Abstract
Reelin and glutamic acid decarboxylase (GAD)67 expressed by cortical γ-aminobutyric acid-ergic interneurons are down-regulated in schizophrenia. Because epidemiological studies of schizophrenia fail to support candidate gene haploinsufficiency of Mendelian origin, we hypothesize that epigenetic mechanisms (i.e., cytosine hypermethylation of CpG islands present in the promoter of these genes) may be responsible for this down-regulation. Protracted l-methionine (6.6 mmol/kg for 15 days, twice a day) treatment in mice elicited in brain an increase of S-adenosyl-homocysteine, the processing product of the methyl donor S-adenosyl-methionine, and a marked decrease of reelin and GAD67 mRNAs in both WT and heterozygous reeler mice. This effect of l-methionine was associated with an increase in the number of methylated cytosines in the CpG island of the reelin promoter region. This effect was not observed for GAD65 or neuronal-specific enolase and was not replicated by glycine doses 2-fold greater than those of l-methionine. Prepulse inhibition of startle declined at a faster rate as the prepulse/startle interval increased in mice receiving l-methionine. Valproic acid (2 mmol/kg for 15 days, twice a day) reverted l-methionine-induced down-regulation of reelin and GAD67 in both WT and heterozygous reeler mice, suggesting an epigenetic action through the inhibition of histone deacetylases. The same dose of valproate increased acetylation of histone H3 in mouse brain nearly 4-fold. This epigenetic mouse model may be useful in evaluating drug efficacy on schizophrenia vulnerability. Hence the inhibition of histone deacetylases could represent a pharmacological intervention mitigating epigenetically induced vulnerability to schizophrenia in individuals at risk.
Studies of heterozygous reeler mice (HRM) have provided preliminary evidence of a relationship between reelin haploinsufficiency, the decrease of dendritic spine expression density in frontal cortex (FC) pyramidal neurons and associated neuropil hypoplasticity, the down-regulation of glutamic acid decarboxylase (GAD)67 expression, and the decrease in γ-aminobutyric acid (GABA) turnover (1–3). Similar neurochemical and structural abnormalities were detected in the FC of schizophrenia postmortem brains (4–9). Hence, HRM may be a model to evaluate the efficacy of novel treatments for schizophrenia by monitoring drug actions on (i) reelin and GAD67 mRNA expression, (ii) GABA turnover, and (iii) cortical neuropil plasticity including dendritic spine expression.
The HRM model several aspects of the molecular neuropathology expressed in schizophrenia, although the mechanisms operative in these pathologies may be different. In fact, demographic studies of schizophrenia inheritance in identical twins show a concordance of ≈50%, which supports an epigenetic model but not gene haploinsufficiency of Mendelian origin. Investigation of a putative epigenetic mechanism attending schizophrenia vulnerability may therefore be in order (10). Along this line of thinking we have hypothesized that a protracted treatment with l-methionine results in an epigenetic hypermethylation of the CpG islands located in a number of gene-promoter regions probably including reelin and GAD67 (11). The selection of reelin as the target for an epigenetic promoter hypermethylation model of schizophrenia vulnerability received support from the finding that hypermethylation of the reelin promoter CpG island down-regulates reelin expression (11) and by evidence that reelin plays an important role in (i) embryonic corticogenesis (12) and (ii) the regulation of adult rodent brain dendritic spine expression density (2, 3, 10).
In looking for an approach to induce promoter CpG-island hypermethylation in mice, we were attracted by several reports on the aggravation of schizophrenia symptoms elicited by a 2-week treatment of these patients with high daily doses (20–40 g) of l-methionine (13). Between 1961 and 1971, 10 studies were published evaluating whether a treatment with l-methionine, a precursor in the biosynthesis of S-adenosyl-methionine (SAM), mitigated any of the symptoms of schizophrenia (for a review see ref. 13). It was disappointing that every one of the 10 studies reported that l-methionine exacerbates the symptoms of schizophrenia (13). Interestingly, the study by Antun et al. (14) excluded that l-methionine caused a “chronic brain syndrome” or other forms of neurotoxicity, theoretically linking the increased brain methylation to the exacerbation of schizophrenia symptoms. Therefore we tested whether by increasing brain SAM content with l-methionine we could decrease the expression of brain GAD67 and reelin mRNAs and proteins by changing the number of 5-methylcytosines in the CpG islands surrounding the promoters of these genes. In WT mice (WTM) treated with l-methionine we did not expect to observe significant behavioral changes, because in nonpsychiatric patients l-methionine treatment was asymptomatic (13). Thus, the experiments with methionine included HRM, in addition to the WTM, because these reelin-haploinsufficient mice exhibit molecular, neuroanatomical, and behavioral changes reminiscent of those found in schizophrenia (refs. 1 and 15 and J. Larson, J. S. Hoffman, A.G., and E.C., unpublished data).
In eukaryotes, gene expression can be regulated by promoter methylation, which is associated with histone tail acetylation and deacetylation (16). A new pharmacological approach used in cancer therapy consists of the use of histone deacetylase (HDAC) inhibitors to elicit histone hyperacetylation and thereby reduce the promoter hypermethylation-induced silencing of oncosuppressor genes (17). Valproate, a drug that is widely used in neuropsychiatry as an anticonvulsant and mood stabilizer and now is gaining clinical consensus as a coadjutant of antipsychotic efficacy in schizophrenia therapy, was shown recently to be an inhibitor of HDAC (18). Here, we have administered valproate in doses that were able to induce histone hyperacetylation and increase reelin and GAD67 expression in brain of WTM and HRM. Moreover, in mice receiving protracted l-methionine treatment, the coadministration of valproate was able to revert the l-methionine-induced decrease of reelin and GAD67 expression.
Materials and Methods
Drug Administration Schedule in WTM and HRM.
A colony of HRM (B6C3Fe strain, The Jackson Laboratory) was established in our laboratory. These mice express a normal and a defective reelin allele with a deletion of ≈150 kb at the 3′ end of the gene (Edinburg mutation) (19). The offspring were PCR-genotyped as described (15). WTM and HRM (60-day-old males), randomly sampled from several contemporaneous litters, received s.c. administrations of l-methionine (1 g/kg, 6.6 mmol/kg), glycine (1 g/kg, 13 mmol/kg), valproate (300 mg/kg, 2 mmol/kg), or the combination of l-methionine and valproate dissolved in bicarbonate solution (0.1 ml/10 g of body weight) for a period of 15 consecutive days (twice daily). Twelve hours after the last injection the animals were killed, and their brains were immediately frozen at −80°C until processing. The doses and schedule of l-methionine administration were selected initially based on schizophrenia patient studies that used up to 3.7 mmol/kg l-methionine administered daily for 2–4 weeks. In preliminary experiments we demonstrated that s.c. injections of l-methionine in doses ranging from 1.65 to 13.2 mmol/kg produced a dose-related increase of SAM and its metabolite S-adenosyl-homocysteine (SAH) with a maximal response at a dose of 6.6 mmol/kg (see Fig. 2B). To establish the dose of valproate required to block HDAC activity effectively in the brain we measured by Western blot the relative content of acetylated histone H3 and H4 with specific antibodies. Valproate (2 mmol/kg, s.c.) produced a maximal increase (4-fold) of acetylated histone H3 2 h after injection (see Fig. 5A).
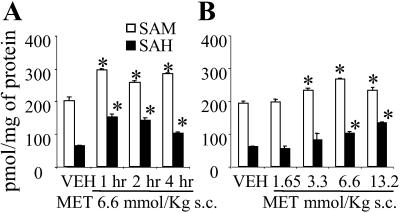
Fig 2.
Increase of SAM and SAH in the cortex of WTM receiving l-methionine (MET). (A) Time course. (B) Dose response was measured 2 h after s.c. injection of l-methionine. Each value is the mean ± SE of four mice. ANOVA P < 0.001 among vehicle-treated (VEH) and l-methionine-treated groups. *, P < 0.05 for comparisons of l-methionine-treated vs. vehicle-treated mice (Bonferroni test).
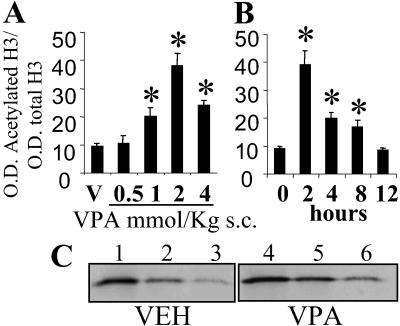
Fig 5.
Acetylated histone H3 nuclear content is increased in FC of WTM treated with valproate. (A) Dose response 2 h after valproate treatment. (B) Time course with 2 mmol/kg valproate. Values are expressed as mean ± SE, n = 3. *, P < 0.01, Student's t test. (C) Western immunoblot of acetylated histone H3 in one vehicle-treated mouse (VEH, lanes 1–3) and one valproate-treated mouse (VPA, 2 mmol/kg, 2 h before; lanes 4–6).
RNA Extraction and Quantitative RT-PCR Analysis.
Total RNA was extracted from brain samples (FC) as described (20).
We selected the FC, defined as the area of neocortex 2 mm anterior to the bregma (21), because in this area it was determined that a decrease in reelin expression is associated with a decrease in dendritic spines (1).
Reelin, GAD67, GAD65, and neuronal-specific enolase (NSE) mRNA content were measured by RT-PCR with internal standards generated by site-directed mutagenesis to introduce a BglII (reelin, GAD67, and NSE) or XbaI (GAD65) restriction endonuclease site midway between the amplification primers (6, 15, 22). The following amplification primers were used: reelin (forward, base pairs 9,211–9,234; reverse, base pairs 9,549–9,572; GenBank accession no. HSU79716; these primers were designed for the 3′ domain of the reelin mRNA, and they were effective in measuring the full-length mRNA of the normal allele but not the truncated mRNA of the mutated allele), GAD67 (forward, base pairs 1,855–1,878; reverse, base pairs 2,246–2,269; GenBank accession no. M818830), GAD65 (forward, base pairs 82–103; reverse, base pairs 507–532; GenBank accession no. M72422), and NSE (forward, base pairs 328–379; reverse, base pairs 792–815; GenBank accession no. M22349).
Western Blot Analysis.
Mouse FC samples were homogenized directly in Laemmli buffer (100 μl/10 mg of tissue) as described (23). The extracted reelin protein and other immunoreactive products were separated by 6% SDS/PAGE and blotted overnight onto Hybond ECL nitrocellulose membranes (Amersham Pharmacia). The membrane blots were blocked for 1 h at room temperature with 3% nonfat dry milk in PBS (10 mM PBS, pH 7.4) and then reacted with G-10 anti-Reln mAb, diluted 1:5,000, for 6 h at 25°C (G-10 anti-reelin mAb is directed against amino acid residues 164–496 in the amino terminus of reelin and was a generous gift of A. M. Goffinet, University of Namur Medical School, Brussels). The immunoreactive bands were detected by using a peroxidase-conjugated goat anti-mouse IgG (Sigma) at 1:1,000 for 1 h followed by the application of the ECL Plus chemiluminescence Western blotting kit (Amersham Pharmacia). The immunoreactive bands were analyzed by chemiluminescent detection by using the Blue Fluorescence/Chemifluorescence Storm system (Molecular Dynamics) with IMAGEQUANT analysis software (23).
Reelin-like immunoreactivity exhibited two distinct molecular forms: one of ≈400 kDa (presumably full-length reelin) and a second of ≈180 kDa (presumably representing a reelin metabolite or processing product). In addition, we detected a minor immunoreactive band of 320 kDa. The intensity of β-actin immunofluorescence was determined on the same blot with a β-actin mAb (1:3,000) (Clone AC-15, Sigma) and used in a comparative measurement of the amount of protein applied to the gels. As external standard, cerebellar extracts containing a known amount of reelin (measured against the reelin-H fusion protein used to prepare the G10 antibody) were applied to each gel. In each sample the relative quantity of reelin-like immunoreactive peptides was estimated from the external standard curve as fmol/mg of tissue (23). Histones were extracted from nuclei prepared as described by Yasmineh and Yunis (24). The tissue was homogenized in 0.32 M sucrose containing 5 mM MgCl2 and 0.2 mM CaCl2 (pH 7.4) and centrifuged at 1,000 × g for 10 min. The pellet then was resuspended in 2.2 M sucrose/5 mM MgCl2/0.2 mM CaCl2 and centrifuged at 15,000 × g for 1 h. The histones present in this nuclear pellet were resuspended in 0.4 M H2SO4, placed on ice for 30 min, and then ethanol-precipitated. The histone pellet was resuspended in Laemmli buffer, and aliquots were applied to a 10–20% gradient Tris-glycine gel (Invitrogen). After blotting, the membranes were incubated with antibodies directed against either histone H3 or H4 (total, 1:1,000) or their acetyl isoforms (1:3,000, Upstate Biotechnology, Lake Placid, NY), followed by incubation with peroxidase-conjugated anti-rabbit antibody (1:1,000, Amersham Pharmacia). The quantification was performed as described above, and the values are expressed as the ratio of the optical density (OD) of the acetylated histone H3 immunoreactivity to the OD of the total histone H3 immunoreactivity.
Bisulfite-Modified DNA Sequencing.
DNA (2 μg) specimens isolated from the FC tissues were digested with EcoRI. The DNA was denatured with 3 M NaOH by incubating at 37°C for 30 min, followed by 3 min of incubation at 95°C, and then placed briefly on ice. Subsequently, the DNA specimens were treated with 5 M sodium bisulfite, which converts deoxycytosine residues to uracil while leaving 5-methylcytosines unaltered (25, 26). Modified material then was amplified by PCR using nested primers designed specifically for the modified B strand of reelin promoter (outer primers, −722 bp, 5′-AAT TAA AAC CAC TAA CAA AAA ATC CCC AA-3′, and −27 bp, 5′-TTT GTG TGT TTT TTT ATT TAT TTT TGG AGG TAT-3′; inner primers, −636 bp, 5′-AAA ATT ACA ACC RAT ATA AAC AAA AAA CAA-3′, and −135 bp, 5′-AAG TTA GTG GGA GAT YGA GGT TTT-3′). The resulting PCR products were gel-purified, and 5′A overhangs were added by incubation with Taq polymerase and 0.2 mM dATP for 30 min at 70°C. Amplicons then were subcloned into pGEM T-Easy (Promega). Four clones from each subject were sequenced at the University of Illinois Core DNA Facility by using T7 long and SP6 primers with fluorescent dideoxy technology.
SAM and SAH Brain Levels.
Brain FC samples (≈50 mg wet weight) were homogenized in 0.4 M HClO4 containing trace amounts of [3H]SAM (≈2,000 cpm, ≈0.02 pmol each sample). After centrifugation the supernatant was applied to a reversed-phase HPLC column, Symmetry C18 4.6 × 250 mm (Waters). The column was equilibrated with 50 mM NaH2PO4 buffer (pH 3.0) containing 8 mM octanesulfonic acid sodium salt plus 12% methanol and was developed at a flow rate of 1 ml/min with a multistep gradient of methanol: 12% for 11 min, up to 25% in 0.5 min and kept at 25% for 28.5 min, up to 75% in 0.5 min and kept at 75% for 5 min, and down to 12% in 0.5 min to wash. SAM was eluted at ≈31 min and SAH at 25 min after sample injection.
Prepulse Inhibition of Startle (PPI).
The startle apparatus and experimental conditions used were the same as described (15). For the PPI procedure, each trial consisted of a 115-dB startle stimulus (30-msec duration) preceded by an 85-dB prepulse that was followed by a silent interval (offset of prepulse to onset of startle pulse) that was varied by trial. There were six possible trial types randomly presented (by block) such that each trial type was presented 12 times during the experiment. The six trial types were no prepulse, 40-msec interval, 70-msec interval, 100-msec interval, 140-msec interval, and 420-msec interval. The time between trials varied randomly and averaged 20 sec. Stimuli were presented over a constant 65-dB white noise background also present during a 5-min adaptation period. Mice were tested between 11:00 and 15:00 h. Peak amplitude with respect to baseline at stimulus onset was measured on each trial. Ratios reflecting the amount of inhibition were calculated by dividing the difference between the mean for the no-prepulse trials and the prepulse trial by the mean for the no-prepulse trials for each of the five interval trial types. PPI data are commonly presented as this ratio to normalize to the amplitude of the startle reflex itself. Locomotor activity was measured as described by Tueting et al. (15).
Statistical Analysis.
All results are expressed as mean ± SEM. Student's t test and one-way or two-way ANOVA, followed by the Bonferroni multiple comparison test, were used to assess the significance of the differences between groups. The criteria of significance (P < 0.05 or 0.01) are indicated in the figure legends.
For the PPI data, regression lines fitting of decline of PPI with the delay in the prepulse/startle interval were calculated for each animal according to the ANOVA test by using as criteria of significance P < 0.01. From each line the time required to reduce the intensity of PPI from 100% to 50% (t1/2) was calculated. Statistically significant differences between t1/2 of vehicle and l-methionine-treated mice were determined according to the Student's t test.
Results
L-Methionine-Induced Down-Regulation of Reelin and GAD67 Expression.
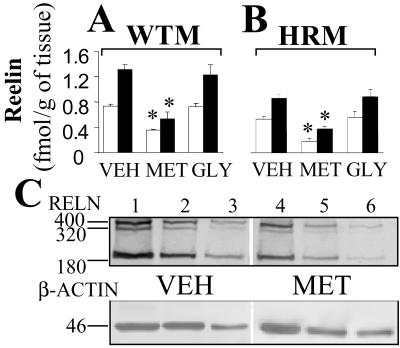
B6C3Fe mice (WTM) treated for 15 days with l-methionine (6.6 mmol/kg twice a day) show a down-regulation in the expression of both reelin and GAD67 mRNAs by ≈40% in the FC when compared with the vehicle-injected group (Table 1). As a positive control, a group of mice was injected twice a day with 13 mmol/kg glycine. This treatment failed to elicit any changes in reelin and GAD67 mRNA expression levels, indicating that the mechanism of l-methionine-induced down-regulation in the expression of both genes is probably related to the selective ability of l-methionine to increase brain levels of SAM. To verify the specificity of the treatment, in the FC of the same mice we studied the expression of two additional genes, GAD65 and NSE. l-methionine failed to change the FC mRNA levels of GAD65 and of NSE (Table 1). To understand whether the mRNA down-regulation induced by l-methionine was phenotypically relevant, we comparatively assayed the reelin protein levels in the FC of WTM receiving vehicle, l-methionine, or glycine. Fig. 1A shows that l-methionine-treated WTM express a significant decrease in the levels of both 400- and 180-kDa reelin immunoreactivity when compared with vehicle- and glycine-treated WTM. The down-regulation of reelin and GAD67 elicited by l-methionine in HRM was similar of that in WTM despite the HRM-deficient expression of these two genes (see Table 1 and Fig. 1B). A decrease of reelin and GAD67 mRNAs of similar magnitude was obtained with l-methionine treatment in FC of Swiss–Webster albino mice (n = 5, data not shown).
Table 1.
The down-regulation of reelin and GAD67 mRNAs induced by protracted l-methionine (MET) treatment is normalized by valproate (VPA) at doses that per se increase reelin and GAD67 mRNA expression in the FC of both WTM and HRM
| Mice | mRNA | VEH | MET | VPA | MET + VPA | GLY |
|---|---|---|---|---|---|---|
| WTM | Reelin | 168 ± 7.0 | 94 ± 5.0 | 260 ± 16 | 198 ± 14 | 175 ± 10 |
| GAD67 | 7.3 ± 0.38 | 3.9 ± 0.11 | 11.5 ± 1.4 | 7.5 ± 14 | 7.0 ± 0.15 | |
| NSE | 306 ± 46 | 320 ± 57 | 305 ± 56 | 299 ± 54 | 300 ± 40 | |
| GAD65 | 44 ± 4.0 | 45 ± 4.0 | 45 ± 5.0 | 43 ± 3.0 | 44 ± 5.0 | |
| HRM | Reelin | 102 ± 3.0 | 62 ± 4 | 164 ± 13 | 125 ± 12 | 110 ± 8.0 |
| GAD67 | 4.1 ± 0.21 | 2.6 ± 0.17 | 6.1 ± 0.62 | 4.7 ± 0.30 | 4.5 ± 0.35 | |
| NSE | 310 ± 56 | 300 ± 62 | 312 ± 60 | 320 ± 49 | 310 ± 50 | |
| GAD65 | 42 ± 5.0 | 40 ± 5.0 | 42 ± 6.0 | 41 ± 4.0 | 48 ± 10 |
l-Methionine (6.6 mmol/kg s.c.), valproate (2 mmol/kg s.c.), vehicle (VEH), 0.1 ml/10 g of body weight s.c.), or glycine (GLY) (13 mmol/kg s.c.) was administered twice a day for 15 days. mRNA was measured by quantitative RT-PCR, and the values were expressed in attomol/μg total RNA. Each value is the mean ± SE of 10 mice except for glycine (n = 5).
, Overall two-way ANOVA for reelin and GAD67 mRNA levels yielded P < 0.001;
, P < 0.05 for Bonferroni comparison between vehicle and l-methionine or vehicle and valproate;
, P < 0.05 for Bonferroni comparison between l-methionine and l-methionine + valproate.
Fig 1.
Protracted l-methionine treatment (6.6 mmol/kg, twice daily for 15 days) induced down-regulation of reelin protein expression in the FC of WTM (A) and HRM (B). Measurements were conducted after 15 days of treatment with vehicle (VEH), l-methionine (MET), or glycine (GLY) at the doses detailed in Table 1. Each value is the mean ± SE of five mice per group. The overall ANOVA (400- and 180-kDa proteins) of vehicle, l-methionine, and glycine groups yielded P < 0.001. Bonferroni multiple comparison: *, P < 0.05 for l-methionine vs. vehicle or glycine. The open and filled bars indicate 400- and 180-kDa reelin-like immunoreactive peptides, respectively. (C) Western immunoblot of reelin and β-actin after 6% SDS/PAGE. Comparison between a vehicle- (lanes 1–3, serial dilutions of the same sample) and an l-methionine-treated WTM (lanes 4–6).
SAM and SAH Levels After l-Methionine Treatment.
To relate the methionine treatment to the changes in reelin and GAD67 mRNA expression and to the methylation of these two gene promoters, we measured SAM levels in the FC of vehicle and l-methionine-treated WTM. SAM content was increased by 50% in mice injected with l-methionine (Fig. 2A). This increase persisted unabated for at least 4 h. SAH, the demethylated metabolite of SAM, was also increased (Fig. 2A). The maximal effective dose of l-methionine was 6.6 mmol/kg (Fig. 2B). A similar FC increase of SAH levels (from 63 ± 2 to 144 ± 24 pmol/mg protein, P < 0.01, Student's t test, n = 5) was expressed 4 h after the last injection in mice that received the l-methionine s.c. treatment twice daily for 15 days. FC SAM levels in these mice were also increased albeit not significantly (from 201 ± 13 to 223 ± 75 pmol/mg protein, n = 5), presumably reflecting a higher conversion rate of SAM into SAH during the protracted l-methionine treatment. Similar results were also obtained in HRM.
Evaluation of l-Methionine-Induced Hypermethylation of the Reelin Gene Promoter.
To evaluate whether the l-methionine-elicited decrease of reelin expression was related to the methylation of the promoter, the bisulfite-modified genomic DNA of the reelin promoter was sequenced in the region between −640 and −140 bp (Fig. 3A). This promoter region (27) is extremely rich in CpG dinucleotides and is a candidate CpG island, yielding a ratio (calculated with GENETOOL software, BioTool, Edmonton, Alberta, Canada) of the number of observed to the number of expected CpG dinucleotides higher than 0.6 (28).
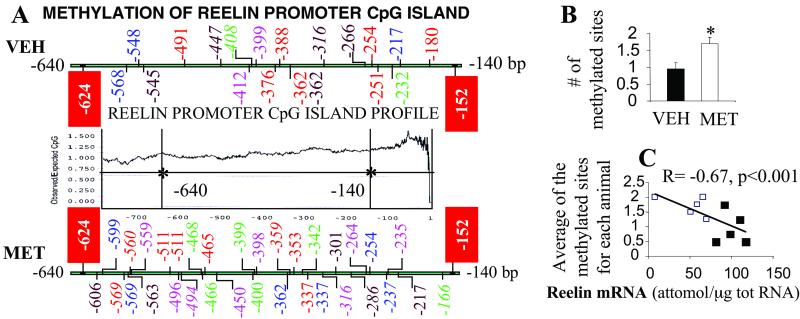
Fig 3.
Protracted l-methionine (MET) treatment induces a reelin promoter CpG island cytosine hypermethylation that is inversely correlated with reelin mRNA expression in FC. (A) Methylated sites in four clones obtained from each of the FC specimens of five HRM receiving vehicle (VEH) and five receiving l-methionine (see Table 1 for dose schedule). Each animal is indicated in a different color; the polymorphisms with respect to the Royaux et al. (27) sequence are indicated in italics. The sequenced region spans from −640 to −140 bp. This region is highly enriched in CpG dinucleotides as shown by the GENETOOL plot. The red boxes indicate two cytosine residues (−624 and −152 bp) that are methylated in nearly every clone (hot spots: −624, vehicle 75%, l-methionine 75%; −152, vehicle 97%, l-methionine 85%). (B) Number of methylated sites (expressed as mean ± SE) in the reelin CpG island of vehicle- and methionine-treated mice. *, P < 0.03, Student's t test (analysis performed without the hot spots). (C) Negative correlation (R = −0.67, P < 0.001) between the reelin promoter mean number of methylated sites and the level of reelin mRNA in the FC of HRM. White squares, l-methionine-treated HRM; black squares, vehicle-treated HRM (analysis was performed without hot spots).
The analysis was performed on genomic DNA extracted from the FC of five l-methionine- and five vehicle-treated HRM. At the 5′ and 3′ ends of the promoter region studied (−624, −152), we identified two CpG dinucleotides that were methylated (“hot spots”) in nearly every clone obtained from vehicle- and l-methionine-treated mice. However, as expected for a functional CpG island, the sequencing of vehicle-treated mice showed an overall CpG hypomethylation (Fig. 3 A and B). In contrast, the CpG-island region of l-methionine-treated mice showed a significant increase (≈2-fold) in the number of methylated cytosines (Fig. 3 A and B). Moreover, the mean of cytosine residues methylated for each animal and the levels of reelin mRNA were correlated (R = −0.67, P < 0.001; Fig. 3C).
L-Methionine Treatment Disrupts the PPI.
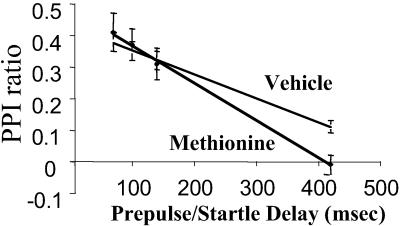
Decreased PPI is considered a reliable measure in both human and rodents of sensory gating deficit. We tested whether PPI was changed by protracted l-methionine administration. In Fig. 4 we show that in vehicle-treated WTM, PPI reached a maximum (ratio = 0.4) when an 85-dB prepulse was followed 70 msec later by a 115-dB startle stimulus and declined linearly to a ratio of ≈0.1 when the interval between prepulse and startle stimulus onset was delayed from 100 to 420 msec. There were no significant differences in PPI between mice receiving a protracted vehicle- or l-methionine-treatment when prepulse and startle stimuli were separated by an interval of 70 msec. However, the decline of PPI with increased time delay between prepulse and startle stimuli was ≈40% faster in l-methionine- than in vehicle-treated mice. In fact, the PPI ratio reached a value attending zero when the interval between prepulse and startle stimuli was delayed from 100 to 420 msec in l-methionine-treated mice.
Fig 4.
Protracted l-methionine treatment accelerates the decline of PPI occurring with increase of the delay in the prepulse/startle intervals. From the experimental data of each WT mouse, lines of regression were calculated statistically according to the ANOVA. Only lines of regression with a P < 0.01 were considered. From these lines, we calculated the time required in each animal to reduce by 50% (t1/2) the PPI observed at a prepulse/startle interval of 70 msec, which represents the maximal PPI value (ratio ≈ 0.4 in the majority of animals). Each point is the mean ± SE of seven animals. t1/2 values were 382 ± 35 msec for vehicle-treated and 225 ± 17 for l-methionine-treated mice (P < 0.001 Student's t test). Similar results were observed in HRM.
Body weight (B.W.) and locomotor activity (L.A.) levels did not differ between vehicle-treated (B.W., 27 ± 1 g; L.A., 2,099 ± 143 units/15 min; n = 10) and l-methionine-treated (B.W., 27 ± 1; L.A., 2,024 ± 358; n = 10) mice, which therefore should not account for differences in PPI responses. Similar results were observed in HRM.
Valproate, at Doses That Increase Acetylated Histone Content, Normalizes l-Methionine-Induced Reelin and GAD67 mRNA Down-Regulation.
Fig. 5 shows that in mice, s.c. injections of valproate (from 0.5 to 4 mmol/kg) produced in 2 h a dose-related increase of acetylated histone H3 FC content in the absence of changes in total H3 immunoreactivity. The increase was evident (two times control) at 1 mmol/kg (a dose that is used clinically as a maintenance dose in cancer therapy (29) and was maximal (four times control values) at a dose of 2 mmol/kg. At this dose the effect of valproate lasted for >8 h (Fig. 5B). This effect of 2 mmol/kg of valproate persisted unabated after repeated treatment for 15 days (twice daily) (vehicle 8.6 ± 0.3 and valproate 30.2 ± 4 ratio OD acetylated H3/total H3; n = 3; P < 0.005 Student's t test). The acetylation of histone H4 seemed to be stimulated only marginally by valproate (data not shown).
WTM and HRM treated for 15 days with valproate did not display gross behavioral abnormalities or weight change. WTM treated with valproate expressed ≈30% more reelin and GAD67 mRNAs in FC with respect to vehicle-treated mice without any change in GAD65 and NSE mRNAs (Table 1). Moreover, valproate administration normalized the l-methionine-induced down-regulation of reelin and GAD67 mRNAs (Table 1). Despite the already existing down-regulation of both reelin and GAD67 mRNAs, in HRM treated with valproate the expression of both mRNAs was increased. Moreover, as in WTM, valproate was effective in HRM in reverting the l-methionine-induced down-regulation of reelin and GAD67 mRNAs (Table 1).
A recent report (30) showed that valproate shares with lithium the capacity to modulate inositol homeostasis, suggesting that this common mechanism could be responsible for the overlapping clinical effects of these drugs. Lithium administered in doses of 5 meq/kg s.c. for 15 days to WTM [a dose able to increase in rats (31) the lithium plasma levels to the recommended therapeutic range], unlike valproate, failed to change FC reelin mRNA expression (lithium 192 ± 18 and vehicle 189 ± 20 attomol/μg of total RNA; n = 5; not significant).
Discussion
The results demonstrate that a protracted treatment with l-methionine but not with glycine down-regulates the phenotypic expression of reelin and GAD67 genes but not that of GAD65 and NSE genes in HRM, WT B6C3Fe mice, and WT Swiss–Webster albino mice.
The down-regulation of reelin expression induced by l-methionine administration was correlated with the number of methylated cytosine residues in the CpG island (−640 to −140) surrounding the promoter region of the cognate gene. The data on cytosine methylation of the reelin promoter have been obtained with DNA extracted from the FC, which includes several neural and glial cell types, and therefore this procedure may account for the different methylation profiles of different clones from the same animal. Additional studies are required to examine whether reelin and GAD67 DNAs extracted from laser-capture microdissected horizontal layer I GABAergic interneurons present a specific promoter CpG-methylation pattern and to study how such methylation modifies reelin and GAD67 transcription rates.
To obtain further indirect support for the concept that reelin and GAD67 mRNA expression are under an epigenetic control mediated by promoter hypermethylation, we attempted to revert the effect of l-methionine with valproate, an HDAC inhibitor (18). It is now established that histone tail hyperacetylation controls the expression of genes with CpG dinucleotide-rich promoter regions, possibly inducing a DNA demethylase activity in methyl CpG-binding proteins (16, 32), thereby indirectly reducing the degree of DNA methylation. Valproate is an antiepileptic, mood-stabilizing drug that at therapeutic doses increases the brain GABAergic tone (33) and expresses a significant acceleration of the onset of the beneficial action of antipsychotics in the treatment of schizophrenia and bipolar disorder patients with psychosis (34, 35). Recently it was reported that valproate, acting as an HDAC inhibitor, induces differentiation and reduces proliferation in cancer cells (29). Here we demonstrate that valproate administered to mice induces a dose-related brain histone H3 hyperacetylation but fails to modify H4 acetylation, suggesting that valproate is an effective HDAC inhibitor also in brain. Moreover, these doses of valproate normalize the down-regulation of reelin and GAD67 induced by protracted l-methionine treatment in both WTM and HRM, supporting the epigenetic hypermethylation hypothesis of the regulation of the expression of these two genes. Valproate given alone increased the expression of reelin and GAD67 but failed to change that of GAD65 and NSE in both WTM and HRM. Perhaps valproate elicits demethylation of reelin and GAD67 promoters in vehicle-treated animals also. However, alternative explanations may apply (for a review see ref. 29). Because Williams et al. (30) recently suggested that valproate shares with lithium the capacity to modify inositol homeostasis, we tested the effects of protracted lithium treatment (5 meq/kg s.c. for 15 days) on the expression of reelin mRNA. Lithium failed to change the levels of reelin mRNA, suggesting that valproate does not induce the demonstrated reelin and GAD67 up-regulation by acting through an inositol depletion.
It is very important to stress that in the hippocampus, cortex, and striatum but not in cerebellum reelin is expressed in GABAergic neurons, and these neurons secrete reelin into the extracellular matrix where it binds with picomolar affinity to integrin receptors expressed in dendritic spine postsynaptic densities of pyramidal neurons (10, 36). The binding of reelin seems to stimulate a rapamycin-sensitive translation of dendritic resident mRNAs (3, 10). It is understood now that the translation of dendritic resident mRNA plays a role in dendritic spine and neuropil plasticity, and thereby reelin might contribute to event-related dendritic spine structural plasticity that may be operative in memory and learning (ref. 37 and J. Larson, J. S. Hoffman, A.G., and E.C., unpublished data).
Along this line of thought we must emphasize that PPI, a sensory gating reflex that is decreased in schizophrenia, also declines at a faster rate in mice treated with l-methionine than in those treated with vehicle, suggesting that hypermethylation of promoters in GABAergic neurons may be operative in the pathogenesis of schizophrenia.
In closing we stress that these results do not elucidate the etiopathogenetic processes that may bring about an altered pattern of methylation of CpG islands of candidate genes in schizophrenia patients but do offer a mouse model to study the pharmacology of epigenetic hypermethylation of reelin and GAD67 promoters. We believe the down-regulation of reelin and GAD67 mRNAs in GABAergic neurons elicited by l-methionine treatment is mitigated by valproate via inhibition of HDACs.
There are 17 known molecular forms of HDACs (17). It will be important to establish which molecular forms are selectively expressed in GABAergic neurons of cortex, striatum, and cerebellum and whether other inhibitors of HDAC are more specific or more potent than valproate in inhibiting those deacetylases that are expressed in GABAergic neurons. A study of the action of l-methionine in the GABAergic neurons of striatum, hippocampus, and cerebellum and of the antagonism of this action by valproate may widen the utility of the mouse model described here.
Acknowledgments
We thank Dr. Floyd E. Bloom (Department of Neuropharmacology, The Scripps Research Institute, La Jolla, CA), Dr. Gabriella D'Arcangelo (Baylor College of Medicine, Houston), and Dr. Daniel R. Weinberger (Clinical Brain Disorders Branch, National Institutes of Health, Bethesda) for constructive criticism and suggestions in the preparation of the manuscript. This work was supported in part by National Institute of Mental Health Grants MH062188 (to A.G.), MH062090 (to E.C.), and MH62682-01 (to D.R.G.).
Abbreviations
HRM, heterozygous reeler mice
FC, frontal cortex
GAD, glutamic acid decarboxylase
GABA, γ-aminobutyric acid
SAM, S-adenosyl-methionine
WTM, WT mice
HDAC, histone deacetylase
SAH, S-adenosyl-homocysteine
NSE, neuronal-specific enolase
PPI, prepulse inhibition of startle
References
- 1.Liu W. S., Pesold, C., Rodriguez, M. A., Carboni, G., Auta, J., Lacor, P., Larson, J., Condie, B. G., Guidotti, A. & Costa, E. (2001) Proc. Natl. Acad. Sci. USA 98 3477-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa E., Davis, J., Grayson, D. R., Guidotti, A., Pappas, G. D. & Pesold, C. (2001) Neurobiol. Dis. 8 723-742. [DOI] [PubMed] [Google Scholar]
- 3.Costa E., Davis, J., Pesold, C., Tueting, P. & Guidotti, A. (2002) Curr. Opin. Pharmacol. 2 56-62. [DOI] [PubMed] [Google Scholar]
- 4.Akbarian S., Kim, J. J., Potkin, S. G., Hagman, J. O., Tafazzoli, A., Bunney, W. E., Jr. & Jones, E. G. (1995) Arch. Gen. Psychiatry 52 258-266. [DOI] [PubMed] [Google Scholar]
- 5.Selemon L. D. & Goldman-Rakic, P. S. (1999) Biol. Psychiatry 45 17-25. [DOI] [PubMed] [Google Scholar]
- 6.Guidotti A., Auta, J., Davis, J. M., DiGiorgi Gerevini, V., Dwivedi, Y., Grayson, D. R., Impagnatiello, F., Pandey, G., Pesold, C., Sharma, R., et al. (2000) Arch. Gen. Psychiatry 57 1061-1069. [DOI] [PubMed] [Google Scholar]
- 7.Glantz L. A. & Lewis, D. A. (2000) Arch. Gen. Psychiatry 57 65-73. [DOI] [PubMed] [Google Scholar]
- 8.Volk D. W., Austin, M. C., Pierri, J. N., Sampson, A. R. & Lewis, D. A. (2000) Arch. Gen. Psychiatry 57 237-245. [DOI] [PubMed] [Google Scholar]
- 9.Rosoklija G., Toomayan, G., Ellis, S. P., Keilp, J., Mann, J. J., Latov, N., Hays, A. P. & Dwork, A. J. (2000) Arch. Gen. Psychiatry 57 349-356. [DOI] [PubMed] [Google Scholar]
- 10.Costa E., Chen, Y., Davis, J., Dong, E., Noh, J. S., Tremolizzo, L., Veldic, M., Grayson, D. R. & Guidotti, A. (2002) Mol. Intervent. 2 47-57. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y., Sharma, R. P., Costa, R. H., Costa, E. & Grayson, D. R. (2002) Nucleic Acids Res. 30 2930-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Arcangelo G., Miao, G. G., Chen, S. C., Soares, H. D., Morgan, J. I. & Curran, T. (1995) Nature 374 719-723. [DOI] [PubMed] [Google Scholar]
- 13.Wyatt R. J., Benedict, A. & Davis, J. (1971) Schizophr. Bull. 4 10-44. [Google Scholar]
- 14.Antun F. T., Burnett, G. B., Cooper, A. J., Daly, R. J., Smythies, J. R. & Zealley, A. K. (1971) J. Psychiatr. Res. 8 63-71. [DOI] [PubMed] [Google Scholar]
- 15.Tueting P., Costa, E., Dwivedi, Y., Guidotti, A., Impagnatiello, F., Manev, R. & Pesold, C. (1999) NeuroReport 10 1329-1334. [DOI] [PubMed] [Google Scholar]
- 16.Robertson K. D. (2002) Oncogene 21 5361-5379. [DOI] [PubMed] [Google Scholar]
- 17.Johnstone R. W. (2002) Nat. Rev. Drug Discov. 1 287-299. [DOI] [PubMed] [Google Scholar]
- 18.Phiel C. J., Zhang, F., Huang, E. Y., Guenther, M. G., Lazar, M. A. & Klein, P. S. (2001) J. Biol. Chem. 276 36734-36741. [DOI] [PubMed] [Google Scholar]
- 19.D'Arcangelo G., Miao, G. G. & Curran, T. (1996) Brain Res. Mol. Brain Res. 39 234-236. [DOI] [PubMed] [Google Scholar]
- 20.Impagnatiello F., Pesold, C., Longone, P., Caruncho, H., Fritschy, J. M., Costa, E. & Guidotti, A. (1996) Mol. Pharmacol. 49 822-831. [PubMed] [Google Scholar]
- 21.Paxinos G. & Franklin, K. B. J., (1997) The Mouse Brain in Stereotaxic Coordinates (Academic, New York).
- 22.Grayson D. R., Bovolin, P. & Santi, R. M. (1993) Methods Neurosci. 12 191-208. [Google Scholar]
- 23.Lacor P. N., Grayson, D. R., Auta, J., Sugaya, I., Costa, E. & Guidotti, A. (2000) Proc. Natl. Acad. Sci. USA 97 3556-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasmineh W. G. & Yunis, S. J. (1974) in Methods in Cell Biology, ed. Prescott, D. M. (Academic, New York), Vol. VIII, pp. 151–177. [Google Scholar]
- 25.Clark S. J., Harrison, J., Paul, C. L. & Frommer, M. (1994) Nucleic Acids Res. 22 2990-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grunau C., Clark, S. J. & Rosenthal, A. (2001) Nucleic Acids Res. 29 E65., 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Royaux I., Lambert de Rouvroit, C., D'Arcangelo, G., Demirov, D. & Goffinet, A. M. (1997) Genomics 46 240-250. [DOI] [PubMed] [Google Scholar]
- 28.Gardiner-Garden M. & Frommer, M. (1987) J. Mol. Biol. 196 261-282. [DOI] [PubMed] [Google Scholar]
- 29.Blaheta R. A. & Cinatl, J., Jr. (2002) Med. Res. Rev. 22 492-511. [DOI] [PubMed] [Google Scholar]
- 30.Williams R. S., Cheng, L., Mudge, A. W. & Harwood, A. J. (2002) Nature 417 292-295. [DOI] [PubMed] [Google Scholar]
- 31.Gillin J. C., Hong, J. S., Yang, H. Y. & Costa, E. (1978) Proc. Natl. Acad. Sci. USA 75 2991-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervoni N., Detich, N., Seo, B., Chakravarty, D. & Szyf, M. (2001) J. Biol. Chem. 277 25026-25031. [DOI] [PubMed] [Google Scholar]
- 33.Loscher W. (1999) Prog. Neurobiol. 58 31-59. [DOI] [PubMed] [Google Scholar]
- 34.Tohen M., Chengappa, K. N., Suppes, T., Zarate, C. A., Jr., Calabrese, J. R., Bowden, C. L., Sachs, G. S., Kupfer, D. J., Baker, R. W., Risser, R. C., et al. (2002) Arch. Gen. Psychiatry 59 62-69. [DOI] [PubMed] [Google Scholar]
- 35.Muller-Oerlinghausen B., Retzow, A., Henn, F. A., Giedke, H. & Walden, J. (2000) J. Clin. Psychopharmacol. 20 195-203. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez M. A., Pesold, C., Liu, W. S., Kriho, V., Guidotti, A., Pappas, G. D. & Costa, E. (2000) Proc. Natl. Acad. Sci. USA 97 3550-3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weeber E. J., Beffert, U., Jones, C., Christian, J. M., Forster, E., Sweatt, J. D. & Herz, J. (2002) J. Biol. Chem. 277 39944-39952. [DOI] [PubMed] [Google Scholar]