of SCL in the zebrafish embryos has been reported to be sufficient for perturbing the normal developmental process, resulting in overproduction of FLK1+ hematopoietic and endothelial progenitors, with the concomitant loss of paraxial mesodermal cells (Gering et al., 1998). Mead et al. reported that when overexpressed in Xenopus animal cap explants, SCL is capable of directing mesoderm to differentiate to HPC, though forced expression in developing Xenopus embryos does not lead to ectopic blood formation (Mead et al., 1998, 2001). In our ES cell culture, overexpression of SCL alone resulted in a somewhat increased percentage (1.5–2 times) of FLK1+ cells at day 4 of the culture (Figure 5B). The generation of PDGFRα+ paraxial mesoderm did not seem to be affected (data not shown). The frequency of primitive hematopoietic progenitors in the FLK1+ population in mesoderm increased 3- to 9-fold in proportion, but that of endothelial progenitors did not increase (Figure 5D and F). The percentage of VE-cadherin+ endothelial cells was the same or slightly increased at day 5.5 of the culture, and the frequency of definitive hematopoietic progenitors in the VE-cadherin+ population was also remarkably increased (Figure 5C and E). Though it would be difficult to specify the reason for the discrepancy between our results and overexpression experiments in Xenopus and zebrafish embryos, we speculate that one explanation might be that the ES cell differentiation system is less affected by cell interactions than RNA injection in the embryos. Moreover, as shown in our previous studies, our culture condition is strongly biased towards differentiation of lateral mesoderm, thereby overcoming the defects present in the embryo (Nakano et al., 1994). Nevertheless, the fact that EC development was not enhanced by forced expression of SCL in the ES cell culture seems to support a hypothesis that the SCL role at the mesoderm stage is to induce HPC competence rather than generation of hemangioblasts, which can give rise to both EC and HPC. If the latter is the case, EC differentiation should also be enhanced.
In conclusion, we showed that SCL acts to specify both primitive and definitive hematopoiesis at the mesodermal stage, and that acquisition of competence to give rise to both hematopoietic lineages has occurred already at that stage irrespective of the program to EC (Figure 6). These data will allow a molecular analysis of the commitment of mesoderm to the hematopoietic and endothelial systems and the role played by SCL in these processes.
Materials and methods
Antibodies
The mAb against E-cadherin, ECCD2, was a gift from Dr Nagafuchi (Kumamoto University, Kumamoto, Japan). The mAbs AVAS12 (anti-FLK1) and VECD1 (anti-VE-cadherin) were purified from hybridoma culture supernatants on a protein G–Sepharose column (Pharmacia, Uppsala, Sweden). These mAbs were labeled with fluorescein isothiocyanate (FITC) or allophycocyanin (APC) by standard methods. The anti-SCL rabbit polyclonal antibody was a gift from Dr Baer (UT Southwestern Medical Center, Dallas, TX). The anti-estrogen receptor rabbit polyclonal antibody (sc-542) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The alkaline phosphatase-conjugated anti-rat IgG, anti-rabbit IgG and peroxidase-conjugated anti-rat IgG antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA).
Plasmid construction
To engineer pCAG-MerCreMer, a 3.1 kb HindIII fragment containing the MerCreMer-coding sequence from pANMerCreMer (a kind gift from Dr Reth, University of Freiburg, Germany) was subcloned into the XhoI site of plasmid pCAGGS (a kind gift from Dr Niwa, CDB, RIKEN, Kobe, Japan). To engineer pCAG-lox-STOP-lox-SCL-IRES-EGFP, a 1.3 kb NotI–XhoI fragment from pBIKS(-)CITE-EGFP (a kind gift from Dr Niwa) was subcloned into the BglII site in pCAGGS to generate pCAGGS-IRES-EGFP. In parallel, a 1.6 kb NotI fragment containing floxed STOP cassette from pBS302 (Gibco-BRL, Rockville, MD) was subcloned into the XhoI site in pCAGGS-IRES-EGFP to generate pCAGGS-lox-STOP-lox-IRES-EGFP. A 1.5 kb EcoRI–BglII SCL cDNA fragment encompassing the SCL-coding sequence was subcloned into the EcoRV site in pCAGGS-lox-STOP-lox-IRES-EGFP to generate pCAGGS-lox-STOP-lox-SCL-IRES-EGFP. To engineer pCAG-SCL-IRES-Puro, a 1.5 kb EcoRI–BglII SCL cDNA fragment was subcloned into the XhoI site in PCAGIPuro (a kind gift from Dr Niwa). pCAG-MerCreMer, pPyCAGIRESzeocinpA (a kind gift from Dr Niwa), pCAGGS-lox-STOP-lox-SCL-IRES-EGFP, pCAGIPuro and pCAG-SCL-IRES-Puro were linearized by PvuI digestion before transfection into ES cells.
Cell culture
J1, SCL-null and EB5 (a subclone of E14 ES cell line; a kind gift from Dr Niwa) ES cell lines were maintained on gelatin-coated culture dishes in G-MEM (Gibco-BRL) supplemented with 1% FCS (Whittaker Bioproducts, Walkersville, MD), 10% KSR (Gibco-BRL), 0.1 mM non-essential amino acids, 1 mM sodium pyruvate (Gibco-BRL), 0.1 mM 2-mercaptoethanol (2ME), 2000 U/ml leukemia inhibitory factor (LIF) (Gibco-BRL). OP9 stromal cell line was maintained in α minimum essential medium (MEM) (Gibco-BRL) supplemented with 20% FCS (HyClone Laboratories, Logan, UT).
Electroporation and selection procedure
SCL-null ES cells were electroporated with linearized pCAG-MerCreMer and pPyCAGIRESzeocinpA and then selected for resistance to Zeocin (30 µg/ml; Sigma, St Louis, MO). Resistant ES cell clones were stained by anti-estrogen receptor polyclonal antibody (sc-542, Santa Cruz Biotechnology) and selected MerCreMer-expressing ES clones. These ES clones were electroporated with linearized pCAGGS-lox-STOP-lox-SCL-IRES-EGFP and PCAGIPuro and then selected for resistance to puromycin (2 µg/ml; Sigma). Resistant ES cell clones were cultured with or without tamoxifen for a day and stained with anti-SCL rabbit polyclonal antibody (a kind gift from Dr Baer) and alkaline phosphatase-conjugated anti-rabbit IgG. Expression of SCL proteins in those clones were detected by using NBT–BCIP substrate solution (Boehringer Mannheim, Mannheim, Germany). Three clones that displayed low background and high induction of Cre activity were selected for further studies. To induce Cre activity, 800 nM 4′hydroxy-tamoxifen (Sigma) was added to the culture. EB5 ES cells were electroporated with linearized pCAG-SCL-IRES-Puro and then selected for resistance to puromycin (2 µg/ml). Resistant ES cell clones were stained with anti-SCL polyclonal antibody to confirm the expression of SCL.
In vitro differentiation of ES cells, cell staining and sorting
The induction of ES cell differentiation was carried out as described previously (Nakano et al., 1994). ES cells were seeded onto confluent OP9 cell layers in six-well plates (Becton Dickinson Labware, Bedford, MA) at a density of 104 cells per well. α-MEM (Gibco-BRL) supplemented with 10% FCS (Gibco-BRL) and 50 µmol/l 2ME in the absence of LIF was used as a culture media (induction medium). The induced cells were harvested in cell dissociation buffer (Gibco-BRL), blocked with normal mouse serum and stained with several combinations of labeled mAbs. Stained cells were resuspended in Hanks’ balanced salt solution (Gibco-BRL) containing 1% bovine serum albumin (Sigma) and 5 µg/ml propidium iodide (Sigma) to exclude dead cells. Cells were analyzed and sorted by FACS Vantage (Becton Dickinson Immuno cytometry Systems, San Jose, CA). Data were analyzed and printed out by using software CellQuest (Becton Dickinson Immunocytometry Systems). Sorted cells were cultured for subsequent induction of hematopoietic or endothelial cells.
Hematopoietic and endothelial progenitor analysis
For the induction of erythro/myeloid cells, sorted cells were transferred into methylcellulose medium with SCF, IL-3, IL-6 and Epo (Methocult GF M3434, Stemcell Technologies Inc., British Columbia, CA). The number of primitive erythroid colonies was scored at day 8 of total differentiation from ES cells, and that of definitive hematopoietic cell colonies was scored at day 14 of total differentiation.
For induction of endothelial cell growth, sorted cells were transferred on to freshly prepared OP9 cell layer at a density of 2 × 103 cells per well of 6-well plates and incubated in induction medium. After 3 days, cultures were fixed in situ by 4% paraformaldehyde and stained with anti-FLK1, anti-VE-cadherin, or anti-CD31 mAbs and peroxidase conjugated anti-rat IgG antibody. FLK1+/VE-cadherin+/CD31+ endothelial cell colonies were revealed by 0.025% DAB substrate solution (Wako, Osaka, Japan) and scored by microscopy (Hirashima et al., 1999).
Northern blotting
Total RNA was prepared from wild-type or SCL-transfected ES cells using Isogen (Nippon Gene, Toyama, Japan). The northern blot analysis was carried out using standard protocols as described previously (Narumi et al., 2000). Probes were labeled with [32P]dCTP by the random priming method. The probes used in this study were derived from a 1.5 kb EcoRI–BglII SCL cDNA fragment containing the SCL-coding sequence. 18S ribosomal RNA was detected by ethidium bromide staining.
Acknowledgments
Acknowledgements
We thank Drs Michael Reth and Richard Baer for generous gifts of pANMerCreMer plasmid and anti-SCL antibody, respectively. We also thank Dr Ruth Yu for critical reading of the manuscript. This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Nos 12670301 and 07CE2005), the Cell Science Research Foundation and Japanese Society for the Promotion of Science ‘Research of Future’ program.
References
- de Bruijn M.F., Ma,X., Robin,C., Ottersbach,K., Sanchez,M.J. and Dzierzak,E. (2002) Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity, 16, 673–683. [DOI] [PubMed] [Google Scholar]
- Dorfman D.M., Wilson,D.B., Bruns,G.A. and Orkin,S.H. (1992) Human transcription factor GATA-2. Evidence for regulation of preproendothelin-1 gene expression in endothelial cells. J. Biol. Chem., 267, 1279–1285. [PubMed] [Google Scholar]
- Elefanty A.G., Robb,L., Birner,R. and Begley,C.G. (1997) Hematopoietic-specific genes are not induced during in vitro differentiation of scl-null embryonic stem cells. Blood, 90, 1435–1447. [PubMed] [Google Scholar]
- Fujimoto T., Ogawa,M., Minegishi,N., Yoshida,H., Yokomizo,T., Yamamoto,M. and Nishikawa,S. (2001) Step-wise divergence of primitive and definitive haematopoietic and endothelial cell lineages during embryonic stem cell differentiation. Genes Cells, 6, 1113–1127. [DOI] [PubMed] [Google Scholar]
- Gering M., Rodaway,A.R., Gottgens,B., Patient,R.K. and Green,A.R. (1998) The SCL gene specifies haemangioblast development from early mesoderm. EMBO J., 17, 4029–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima M., Kataoka,H., Nishikawa,S. and Matsuyoshi,N. (1999) Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood, 93, 1253–1263. [PubMed] [Google Scholar]
- Hwang L.Y., Siegelman,M., Davis,L., Oppenheimer-Marks,N. and Baer,R. (1993) Expression of the TAL1 proto-oncogene in cultured endothelial cells and blood vessels of the spleen. Oncogene, 8, 3043–3046. [PubMed] [Google Scholar]
- Jaffredo T., Gautier,R., Eichmann,A. and Dieterlen-Lievre,F. (1998) Intraaortic hemopoietic cells are derived from endothelial cells during ontogeny. Development, 125, 4575–4583. [DOI] [PubMed] [Google Scholar]
- Kabrun N., Buhring,H.J., Choi,K., Ullrich,A., Risau,W. and Keller,G. (1997) Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development, 124, 2039–2048. [DOI] [PubMed] [Google Scholar]
- Kallianpur A.R., Jordan,J.E. and Brandt,S.J. (1994) The SCL/TAL-1 gene is expressed in progenitors of both the hematopoietic and vascular systems during embryogenesis. Blood, 83, 1200–1208. [PubMed] [Google Scholar]
- Kennedy M., Firpo,M., Choi,K., Wall,C., Robertson,S., Kabrun,N. and Keller,G. (1997) A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature, 386, 488–493. [DOI] [PubMed] [Google Scholar]
- Lacaud G., Robertson,S., Palis,J., Kennedy,M. and Keller,G. (2001) Regulation of hemangioblast development. Ann. N. Y. Acad. Sci., 938, 96–108. [DOI] [PubMed] [Google Scholar]
- Lacaud G. et al. (2002) Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood, 100, 458–466. [DOI] [PubMed] [Google Scholar]
- Liao E.C., Paw,B.H., Oates,A.C., Pratt,S.J., Postlethwait,J.H. and Zon,L.I. (1998) SCL/Tal-1 transcription factor acts downstream of cloche to specify hematopoietic and vascular progenitors in zebrafish. Genes Dev., 12, 621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead P.E., Kelley,C.M., Hahn,P.S., Piedad,O. and Zon,L.I. (1998) SCL specifies hematopoietic mesoderm in Xenopus embryos. Development, 125, 2611–2620. [DOI] [PubMed] [Google Scholar]
- Mead P.E., Deconinck,A.E., Huber,T.L., Orkin,S.H. and Zon,L.I. (2001) Primitive erythropoiesis in the Xenopus embryo: the synergistic role of LMO-2, SCL and GATA-binding proteins. Development, 128, 2301–2308. [DOI] [PubMed] [Google Scholar]
- Medvinsky A. and Dzierzak,E. (1996) Definitive hematopoiesis is autonomously initiated by the AGM region. Cell, 86, 897–906. [DOI] [PubMed] [Google Scholar]
- Muller A.M., Medvinsky,A., Strouboulis,J., Grosveld,F. and Dzierzak,E. (1994) Development of hematopoietic stem cell activity in the mouse embryo. Immunity, 1, 291–301. [DOI] [PubMed] [Google Scholar]
- Nakano T. (1996) In vitro development of hematopoietic system from mouse embryonic stem cells: a new approach for embryonic hematopoiesis. Int. J. Hematol., 65, 1–8. [DOI] [PubMed] [Google Scholar]
- Nakano T., Kodama,H. and Honjo,T. (1994) Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science, 265, 1098–1101. [DOI] [PubMed] [Google Scholar]
- Narumi O., Mori,S., Boku,S., Tsuji,Y., Hashimoto,N., Nishikawa,S. and Yokota,Y. (2000) OUT, a novel basic helix–loop–helix transcription factor with an Id-like inhibitory activity. J. Biol. Chem., 275, 3510–3521. [DOI] [PubMed] [Google Scholar]
- Nishikawa S.I., Nishikawa,S., Hirashima,M., Matsuyoshi,N. and Kodama,H. (1998a) Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development, 125, 1747–1757. [DOI] [PubMed] [Google Scholar]
- Nishikawa S.I., Nishikawa,S., Kawamoto,H., Yoshida,H., Kizumoto,M., Kataoka,H. and Katsura,Y. (1998b) In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity, 8, 761–769. [DOI] [PubMed] [Google Scholar]
- North T.E., de Bruijn,M.F., Stacy,T., Talebian,L., Lind,E., Robin,C., Binder,M., Dzierzak,E. and Speck,N.A. (2002) Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity, 16, 661–672. [DOI] [PubMed] [Google Scholar]
- Robb L., Lyons,I., Li,R., Hartley,L., Kontgen,F., Harvey,R.P., Metcalf,D. and Begley,C.G. (1995) Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc. Natl Acad. Sci. USA, 92, 7075–7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S.M., Kennedy,M., Shannon,J.M. and Keller,G. (2000) A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development, 127, 2447–2459. [DOI] [PubMed] [Google Scholar]
- Shivdasani R.A., Mayer,E.L. and Orkin,S.H. (1995) Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature, 373, 432–434. [DOI] [PubMed] [Google Scholar]
- Vallier L., Mancip,J., Markossian,S., Lukaszewicz,A., Dehay,C., Metzger,D., Chambon,P., Samarut,J. and Savatier,P. (2001) An efficient system for conditional gene expression in embryonic stem cells and in their in vitro and in vivo differentiated derivatives. Proc. Natl Acad. Sci. USA, 98, 2467–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrou C., Zhang,Y., Zurn,C., Schamel,W.W. and Reth,M. (1999) Comparison of the tamoxifen regulated chimeric Cre recombinases MerCreMer and CreMer. Biol. Chem., 380, 1435–1438. [DOI] [PubMed] [Google Scholar]
- Visvader J.E., Fujiwara,Y. and Orkin,S.H. (1998) Unsuspected role for the T-cell leukemia protein SCL/tal-1 in vascular development. Genes Dev., 12, 473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Pannell,R., Forster,A. and Rabbitts,T.H. (2000) The oncogenic LIM-only transcription factor Lmo2 regulates angiogenesis but not vasculogenesis in mice. Proc. Natl Acad. Sci. USA, 97, 320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]