Abstract
Emission of methyl benzoate, one of the most abundant scent compounds of bee-pollinated snapdragon flowers, occurs in a rhythmic manner, with maximum emission during the day, and coincides with the foraging activity of bumblebees. Rhythmic emission of methyl benzoate displays a “free-running” cycle in the absence of environmental cues (in continuous dark or continuous light), indicating the circadian nature of diurnal rhythmicity. Methyl benzoate is produced in upper and lower snapdragon petal lobes by enzymatic methylation of benzoic acid in the reaction catalyzed by S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase (BAMT). When a detailed time-course analysis of BAMT activity in upper and lower petal lobes during a 48-hr period was performed, high BAMT activity was found at night as well as in continuous darkness, indicating that the BAMT activity is not an oscillation-determining factor. Analysis of the level of benzoic acid during a 24-hr period revealed oscillations in the amount of benzoic acid during the daily light/dark cycle that were retained in continuous darkness. These data clearly show that the total amount of substrate (benzoic acid) in the cell is involved in the regulation of the rhythmic emission of methyl benzoate. Our results also suggest that similar molecular mechanisms are involved in the regulation of methyl benzoate production in diurnally (snapdragon) and nocturnally (tobacco and petunia) emitting plants.
INTRODUCTION
Flowers of many plant species attract pollinators by producing different complex mixtures of volatile compounds that give each species a unique and characteristic fragrance. Such volatile compounds emitted from flowers may function as both long-distance and short-distance attractants and play a prominent role in the localization and selection of flowers by insects, especially in moth-pollinated flowers, which are detected and visited at night (Dobson, 1994). Plants need to, and often do, vary the floral scent they emit during the life span of the flower, both in total output and in specific composition. These changes occur in relation to flower age (Tollsten, 1993; Pichersky et al., 1994; Wang et al., 1997; Dudareva et al., 1998, 2000), pollination status (Tollsten, 1993; Schiestl et al., 1997), environmental conditions (Jakobsen and Olsen, 1994), and diurnal endogenous rhythms (summarized by Dudareva et al., 1999). In addition to reduced costs for scent production, these intraspecific changes in floral fragrances also can influence the activity of flower visitors. For example, a quantitative and/or qualitative change of floral bouquets after pollination might lead to a lower attractiveness of these flowers and help to direct pollinators to the unpollinated flowers, thereby maximizing the reproductive success of the plant.
It is an evolutionary advantage that plants have scent output at maximal levels only when potential pollinators are active. Plants that are pollinated by insects with maximum activity during the day (e.g., bees) show a diurnal rhythmicity, whereas flowers pollinated by nocturnal insects (e.g., moths) tend to have maximal scent output at night (Matile and Altenburger, 1988; Loughrin et al., 1990; Nielsen et al., 1995). In addition, some plants emit one set of compounds during the day and another at night (Matile and Altenburger, 1988; Loughrin et al., 1992). Moreover, it has been found that within the flower, some compounds are emitted in a rhythmic manner during a 24-hr period, whereas others are not, suggesting that different mechanisms regulate the biosynthesis and/or emission of these volatile compounds (Loughrin et al., 1991; Nielsen et al., 1995). The rhythmic release of flower scent during the day and night periods generally coincides with the flight period of potential pollinators. However, there are examples of flowers that are pollinated primarily by diurnally or nocturnally active insects that show no differences in day and night scent production (Pichersky et al., 1994; Schiestl et al., 1997; Dudareva et al., 1999).
Regulation of fragrance emission cycling can be induced either by illumination or darkness or by an endogenous clock. Nocturnally pollinated plants exhibit a circadian, endogenously controlled rhythmicity in their nocturnal emission patterns. This rhythmicity is maintained upon exposure to continuous light or dark (Altenburger and Matile, 1988, 1990; Matile and Altenburger, 1988; Loughrin et al., 1991). In contrast, diurnal rhythmicity in the emission of volatile compounds by plants was reported to be noncircadian and controlled by irradiation levels (Altenburger and Matile, 1990; Jakobsen and Olsen, 1994; Jakobsen et al., 1994). However, a recent investigation indicates that the emission of volatile compounds by diurnally emitting plants (rose flowers) is controlled by a circadian clock (Helsper et al., 1998).
Although the rhythmic release of floral volatile compounds has been demonstrated in several different species, the molecular mechanisms responsible for this process remain unknown. Investigations of the regulation of rhythmic emission of plant volatile compounds have been seriously hampered by the lack of characterized biosynthetic enzymes and genes involved in the synthesis of scent compounds. Recent studies of the biogenesis of floral scent production in Clarkia breweri (summarized by Dudareva et al., 1999; Dudareva and Pichersky, 2000) and snapdragon (Dudareva et al., 2000) represent, to our knowledge, the only examples to date for which the isolation of enzymes and genes responsible for the formation of scent volatile compounds in the flower has been accomplished. It has been shown that volatile compounds are synthesized de novo in epidermal cells of organs from which they are emitted (Dudareva and Pichersky, 2000; Kolosova et al., 2001) and that the enzyme activities involved in the formation of these volatile compounds and, indirectly, their emission are regulated at the transcriptional level.
C. breweri flowers, despite being pollinated by moths, do not exhibit oscillation differences in emission between day and night. Snapdragon flowers, on the other hand, are pollinated by bees and have a marked peak of emission during the day (Dudareva et al., 2000). One of the major components of snapdragon floral scent is the volatile ester methyl benzoate, which is synthesized by enzymatic methylation of benzoic acid in the reaction catalyzed by S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase (BAMT) (Dudareva et al., 2000; Murfitt et al., 2000). Rhythmic emission of methyl benzoate from snapdragon flowers and the availability of the enzyme responsible for its formation place us in a unique position to analyze the molecular mechanisms of rhythmic emission. In this study, we investigate how plants regulate the rhythmic emission of volatile compounds. We show that the rhythmic emission of methyl benzoate in snapdragon is under the control of the biological clock and is regulated by the level of supplied substrate for the final reaction in methyl benzoate production. Moreover, our data indicate that similar molecular mechanisms are involved in the regulation of methyl benzoate production in diurnally (snapdragon) and nocturnally (tobacco and petunia) emitting plants.
RESULTS
Is the Rhythmic Emission of Methyl Benzoate in Snapdragon Flowers Controlled by a Circadian Clock?
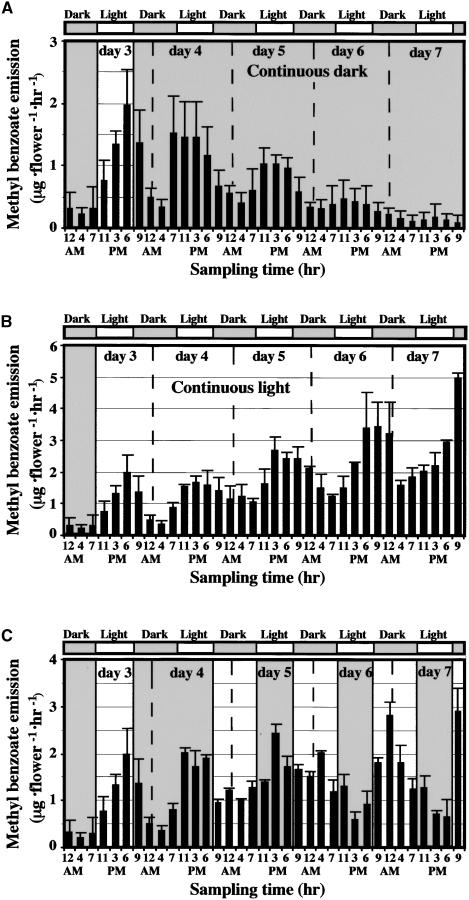
We have shown previously that emission of methyl benzoate from snapdragon flowers follows diurnal cycles, with the highest emission rate between 9 am and 4 pm, and is correlated with light intensity (Dudareva et al., 2000). To determine whether the rhythmicity in methyl benzoate emission is controlled by a circadian clock, plants were grown in a 12-hr-light/12-hr-dark period at a constant temperature of 25°C and then transferred to conditions of either continuous light or continuous dark. Floral volatile compounds were collected at seven time points during 24-hr intervals for 120 hr (Figures 1A and 1B). Because the emission of methyl benzoate is regulated developmentally during the life span of the flower and young flowers produce low amounts of volatile ester (Dudareva et al., 2000), flowers used for headspace analysis were 3 days old at the beginning of the experiment. Oscillations in methyl benzoate emission continued for at least three cycles in continuous darkness, although the amplitude of emission decreased gradually. The dampening of the amplitude exhibits the typical pattern of a “free-running” cycle (Piechulla, 1988, 1989), indicating that an endogenous (circadian) rhythm influences this process (Figure 1A). This dampening phenomenon has been observed for some physiological oscillations (Thomas and Vince-Prue, 1997; Engelmann and Johnsson, 1998; Webb, 1998) and also for the expression of several genes (summarized by Fejes and Nagy, 1998). Circadian oscillations in methyl benzoate emission also were maintained during 4 days of continuous illumination, establishing circadian clock control over the temporal pattern of volatile ester emission (Figure 1B). Inversion of the photoperiod by extending one 12-hr-dark period led to a shift of maximum methyl benzoate emission by ∼12 hr. During the adaptation to an altered photoperiod, the emission level continued to oscillate and the shift was completed in ∼24 hr, indicating that plants are able to respond to the changed light/dark cycle within a relatively short time by modulating their emission patterns (Figure 1C).
Figure 1.
Emission of Methyl Benzoate from Snapdragon Flowers Exposed to Different Light/Dark Conditions Measured by Headspace Analysis.
(A) Intact flowers were exposed to the normal day/night cycle (12-hr day/12-hr night) followed by continuous dark for 4 days.
(B) Intact flowers were exposed to the normal day/night cycle (12-hr day/12-hr night) followed by continuous light (100 μmol·m−2·sec−1 light intensity) for 4 days.
(C) Intact flowers were exposed to the normal day/night cycle (12-hr day/12-hr night) followed by an inverted light/dark regimen. Inversion of photoperiod was achieved by extension of the dark period for 12 hr.
Headspace collections were performed at seven time points during the 24-hr interval. Shaded and light areas correspond to dark and light, respectively. The corresponding theoretical light/dark cycle is indicated at the top of each panel by open bars (light period) and shaded bars (dark period). Each graph represents the average of three to five independent experiments. Standard deviations are indicated by vertical bars.
Do the Levels of BAMT Activity, BAMT mRNA, and Benzoic Acid Oscillate during the Light/Dark Cycle?
The volatile ester methyl benzoate is produced in upper and lower snapdragon petal lobes by enzymatic methylation of benzoic acid in the reaction catalyzed by BAMT (Dudareva et al., 2000). Low emission of methyl benzoate at night (Figure 2A) could be attributed to reduced biosynthesis, reduced release of the volatile ester, or other light-related reactions. To test these hypotheses, we first performed a detailed time-course analysis of BAMT activity in upper and lower petal lobes of 3- and 4-day-old flowers during two normal light/dark cycles (a 48-hr period) (Figure 2B). We found only slight variations in BAMT activity during the daily light/dark cycle, which displayed a clear rhythmic nature (Figure 2B). BAMT activity increased gradually during the day, achieved a maximum at approximately 6 pm, and then decreased at night. The level of BAMT activity at night was high and comparable with the maximum BAMT level during the day (e.g., in 4-day-old flowers, BAMT activity at night was ∼70% of the maximum daytime level: 16.6 and 23.7 pkat/g fresh weight at night and day, respectively; Figure 2B), whereas methyl benzoate emission decreased at least eight times (2.85 μg·flower−1·hr−1 at 3 pm and 0.35 μg·flower−1·hr−1 at 12 am; Figure 2A). Although BAMT activity decreased only slightly at night and was not sufficient to account for all of the changes in emission, it still showed a strong correlation with methyl benzoate emission (Figures 2A and 2B). An increase of the maximum amplitude in 4-day-old flowers relative to 3-day-old flowers (Figure 2) reflects the developmental changes in methyl benzoate emission and BAMT activity (Dudareva et al., 2000).
Figure 2.
Emission of Methyl Benzoate and BAMT Activity in Snapdragon Flowers during Two Normal Light/Dark Cycles.
(A) Rhythmic emission of methyl benzoate from 3- and 4-day-old snapdragon flowers during a 48-hr period under normal light/dark conditions.
(B) BAMT activity in upper and lower petal lobes of 3- and 4-day-old snapdragon flowers during a 48-hr period. Maximum BAMT activity value, equal to 100%, was 6 pkat/flower or 23.7 pkat/g fresh weight.
An increase of the maximum amplitude in 4-day-old flowers relative to 3-day-old flowers reflects the developmental changes in methyl benzoate emission (A) and BAMT activity (B) (Dudareva et al., 2000). Shaded and light areas correspond to dark and light, respectively. Each graph represents the average of three independent experiments. Standard deviations are indicated by vertical bars.
Analysis of BAMT activity in plants transferred to continuous darkness for 72 hr revealed that constant dark had little effect on BAMT activity, which continued to display rhythmic variations similar to those observed in plants grown under light/dark cycles (Figures 3 and 2B). Although methyl benzoate emission decreased by 70% during the second cycle in the absence of environmental cues and was very low in the third dark cycle (Figure 1A), the level of BAMT activity remained high during 3 days in continuous darkness (Figure 3). Moreover, although BAMT activity was not directly light dependent, the highest level of activity was observed during the light period of the day/night cycle (Figure 2B) and the theoretical light period when plants were kept in constant dark (Figure 3). Furthermore, the oscillations in BAMT activity became more pronounced during the third cycle in free-running conditions (Figure 3). Similar fluctuations in BAMT activity were found when plants were kept in continuous light (data not shown).
Figure 3.
BAMT Activity in Snapdragon Flowers Exposed to Continuous Dark for 3 Days.
Intact flowers were grown in the normal day/night cycle (12-hr day/12-hr night) and then exposed to continuous dark for 3 days. Flowers were 4 days old when they were exposed to continuous dark. BAMT activity was measured in combined upper and lower petal lobes, tissue highly specialized in floral scent production (Dudareva et al., 2000) at the times indicated at bottom. The corresponding theoretical light/dark cycle is indicated at top by open bars (light period) and hatched bars (dark period). Maximum BAMT activity value, equal to 100%, was 6.21 pkat/flower or 24.7 pkat/g fresh weight. Each point is the average of three experiments. Standard deviations are indicated by vertical bars.
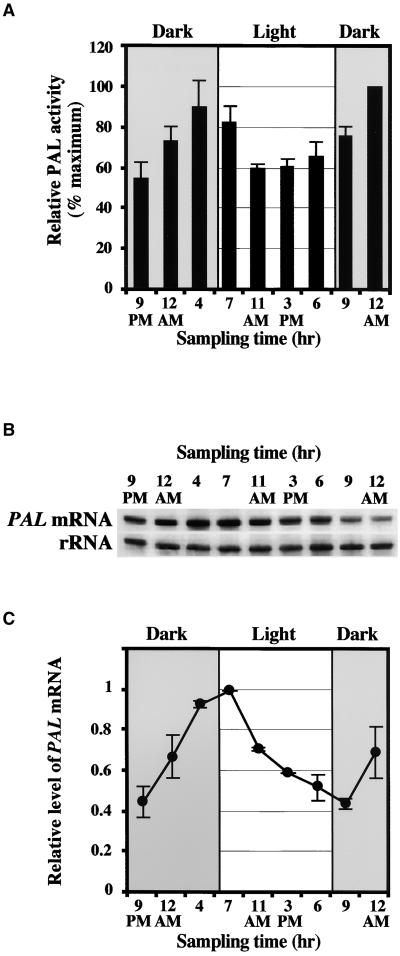
To determine whether these slight fluctuations of BAMT activity during a light/dark cycle are a function of the rhythmic regulation of BAMT gene expression, the accumulation of BAMT mRNA was examined by RNA gel blot hybridization. Total RNA was isolated from upper and lower petal lobes at nine time points during a 27-hr interval and hybridized with the BAMT probe (Figure 4A). BAMT expression levels were quantified and normalized for any loading differences, and the relative values are shown in Figure 4B. The changes in the BAMT mRNA levels were more pronounced than were the changes in BAMT activity. The level of BAMT mRNA increased during the light period, and maximum BAMT transcript accumulation was detected at approximately 3 to 6 pm; the level then declined at least twofold during the dark period (4 am), showing an oscillation pattern. This cyclic profile of transcript accumulation correlated strongly with BAMT activity (Figure 4B) and methyl benzoate emission (Figure 2A), indicating pretranslational regulation of enzyme activity. The rhythmic pattern of BAMT mRNA accumulation was retained under continuous dark (Figure 4C), suggesting that the cyclic expression of BAMT is under circadian control.
Figure 4.
BAMT mRNA Expression in Petal Tissue (Upper and Lower Lobes) of 3-Day-Old Flowers during a Normal Light/Dark Cycle and in Continuous Dark.
(A) RNA gel blot analysis of steady state BAMT mRNA levels in snapdragon petals during a normal light/dark cycle. Total RNA was isolated from upper and lower petal lobes of 3-day-old flowers at the times indicated at top, and 5 μg of total RNA was loaded in each lane. The top gel represents the results of hybridization with a BAMT probe. Autoradiography was performed overnight. The blot was rehybridized with an 18S rRNA probe (bottom) to standardize samples.
(B) Correlation between BAMT activity and BAMT mRNA levels in upper and lower petal lobes during a normal light/dark cycle. Hatched bars show BAMT activity, and the line shows a plot of the variations in BAMT mRNA level. Maximum BAMT activity value, equal to 100%, was 5.25 pkat/flower or 20.8 pkat/g fresh weight. For BAMT mRNA, values were obtained by scanning RNA gel blots with a phosphorimager and corrected by standardizing for the amounts of 18S rRNA measured in the same runs. The maximum transcript level was taken as 1. Each point is the average of four different experiments (including the one shown in [A]). Standard errors are indicated by vertical bars.
(C) RNA gel blot analysis of steady state BAMT mRNA levels in snapdragon flowers exposed to continuous dark. Total RNA was isolated from upper and lower petal lobes of 5-day-old flowers exposed to the second constant dark cycle at the times indicated at top, and 5 μg of total RNA was loaded in each lane. The top gel represents the results of hybridization with a BAMT probe. Autoradiography was performed overnight. The blot was rehybridized with an 18S rRNA probe (bottom gel) to standardize samples. RNA gel blots were scanned with a phosphorimager, and values were used to generate a graph of fluctuation in relative BAMT mRNA levels during a 24-hr period of continuous darkness. Each point is the average of three independent experiments. Standard errors are indicated by vertical bars.
The low emission of methyl benzoate and high BAMT activity at night raises at least three questions. (1) Is methyl benzoate still being made by floral tissue at night and then released during the day? (2) Is benzoic acid involved in the regulation of rhythmic emission of methyl benzoate; could an absence of substrate (free benzoic acid) be the cause of low methyl benzoate emission at night? (3) Is benzoic acid present in the cell at night but unavailable to the BAMT protein because of different compartmentation?
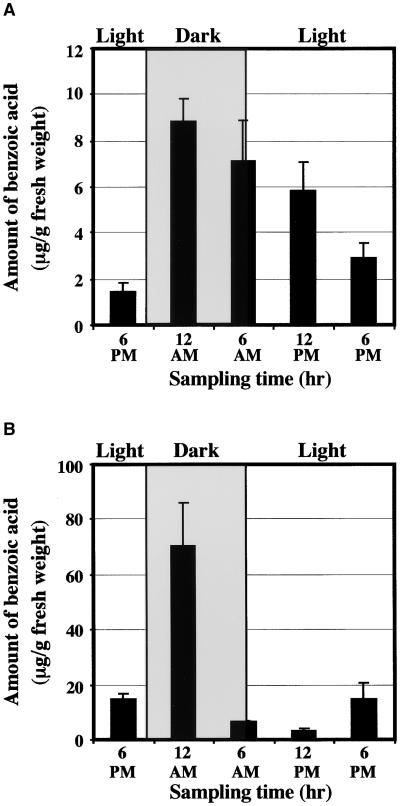
We did not find any accumulation of methyl benzoate within petal tissue at night. The amount of methyl benzoate determined by hexane extraction and gas chromatography–mass spectrometry (GC-MS) analyses was 0.9 μg/g fresh weigh at night and 1.6 μg/g fresh weight during the day. However, we did find oscillations in the level of benzoic acid during the daily light/dark cycle, with maximal and minimal accumulation during the day and night, respectively (Figure 5A). The level of benzoic acid was five times higher during the day (6 μg/g fresh weight) than at night (1.1 μg/g fresh weight), suggesting that benzoic acid is involved in the regulation of the rhythmic emission of methyl benzoate. To determine whether the level of benzoic acid is under circadian control, we analyzed the endogenous pool of benzoic acid in petal tissue of snapdragon flowers that were transferred to continuous darkness for 48 hr. Figure 5B shows that the petal concentration of benzoic acid continued to cycle under free-running conditions, indicating that the supply of substrate in the cell is regulated by a circadian clock.
Figure 5.
Oscillation of the Amount of Benzoic Acid in Upper and Lower Lobes of Snapdragon Petals during a Normal Light/Dark Cycle and in Continuous Dark.
(A) Diurnal changes in the amount of benzoic acid in upper and lower lobes of snapdragon petals. Benzoic acid was extracted from upper and lower petal lobes of 3-day-old snapdragon flowers grown under a normal light/dark cycle at the times indicated at bottom by supercritical carbon dioxide extraction and analyzed by HPLC. Maximum benzoic acid value, equal to 100%, was 6 μg/g fresh weight. Shaded and light areas correspond to dark and light periods, respectively.
(B) Oscillation of the amount of benzoic acid in snapdragon flowers exposed to continuous dark for 3 days. Intact flowers were grown in the normal day/night cycle (12-hr day/12-hr night) and then exposed to continuous dark for 2 days. Flowers were 4 days old when they were exposed to continuous dark. Benzoic acid was extracted from upper and lower petal lobes of snapdragon flowers at the times indicated at bottom by supercritical carbon dioxide extraction and analyzed by HPLC. The corresponding theoretical light/dark cycle is indicated at top by open bars (light period) and hatched bars (dark period).
Each graph represents the average of three independent experiments. Standard deviations are indicated by vertical bars.
How Is the Oscillation of the Endogenous Pool of Benzoic Acid Regulated during the Daily Light/Dark Cycle?
The low amount of benzoic acid at night could be attributed to a low rate of benzoic acid biosynthesis at night or to esterification of benzoic acid to benzoyl glucose. To date, the exact biochemical pathway leading to benzoic acid biosynthesis in plants remains unclear. Benzoic acid can be synthesized either via a β-oxidation pathway with formation of four CoA ester intermediates or, alternatively, via nonoxidative side chain cleavage with benzaldehyde as a key intermediate (Jarvis et al., 2000). However, the first committed step in both pathways is the conversion of l-phenylalanine to trans-cinnamic acid by l-phenylalanine ammonia–lyase (PAL; EC 4.3.1.5). To determine whether the rhythmic accumulation of benzoic acid is a function of the rhythmic regulation of PAL and/or other enzymes catalyzing the subsequent steps in the benzoic acid pathway, the levels of PAL activity and PAL mRNA expression were analyzed in the upper and lower petals of 3-day-old snapdragon flowers during the daily light/dark cycle. PAL activity peaked just before the dark-to-light transition and remained relatively high during the light period (60 to 70% of the maximum level; Figure 6A). In the same time-course experiments, samples were collected for isolation of total RNA, and PAL mRNA levels during the cycle were analyzed by RNA gel blot hybridization (Figure 6B). The PAL cDNA clone, used as a probe, was obtained through an ongoing snapdragon expressed sequence tag project from a petal-specific library constructed from mRNA isolated from petals (upper and lower lobes) of 1- to 5-day-old snapdragon flowers, tissue highly specialized for floral scent biosynthesis (Dudareva et al., 2000). Additionally, we checked the tissue specificity of this clone and found that it is expressed only in upper and lower lobes of snapdragon flowers; no detectable signals were found in pistils, stamens, sepals, leaves, or tubes (data not shown).
Figure 6.
PAL Activity and PAL mRNA Expression in Petal Tissue (Upper and Lower Lobes) of 3-Day-Old Flowers during a Normal Light/Dark Cycle.
(A) PAL activity in upper and lower petal lobes during a normal light/dark cycle. Maximum PAL activity value, equal to 100%, was 72.3 pkat/flower or 287 pkat/g fresh weight. Shaded and light areas correspond to dark and light periods, respectively. Each point is the average of three independent experiments. Standard deviations are indicated by vertical bars.
(B) RNA gel blot analysis of steady state PAL mRNA levels in snapdragon petals during a normal light/dark cycle. Total RNA was isolated from upper and lower petal lobes of 3-day-old flowers at the times indicated at top, and 5 μg of total RNA was loaded in each lane. The top gel represents the results of hybridization with a PAL probe. Autoradiography was performed overnight. The blot was rehybridized with an 18S rRNA probe (bottom gel) to standardize samples.
(C) Plot of the variations in PAL mRNA levels in upper and lower petal lobes during a normal light/dark cycle. Values were obtained by scanning RNA gel blots (including the one shown in [B]) with a phosphorimager and corrected by standardizing for the amounts of 18S rRNA measured in the same runs. The maximum transcript level was set at 1. Each point is the average of three independent experiments (including the one shown in [B]). Standard errors are indicated by vertical bars. Shaded and light areas correspond to dark and light periods, respectively.
The normalized values for PAL mRNA levels during the light/dark cycle are shown in Figure 6C. The level of PAL mRNA increased markedly before dawn, between 4 and 7 am, and then began to decline, returning to its basal level after 24 hr (at 9 pm). This oscillation pattern of PAL mRNA expression correlates strongly with the PAL activity profile (Figures 6A and 6C), indicating that enzyme activity is regulated at the level of gene expression. Moreover, the rhythmic pattern of PAL mRNA accumulation was retained under continuous dark (data not shown), suggesting that the cyclic expression of PAL is under the control of the circadian clock.
To determine whether esterification is involved in the regulation of the benzoic acid pool during the daily light/dark cycle, benzoic acid extracts obtained at 4 am (the lowest level; night samples) and 6 pm (the highest level; day samples) were subjected to saponification (1 N NaOH for 30 min at room temperature) to release free acid from its ester forms (CoA thioesters and/or glucose esters). Our results revealed only a slight increase (<10%) in the amount of free benzoic acid in day samples and an increase of up to twofold in night samples. This suggests that only a small pool of conjugated benzoic acid exists in snapdragon cells during the day, which increases at night.
Are Similar Molecular Mechanisms Involved in the Regulation of Circadian Methyl Benzoate Emission in Other Plant Species?
It has been shown that methyl benzoate is one of the most abundant scent compounds emitted from Nicotiana suaveolens flowers and that its night emission is under the control of the circadian clock, although the results were not quantitative (Loughrin et al., 1991). To compare the regulation of the rhythmic emission of methyl benzoate in different plant species, we first performed a quantitative analysis of the emission of this volatile ester from Nicotiana suaveolens flowers during two normal light/dark cycles. The daily maximum of methyl benzoate emission from tobacco flowers occurred from 12 midnight to 6 am, just before dawn (Figure 7A). We also found that Petunia cv Mitchell flowers emitted methyl benzoate with maximum emission around midnight (Figure 8A). To determine whether similar molecular mechanisms are involved in the regulation of diurnal and nocturnal methyl benzoate emission, we checked BAMT activity in tobacco (Figure 7B) and petunia (Figure 8B) flowers. The levels of BAMT activity in both species were high during the day, although methyl benzoate was emitted predominantly during the night. Moreover, as in snapdragon flowers (Figure 2B), the fluctuations in BAMT activity during daily light/dark cycles displayed a rhythmic nature (Figures 7B and 8B).
Figure 7.
Rhythmic Emission of Methyl Benzoate and BAMT Activity in Nicotiana suaveolens Flowers during Two Normal Light/Dark Cycles.
(A) Rhythmic emission of methyl benzoate from 1- and 2-day-old tobacco flowers during a 48-hr period under normal light/dark conditions. Headspace collections were performed at seven time points during a 24-hr period. Day 1 for tobacco flowers began at 6 pm, when flowers began to open. Each point is the average of three independent experiments. Standard errors are indicated by vertical bars.
(B) BAMT activity in petal tissue of 1- and 2-day-old tobacco flowers during a 48-hr period. Maximum BAMT activity value, equal to 100%, was 4.98 pkat/flower or 110 pkat/g fresh weight. Each point is the average of two independent experiments. Standard errors are indicated by vertical bars.
An increase of the maximum amplitude in 2-day-old flowers relative to 1-day-old flowers reflects the developmental changes in methyl benzoate emission (A) and BAMT activity (B) (N. Kolosova and N. Dudareva, personal communication). Shaded and light areas correspond to dark and light periods, respectively.
Figure 8.
Rhythmic Emission of Methyl Benzoate, BAMT, and PAL Activities in Petunia cv Mitchell Flowers during Two Normal Light/Dark Cycles.
(A) Rhythmic emission of methyl benzoate from 1- and 2-day-old petunia flowers during a 48-hr period under normal light/dark conditions. Headspace collections were performed at seven time points during a 24-hr period. Each point is the average of four independent experiments. Standard errors are indicated by vertical bars.
(B) BAMT activity in petal tissue of 1- and 2-day-old petunia flowers during a 48-hr period. Maximum BAMT activity value, equal to 100%, was 22.1 pkat/flower or 72.6 pkat/g fresh weight. Each point is the average of five independent experiments. Standard errors are indicated by vertical bars.
(C) PAL activity in petal tissue of 1- and 2-day-old petunia flowers during a 48-hr period. Maximum PAL activity value, equal to 100%, was 68.5 pkat/flower or 225 pkat/g fresh weight. Each point is the average of four independent experiments. Standard errors are indicated by vertical bars.
An increase of the maximum amplitude in 2-day-old flowers relative to 1-day-old flowers reflects the developmental changes in methyl benzoate emission (A), BAMT (B), and PAL (C) activities (N. Kolosova and N. Dudareva, personal communication). Shaded and light areas correspond to dark and light periods, respectively.
The level of benzoic acid was analyzed at five time points during a daily light/dark cycle in 1-day-old tobacco and petunia flowers (Figure 9). The amount of benzoic acid in petal tissue of both species was highest at night (12 am), decreased during the day, and correlated with nocturnal rhythmicity in methyl benzoate emission (Figures 7A and 8A). Although ∼10-fold differences in methyl benzoate emission during a daily light/dark cycle were found in tobacco and petunia (Figures 7A and 8A), changes in day and night levels of benzoic acid were more pronounced in petunia flowers. The 23-fold difference in the amount of free benzoic acid during night and day (70 and 3 μg/g fresh weight at night and day, respectively; Figure 9B) was sufficient to explain the changes in emission in petunia flowers. However, in tobacco flowers, the pool of free benzoic acid changed only threefold during the night/day cycle (Figure 9A). Because Nicotiana suaveolens flowers exhibited a nocturnal rhythm of opening and closing, the day/night changes in petal water potential might affect methyl benzoate production.
Figure 9.
Diurnal Changes in the Amount of Benzoic Acid in Petal Tissue of (A) Amount in Nicotiana suaveolens flowers. (B) Amount in Petunia cv Mitchell flowers.
Benzoic acid was extracted from petal tissue of 1-day-old flowers grown under a normal light/dark cycle, at the times indicated at bottom, by supercritical carbon dioxide extraction and analyzed by HPLC. Shaded and light areas correspond to dark and light periods, respectively. Each point is the average of three independent experiments. Standard errors are indicated by vertical bars.
The amount of benzoic acid in petunia increased twofold after saponification of night samples with 1 N NaOH and increased only 40% after treatment of day samples with 1 N NaOH. These results indicate that the 23-fold difference in the amount of free benzoic acid during night and day most likely is the result of differences in benzoic acid biosynthesis and not the esterification process. When PAL activity was measured in petunia flowers during two daily light/dark cycles, the oscillation pattern was observed with maximum activity at night, approximately 9 pm, several hours ahead of maximum methyl benzoate emission and benzoic acid accumulation (Figures 8C and 9B).
DISCUSSION
Circadian Nature of Methyl Benzoate Emission in Snapdragon Flowers
Methyl benzoate is one of the major scent components of bee-pollinated snapdragon flowers, and its emission is subject to diurnal changes that coincide with the foraging activity of bumblebees (Heinrich, 1979; Dudareva et al., 2000). Diurnal rhythmicity in the emission of volatile compounds by plants was reported to be noncircadian and controlled by irradiation levels (Altenburger and Matile, 1990; Jakobsen and Olsen, 1994; Jakobsen et al., 1994). In the present study, we have shown that the rhythmic emission of methyl benzoate displays a free-running cycle in the absence of environmental cues (in continuous dark or continuous light) (Figures 1A and 1B), indicating the circadian nature of emission. Inversion of the photoperiod by extension of one 12-hr-dark period results in the shifting of the phase of methyl benzoate emission (Figure 1C), as expected for circadian rhythmicity. These data reveal that the oscillations in methyl benzoate emission in snapdragon fulfill the criteria established previously for circadian rhythmicity, which include (1) oscillation with circadian periodicity, (2) persistence in constant conditions with an ∼24-hr period, and (3) an induced phase shift in cycling under a changed daily light/dark cycle (Thomas and Vince-Prue, 1997; Johnson et al., 1998; Somers, 1999). Our results show that circadian, diurnal rhythmicity in the emission of floral volatile compounds exists not only in flowers of Rosa hybrida cv Honesty (Helsper et al., 1998) but also in snapdragon.
Regulation of Circadian Methyl Benzoate Emission in Snapdragon Flowers
Analysis of BAMT activity in upper and lower petal lobes of snapdragon flowers during two daily light/dark cycles revealed only slight variations in enzyme activity that display a clear rhythmic pattern (Figure 2B). High BAMT activity with low concomitant emission of methyl benzoate at night (Figure 2), as well as in continuous darkness (Figures 1A and 3), suggests that BAMT activity is not an oscillation-determining factor and that important control might occur at the level of substrate availability for the reaction. Because benzoic acid is the direct precursor of methyl benzoate (Dudareva et al., 2000; Murfitt et al., 2000), the endogenous pool of benzoic acid was measured during a daily light/dark cycle. Our data show that the endogenous pool of benzoic acid exhibits a cycle with the highest level during the day and the lowest level at night (Figure 5A), which is maintained in continuous dark (Figure 5B). Diurnal fluctuation of benzoic acid levels strongly correlates with methyl benzoate emission (Figure 2A), suggesting that, similar to emission during the life span of the flower (Dudareva et al., 2000), circadian rhythmic emission of methyl benzoate in snapdragon is controlled by both the level of supplied substrate and, to a lesser extent, BAMT activity.
Rhythmic fluctuations in the level of benzoic acid during a daily light/dark cycle may be a function of the rhythmic regulation of PAL and/or other enzymes catalyzing the subsequent steps in benzoic acid biosynthesis or the esterification of benzoic acid to benzoyl glucose. When PAL activity was measured in upper and lower petal lobes of 3-day-old snapdragon flowers during the day/night cycle, it displayed a rhythmic oscillation that was out of phase with BAMT activity by almost 12 hr (Figures 2B and 6A). The highest PAL activity was found just before dawn, whereas BAMT activity peaked at 3 to 6 pm (Figure 2B).
Very often, enzyme activity and protein levels exhibit less marked variations than corresponding mRNA pools during the 24-hr period (Piechulla and Gruissem, 1987; Galangau et al., 1988; Carpenter et al., 1994). When BAMT and PAL mRNA levels were analyzed in snapdragon flowers during a 24-hr period (Figures 4A, 4B, 6B, and 6C), rhythmic oscillations were found in transcript levels that were more pronounced than were fluctuations in the corresponding enzyme activity. These oscillations continued in constant dark (Figure 4C; data not shown for PAL mRNA), suggesting that a free-running internal circadian clock regulates the BAMT and PAL mRNA abundances.
Recently, using DNA microarray technology, it was found that only a small proportion of genes are cycling. In Arabidopsis, for example, the number of genes that exhibit circadian changes in steady state mRNA levels varies from 2% (Schaffer et al., 2001) to 6% of almost 8000 unique genes (Harmer et al., 2000). In addition, 11% of the genes showed a diurnal expression pattern (Schaffer et al., 2001). Clustering of cycling genes based on their expression profiles revealed a number of representative expression patterns (Schaffer et al., 2001). Our results show that in snapdragon, PAL and BAMT belong to a group of cycling genes, and their gene expression follows different oscillation patterns. While PAL mRNA accumulates to high levels by ∼7 am (Figures 6B and 6C), the accumulation of BAMT transcripts occurs between 3 and 6 pm (Figures 4A and 4B). The BAMT gene does not exhibit the pattern of modulation typical for genes whose transcription is controlled by light, in which expression occurs immediately after light exposure with sunrise and high levels of mRNAs are reached in the subsequent hours. On the other hand, PAL gene expression shows a profile typical for light-dependent genes with high expression in the morning (Figures 6B and 6C). Moreover, PAL gene expression in snapdragon is similar to that in Arabidopsis, in which the PAL mRNA transcript level peaks before dawn and is under the control of the circadian clock, as determined by high-density oligonucleotide microarrays (Harmer et al., 2000).
Although PAL is involved in benzoic acid biosynthesis, it does not appear to closely reflect the fluctuations observed in benzoic acid during the day/night cycle in snapdragon flowers. The peak level of PAL mRNA and PAL activity occurs several hours before maximum benzoic acid accumulation and methyl benzoate emission (Figures 2A, 5A, and 6). This lag phase of several hours seems to be required for the subsequent steps of benzoic acid biosynthesis to occur and to increase the pool of benzoic acid. PAL also supplies precursors for anthocyanin production, but anthocyanin biosynthesis occurs in the early stages of flower development during the formation of colored flowers, especially during the period of petal cell expansion (Jackson et al., 1992). Therefore, throughout the period of flower development used in our research (3 to 6 days after anthesis), it is possible that PAL activity may be directed mostly to benzoic acid biosynthesis. This notion is also supported by the absence of PAL mRNA expression in the tube, a petal tissue that has little involvement in the scent production of snapdragon flowers (Dudareva et al., 2000). In most plant species, PAL is encoded by a multigene family (Appert et al., 1994; Wanner et al., 1995); however, the number of different forms of PAL in the snapdragon genome remains unknown. Although we found rhythmic fluctuations in PAL activity and circadian oscillations in PAL mRNA levels, not much is known at present about other enzymes involved in the benzoic acid biosynthetic pathway.
A positive correlation between transcript accumulations and corresponding enzyme activities indicates that oscillations in BAMT and PAL during a day/night cycle are regulated at the level of gene expression. Circadian regulation of gene expression can occur at different levels, including transcription (Millar and Kay, 1991; Liu et al., 1996), transcript abundance (Fujiwara et al., 1996; Zheng et al., 1998), translation (Mittag et al., 1994), and post-translational processing (Nimmo, 1998). The day/night oscillation observed for the steady state levels of BAMT and PAL transcripts may be caused by either transcriptional regulation or differential (day/night) post-transcriptional stability of these messages. However, in most cases in which a day/night modulation in mRNA has been reported, the regulation occurs at the transcriptional level (for review, see Kreps and Kay, 1997).
We do not exclude the possibility that the glucosylation of benzoic acid to benzoyl glucose also may play a role in regulating the levels of free benzoic acid in cells. The involvement of glucosylation in salicylic acid metabolism was shown in Tobacco mosaic virus–inoculated tobacco leaves (Enyedi and Raskin, 1993; Lee and Raskin, 1999; Chong et al., 2001). A UDP-glucose:salicylic acid glucosyltransferase (EC 2.4.1.35) and its corresponding cDNA clone recently were isolated and purified from tobacco leaves 6 hr after salicylic acid infiltration (Lee and Raskin, 1999). This enzyme catalyzes the conversion of salicylic acid to glycosyl salicylate (an ester form) and, to a lesser extent, salicylic acid 2-O-β-d-glucoside and uses benzoic acid as the most favored substrate (Lee and Raskin, 1999). When expression of a putative homolog of salicylic acid glucosyltransferase obtained from a snapdragon petal-specific cDNA library through an ongoing expressed sequence tag project was analyzed during a daily light/dark cycle, very weak hybridization signals were obtained after 15 days of exposure. Although the signals were very weak, quantification of these results showed the highest expression at night (C.M. Kish and N. Dudareva, personal communication). This finding is consistent with our data showing that the esterification process might be involved in the regulation of the endogenous pool of benzoic acid, but it plays only a minor role in our system.
Similar Molecular Mechanisms Are Involved in the Control of the Rhythmic Emission of Methyl Benzoate in Nocturnally and Diurnally Emitting Plants
When BAMT activity was analyzed in Nicotiana suaveloens and Petunia cv Mitchell flowers, which emit methyl benzoate at night, only slight variations were found in enzymatic activity during the daily day/night cycle, similar to those observed in snapdragon flowers (Figures 7B and 8B). Snapdragon and tobacco flowers showed their highest levels of BAMT activity and methyl benzoate emission at approximately 3 to 6 pm and 12 to 4 am, respectively (Figures 2 and 7), whereas in petunia, BAMT peaked at approximately 3 to 9 pm, which is ∼6 hr before the peak of methyl benzoate emission (12 to 4 am) (Figures 8A and 8B). High levels of BAMT activity in tobacco and petunia plants during the day with low concomitant emission of methyl benzoate suggest that in both nocturnally and diurnally (snapdragon) emitting plants, BAMT activity is not an oscillation-determining factor. The level of benzoic acid in tobacco and petunia flowers changes rhythmically during the daily light/dark cycle, with maximum levels at night, and shows correlation with methyl benzoate emission (Figures 7A, 8A, and 9). These data clearly indicate that, as in snapdragon, the level of benzoic acid in petal tissue plays a major role in the control of methyl benzoate circadian emission. PAL activity in petunia more closely reflects methyl benzoate emission than it does in snapdragon and peaks at the beginning of the dark phase, at approximately 9 pm, only 3 hr before maximum emission (Figure 8C). There also was a strong positive correlation between steady state PAL mRNA and activity levels in petunia (data not shown), indicating that, as in snapdragon, PAL is regulated during the day/night cycle at the level of gene expression. However, unlike in snapdragon flowers and Arabidopsis (Harmer et al., 2000), the PAL activity and mRNA levels in petunia did not show the oscillation pattern typical for light-regulated genes.
Overall, our results suggest that, at least in tobacco, petunia, and snapdragon flowers, the rhythmic production and emission of methyl benzoate is regulated primarily by the level of substrate, which in turn could be regulated at the level of expression of genes encoding the key enzymes of benzoic acid biosynthesis. Knowledge about the biochemical pathway(s) leading to benzoic acid in snapdragon and petunia flowers will provide further information regarding their roles in the regulation of the rhythmic emission of methyl benzoate.
METHODS
Plant Material, Growth Conditions, Headspace Collection, and Gas Chromatography
Snapdragon flowers (Antirrhinum majus cv Maryland True Pink; Ball Seed Co., Chicago, IL), petunia (Petunia hybrida cv Mitchell; Ball Seed Co.), and tobacco (Nicotiana suaveolens; North Central Regional Plant Introduction Station, United States Department of Agriculture, Ames, IA) were grown under standard greenhouse conditions, as described previously (Dudareva et al., 2000). For the headspace collections of floral volatile compounds, plants were placed in growth chambers (model E8; Conviron, Asheville, NC) at 25°C, 40% relative humidity, and a 12-hr photoperiod. Light intensity was 350 μmol·m−2·sec−1 for a normal day/night cycle and 100 μmol·m−2·sec−1 for the experiments in continuous light. Volatile compounds emitted from snapdragon, petunia, and tobacco flowers were determined by headspace analysis, as described previously (Raguso and Pichersky, 1995; Raguso and Pellmyr, 1998) at seven time points during a 24-hr period. Trapped floral scent compounds were analyzed by gas chromatography–mass spectrometry (GC-MS) with a FinniganMAT GCQ instrument (Thermoquest, San Jose, CA), as described previously (Dudareva et al., 2000), and by GC with an Agilent 6890 Series Gas Chromatograph (Agilent Technologies, Wilmington, DE) using an HP-5 5% phenyl methyl siloxane column (30.0 m × 320 μm × 0.25 μm). Column temperature was held at 40°C for 3 min and then increased to 180°C at 8°C min−1. Quantification was based on flame ionization detector peak areas and the internal standard. Ten microliters of a 0.03% (v/v) solution of toluene in hexane was added to each sample as an internal standard. Specific correction factors were developed from the methyl benzoate (Aldrich, Milwaukee, WI) standard curve and applied to peak areas of methyl benzoate in samples.
Enzyme Assays
For enzyme assays, petal tissue was disrupted by grinding with liquid nitrogen in a prechilled mortar. The ground material was suspended in an extraction buffer (5:1 [v/w] buffer/tissue) containing 50 mM Bis-Tris-HCl, pH 6.9, 10 mM β-mercaptoethanol, 5 mM Na2S2O5, 1% (w/v) polyvinylpyrrolidone, 1 mM phenylmethylsulfonyl fluoride, and 10% (v/v) glycerol and additionally homogenized in a chilled glass homogenizer. The slurry was centrifuged for 10 min to produce a supernatant that contained enzyme activity. S-Adenosyl-l-methionine: benzoic acid carboxyl methyltransferase (BAMT) assays for snapdragon were performed as described previously (Dudareva et al., 2000). BAMT assays for petunia and tobacco were performed using 34 μM S-methyl-14C-adenosyl-l-methionine (58.5 mCi/mmol; DuPont–New England Nuclear Life Science Products, Boston, MA) and 0.34 mM S-adenosyl-l-methionine in assay buffer (50 mM Tris-HCl, pH 7.5, and 3 mM β-mercaptoethanol) containing 4 mM benzoic acid and 0.5 mM EDTA in conditions described previously (Dudareva et al., 2000).
l-Phenylalanine ammonia–lyase (PAL) activity was determined by measuring the formation of U-14C-cinnamic acid from U-14C-l-phenylalanine, as described previously (Gang et al., 2001) with some modifications. The standard reaction mixture consisted of 0.1 M sodium borate, pH 8.8, 2.4 μM U-14C-l-phenylalanine (460 mCi/mmol; Sigma, St. Louis, MO), 2 mM l-phenylalanine, and protein extract.
The assay of each enzyme activity at each time point was run in duplicate on at least three independent enzyme preparations. The standard reaction mixture (100 μL) included 20 μL of crude extract containing 30 to 60 μg of protein as determined by the Bradford method (Bradford, 1976) using the Bio-Rad (Hercules, CA) protein reagent and BSA as a standard. Fifty microliters of the radioactively labeled products were counted in a liquid scintillation counter (model LS 3801; Beckman, Fullerton, CA). The raw data (counts per minute) were converted to picokatals (picomoles of product produced per second) based on the specific activity of the substrate and the efficiency of counting. Boiled assays, substrateless assays, and assays containing all reaction components without protein added were used as controls to measure the background radioactivity and possible endogenous activity.
Extraction and Quantification of Methyl Benzoate in Snapdragon Flower Petals
Freshly excised snapdragon flower petals were weighed and then ground to powder in liquid nitrogen. Petal powder was suspended in HPLC-grade hexane (1:3 [w/v] tissue/hexane), and samples were shaken (150 rpm) overnight at room temperature. Extracts were filtered through 0.2-μm nylon filters (Nalgene, Rochester, NY) to eliminate insoluble debris and then concentrated to 200 μL. The samples were analyzed by GC-MS in conditions described previously (Dudareva et al., 2000). The mass spectrometer scanned from 41 to 400 atomic mass units. Components were first identified through a computer database containing several thousand mass spectra and confirmed by comparison of retention times and mass spectra of authentic standards. For quantitative determination, 10 μL of a 0.03% (v/v) solution of toluene in hexane was added to each sample as an internal standard.
Extraction and Quantification of Endogenous Benzoic Acid
Benzoic acid was extracted from snapdragon, tobacco, and petunia petal tissues by supercritical carbon dioxide extraction, as described previously (Dudareva et al., 2000). Samples for HPLC analysis were prepared as described previously (Dudareva et al., 2000). Twenty-five-microliter samples were injected, and the compounds were separated on a C18 reversed phase HPLC column (Discovery C18, 5 μm; 4.6 mm × 25 cm; Supelco, Bellefonte, PA) maintained at room temperature. Benzoic acid was separated during a 25-min gradient of acetonitrile as described previously (Graham, 1991). Under these conditions, the retention time for benzoic acid was 15 min and the limit of detection was 3 μg/mL (75 ng of benzoic acid per injection). Solutions containing 3 to 240 μg/mL authentic benzoic acid were used to prepare the calibration curve. All data were corrected for benzoic acid recovery, as determined using internally spiked samples.
To determine whether the conjugated benzoic acid was present in petal tissue, extracts were dried, resuspended in the same amount of water, and saponified with 1 N NaOH for 30 min at room temperature, as described previously (Chong et al., 2001). After neutralization, samples were analyzed by HPLC as described above.
RNA Isolation and RNA Gel Blot Analysis
Total RNA from upper and lower petals of 3-day-old snapdragon flowers was isolated and analyzed as described previously (Dudareva et al., 1996, 1998, 2000) at nine time points during the daily light/dark cycle and at eight time points in continuous dark conditions. A 1.3-kb EcoRI fragment containing the coding region of the BAMT gene and a 2.6-kb EcoRI fragment containing the PAL coding region were used as probes for RNA gel blot analysis. Five micrograms of total RNA were loaded in each lane. Quantification of hybridization levels was performed using a Storm 860 phosphorimager (Molecular Dynamics, Sunnyvale, CA). The BAMT and PAL mRNA transcript levels were normalized to rRNA levels to overcome error in RNA quantitation by spectrophotometry. The values for each point were normalized further to the highest value within an experiment, which was defined as 1.0, and then plotted as relative RNA level. Statistical analysis was performed using Excel (Microsoft, Redmond, WA).
Acknowledgments
We thank the North Central Regional Plant Introduction Station, United States Department of Agriculture (Ames, IA) for supplying Nicotiana suaveolens germplasm. We thank Florence Negre for assistance in the supercritical extraction of benzoic acid. This work was supported by National Science Foundation Grant IBN-9904910 and by grants from the Fred Gloeckner Foundation. This article is Contribution 16504 from the Purdue University Agricultural Experimental Station.
References
- Altenburger, R., and Matile, P. (1988). Circadian rhythmicity of fragrance emission in flowers of Hoya carnosa R. Br. Planta 174, 248–252. [DOI] [PubMed] [Google Scholar]
- Altenburger, R., and Matile, P. (1990). Further observations on rhythmic emission of fragrance in flowers. Planta 180, 194–197. [DOI] [PubMed] [Google Scholar]
- Appert, C., Logemann, E., Hahlbrock, K., Schmid, J., and Amrhein, N. (1994). Structural and catalytic properties of the four phenylalanine ammonia-lyase isoenzymes from parsley (Petroselinum crispum Nym.). Eur. J. Biochem. 225, 491–499. [DOI] [PubMed] [Google Scholar]
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Carpenter, C.D., Kreps, J.A., and Simon, A.E. (1994). Genes encoding glycine-rich Arabidopsis thaliana proteins with RNA-binding motifs are influenced by cold treatment and an endogenous circadian rhythm. Plant Physiol. 104, 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, J., Pierrel, M.-A., Atanossova, R., Werck-Reichert, D., Fritig, B., and Saindrenan, P. (2001). Free and conjugated benzoic acid in tobacco plants and cell cultures: Induced accumulation upon elicitation of defense responses and role as salicylic acid precursors. Plant Physiol. 125, 318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, H.E.M. (1994). Floral volatiles in insect biology. In Insect–Plant Interactions, Vol. 5, E. Bernays, ed (Boca Raton, FL: CRC Press), pp. 47–81.
- Dudareva, N., and Pichersky, E. (2000). Biochemical and molecular aspects of floral scents. Plant Physiol. 122, 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva, N., Cseke, L., Blanc, V.M., and Pichersky, E. (1996). Evolution of floral scent in Clarkia: Novel patterns of S-linalool synthase gene expression in the C. breweri flower. Plant Cell 8, 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudareva, N., D'Auria, J.C., Nam, K.H., Raguso, R.A., and Pichersky, E. (1998). Acetyl CoA:benzyl alcohol acetyltransferase–an enzyme involved in floral scent production in Clarkia breweri. Plant J. 14, 297–304. [DOI] [PubMed] [Google Scholar]
- Dudareva, N., Piechulla, B., and Pichersky, E. (1999). Biogenesis of floral scent. Hortic. Rev. 24, 31–54. [Google Scholar]
- Dudareva, N., Murfitt, L.M., Mann, C.J., Gorenstein, N.L., Kolosova, N., Kish, C.M., Bonham, C., and Wood, K. (2000). Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 12, 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann, W., and Johnsson, A. (1998). Rhythms in organ movement. In Biological Rhythms and Photoperiodism in Plants, P.J. Lumsden and A.J. Millar, eds (Oxford, UK: BIOS Scientific Publishers), pp. 35–50.
- Enyedi, A.J., and Raskin, I. (1993). Induction of UDP-glucose:salicylic acid glucosyltransferase activity in tobacco mosaic virus–inoculated tobacco (Nicotiana tabacum) leaves. Plant Physiol. 101, 1375–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejes, E., and Nagy, F. (1998). Molecular analysis of circadian clock–regulated gene expression in plants: Features of the ‘output’ pathways. In Biological Rhythms and Photoperiodism in Plants, P.J. Lumsden and A.J. Millar, eds (Oxford, UK: BIOS Scientific Publishers), pp. 99–118.
- Fujiwara, S., Ishida, N., and Tsuzuki, M. (1996). Circadian expression of the carbonic anhydrase gene, Cah1, in Chlamydomonas reinhardtii. Plant Mol. Biol. 32, 745–749. [DOI] [PubMed] [Google Scholar]
- Galangau, F., Daniel-Vedele, F., Moureaux, T., Dorbe, M.-F., Leydecker, M.-T., and Caboche, M. (1988). Expression of leaf nitrate reductase genes from tomato and tobacco in relation to light-dark regimes and nitrate supply. Plant Physiol. 88, 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gang, D.R., Wang, J., Dudareva, N., Nam, K.H., Simon, J., Lewinsohn, E., and Pichersky, E. (2001). An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol. 125, 539–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, T.L. (1991). A rapid, high resolution high performance liquid chromatography profiling procedure for plant and microbial aromatic secondary metabolites. Plant Physiol. 95, 584–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer, S.L., Hogenesch, J.B., Straume, M., Chang, H.-S., Zhu, T., Wang, X., Kreps, J.A., and Kay, S.A. (2000). Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290, 2110–2113. [DOI] [PubMed] [Google Scholar]
- Heinrich, B. (1979). Bumblebee Economics. (Cambridge, MA: Harvard University Press).
- Helsper, J.P.F.G., Davies, J.A., Bouwmeester, H.J., Krol, A.F., and van Kampen, M.H. (1998). Circadian rhythmicity in emission of volatile compounds by flowers of Rosa hybrida L. cv. Honesty. Planta 207, 88–95. [Google Scholar]
- Jackson, D., Roberts, K., and Martin, C. (1992). Temporal and spatial control of expression of anthocyanin biosynthetic genes in developing flowers of Antirrhinum majus. Plant J. 2, 425–434. [Google Scholar]
- Jakobsen, H.B., and Olsen, C.E. (1994). Influence of climatic factors on rhythmic emission of volatiles from Trifolium repens L. flowers in situ. Planta 192, 365–371. [Google Scholar]
- Jakobsen, H.B., Friis, P., Nielsen, J.K., and Olsen, C.E. (1994). Emission of volatiles from flowers and leaves of Brassica napus in situ. Phytochemistry 37, 695–699. [Google Scholar]
- Jarvis, A.P., Schaaf, O., and Oldham, N.J. (2000). 3-Hydroxy-3-phenylpropanoic acid is an intermediate in the biosynthesis of benzoic acid and salicylic acid but benzaldehyde is not. Planta 212, 119–126. [DOI] [PubMed] [Google Scholar]
- Johnson, C.H., Knight, M., Trewavas, A., and Kondo, T. (1998). A clockwork green: Circadian programs in photosynthetic organisms. In Biological Rhythms and Photoperiodism in Plants, P.J. Lumsden and A.J. Millar, eds (Oxford, UK: BIOS Scientific Publishers), pp. 1–34.
- Kolosova, N., Sherman, D., Karlson, D., and Dudareva, N. (2001). Cellular and subcellular localization of S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methylbenzoate in snapdragon flowers. Plant Physiol. 126, 956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreps, J.A., and Kay, S.A. (1997). Coordination of plant metabolism and development by the circadian clock. Plant Cell 9, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H., and Raskin, I. (1999). Purification, cloning, and expression of a pathogen inducible UDP-glucose:salicylic acid glucosyltransferase from tobacco. J. Biol. Chem. 274, 36637–36642. [DOI] [PubMed] [Google Scholar]
- Liu, Z., Taub, C.C., and McClung, C.R. (1996). Identification of an Arabidopsis thaliana ribulose-1,5-bisphosphate carboxylase/oxygenase activase (RCA) minimal promoter regulated by light and the circadian clock. Plant Physiol. 112, 43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughrin, J.H., Hamilton-Kemp, T.R., Anderson, R.A., and Hildebrand, D.F. (1990). Volatiles from flowers of Nicotiana sylvestris, N. otophora and Malus domestica: Headspace components and day/night changes in their relative concentrations. Phytochemistry 29, 2473–2477. [Google Scholar]
- Loughrin, J.H., Hamilton-Kemp, T.R., Anderson, R.A., and Hildebrand, D.F. (1991). Circadian rhythm of volatile emission from flowers of Nicotiana sylvestris and N. suaveolens. Physiol. Plant. 83, 492–496. [Google Scholar]
- Loughrin, J.H., Hamilton-Kemp, T.R., Burton, H.R., Anderson, R.A., and Hildebrand, D.F. (1992). Glycosidically bound volatile components of Nicotiana sylvestris and N. suaveolens flowers. Phytochemistry 31, 1537–1540. [Google Scholar]
- Matile, P., and Altenburger, R. (1988). Rhythms of fragrance emission in flowers. Planta 174, 242–247. [DOI] [PubMed] [Google Scholar]
- Millar, A.J., and Kay, S.A. (1991). Circadian control of cab gene transcription and mRNA accumulation in Arabidopsis. Plant Cell 3, 541–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittag, M., Lee, D.-H., and Hastings, W. (1994). Circadian expression of the luciferin-binding protein correlates with the binding of a protein to the 3′ untranslated region of its mRNA. Proc. Natl. Acad. Sci. USA 91, 5257–5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murfitt, L.M., Kolosova, N., Mann, C.J., and Dudareva, N. (2000). Purification and characterization of S-adenosyl-l-methionine:benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methyl benzoate in flowers of Antirrhinum majus. Arch. Biochem. Biophys. 382, 145–151. [DOI] [PubMed] [Google Scholar]
- Nielsen, J.K., Jakobsen, H.B., Hansen, P.F.K., Moller, J., and Olsen, C.E. (1995). Asynchronous rhythms in the emission of volatiles from Hesperis matronalis flowers. Phytochemistry 38, 847–851. [Google Scholar]
- Nimmo, H.G. (1998). Circadian regulation of a plant protein kinase. Chronobiol. Int. 15, 109–118. [DOI] [PubMed] [Google Scholar]
- Pichersky, E., Raguso, R.A., Lewinsohn, E., and Croteau, R. (1994). Floral scent production in Clarkia (Onagraceae). I. Localization and developmental modulation of monoterpene emission and linalool synthase activity. Plant Physiol. 106, 1533–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechulla, B. (1988). Plastid and nuclear mRNA fluctuations in tomato leaves: Diurnal and circadian rhythms during extended dark and light periods. Plant Mol. Biol. 11, 345–353. [DOI] [PubMed] [Google Scholar]
- Piechulla, B. (1989). Changes of the diurnal and circadian (endogenous) mRNA oscillations of the chlorophyll a/b binding protein in tomato leaves during altered day/night (light/dark) regimes. Plant Mol. Biol. 12, 317–327. [DOI] [PubMed] [Google Scholar]
- Piechulla, B., and Gruissem, W. (1987). Diurnal mRNA fluctuations of nuclear and plastid genes in developing tomato fruits. EMBO J. 6, 3593–3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguso, R.A., and Pellmyr, O. (1998). Dynamic headspace analysis of floral volatiles: A comparison of methods. OIKOS 81, 238–254. [Google Scholar]
- Raguso, R.A., and Pichersky, E. (1995). Floral volatiles from Clarkia breweri and C. concinna (Onagraceae): Recent evolution of floral scent and moth pollination. Plant Syst. Evol. 194, 55–67. [Google Scholar]
- Schaffer, R., Landgraf, J., Accerbi, M., Simon, V., Larson, M., and Wisman, E. (2001). Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis. Plant Cell 13, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, F.P., Ayasse, M., Paulus, H.F., Erdmann, D., and Francke, W. (1997). Variation of floral scent emission and post pollination changes in individual flowers of Ophrys sphegodes subsp. Sphegodes. J. Chem. Ecol. 23, 2881–2895. [Google Scholar]
- Somers, D.E. (1999). The physiology and molecular bases of the plant circadian clock. Plant Physiol. 121, 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, B., and Vince-Prue, D. (1997). Photoperiodism in Plants. In Photoperiodic Timekeeping, B. Thomas and D. Vince-Prue, eds (San Diego, CA: Academic Press), pp. 29–62.
- Tollsten, L. (1993). A multivariate approach to post-pollination changes in the floral scent of Platanthera bifolia (Orchidaceae). Nord. J. Bot. 13, 495–499. [Google Scholar]
- Wang, J., Dudareva, N., Bhakta, S., Raguso, R.A., and Pichersky, E. (1997). Floral scent production in Clarkia breweri (Onagraceae). II. Localization and developmental modulation of the enzyme S-adenosyl-l-methionine:(iso)eugenol O-methyl transferase and phenylpropanoid emission. Plant Physiol. 114, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner, L.A., Li, G., Ware, D., Somssich, I.E., and Davis, K.R. (1995). The phenylalanine ammonia-lyase gene family in Arabidopsis thaliana. Plant Mol. Biol. 27, 327–338. [DOI] [PubMed] [Google Scholar]
- Webb, A.A.R. (1998). Stomatal rhythms. In Biological Rhythms and Photoperiodism in Plants, P.J. Lumsden and A.J. Millar, eds (Oxford, UK: BIOS Scientific Publishers), pp. 69–79.
- Zheng, C.C., Porat, R., Lu, P.Z., and O'Neill, S.D. (1998). PNZIP is a novel mesophyll-specific cDNA that is regulated by phytochrome and a circadian rhythm and encodes a protein with a leucine zipper motif. Plant Physiol. 116, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]