Abstract
Wild deer are one of the important natural reservoir hosts of several species of Ehrlichia and Anaplasma that cause human ehrlichiosis or anaplasmosis in the United States and Europe. The primary aim of the present study was to determine whether and what species of Ehrlichia and Anaplasma naturally infect deer in Japan. Blood samples obtained from wild deer on two major Japanese islands, Hokkaido and Honshu, were tested for the presence of Ehrlichia and Anaplasma by PCR assays and sequencing of the 16S rRNA genes, major outer membrane protein p44 genes, and groESL. DNA representing four species and two genera of Ehrlichia and Anaplasma was identified in 33 of 126 wild deer (26%). DNA sequence analysis revealed novel strains of Anaplasma phagocytophilum, a novel Ehrlichia sp., Anaplasma centrale, and Anaplasma bovis in the blood samples from deer. None of these have been found previously in deer. The new Ehrlichia sp., A. bovis, and A. centrale were also detected in Hemaphysalis longicornis ticks from Honshu Island. These results suggest that enzootic cycles of Ehrlichia and Anaplasma species distinct from those found in the United States or Europe have been established in wild deer and ticks in Japan.
Human ehrlichioses (also called anaplasmoses), which are emerging potentially fatal infectious diseases, are caused by obligatory intracellular gram-negative bacteria in the family Anaplasmataceae (17, 42). Members of this family also cause economically devastating diseases such as heartwater and bovine anaplasmosis in livestock and diseases such as canine ehrlichiosis and Potomac horse fever in companion, military, and police working animals (12, 17, 28, 49). In the family Anaplasmataceae, five Ehrlichia species and five Anaplasma species are officially recognized (17). All of them are maintained in nature through an enzootic cycle between bloodsucking ticks and vertebrate hosts, primarily wild mammals. Vertebrate host infection is essential for them, since these bacteria are rarely transmitted vertically in ticks (7, 34). Seven Ehrlichia and Anaplasma species are recognized in the continent of North America (Ehrlichia chaffeensis, Ehrlichia canis, Ehrlichia ewingii, Anaplasma phagocytophilum, Anaplasma platys, Anaplasma marginale, and Anaplasma centrale) (3, 4, 30, 31, 37, 51). Thus far, only Ehrlichia chaffeensis, Ehrlichia ewingii, and Anaplasma phagocytophilum have been known to cause potentially fatal human diseases in the United States (9, 17, 42). In Europe, six Ehrlichia and Anaplasm species (A. phagocytophilum, E. chaffeensis, E. canis, A.platys, A. centrale, and A. marginale) have been reported (1, 8, 16, 18, 39, 45, 46, 53). However, human infection in Europe was molecularly confirmed only for A. phagocytophilum (8), but several serologically confirmed human monocytic and granulocytic ehrlichioses have been reported (8, 15, 57). In Africa, sixspecies (Ehrlichia ruminantium, E. canis, A. marginale, Anaplasma bovis, A. platys, and A. centrale) have been reported (2, 13, 29, 41, 50). Human ehrlichiosis caused by Ehrlichia sp. was serologically detected in Mali (58). In Asia, seven Ehrlichia and Anaplasm species (E. canis, E. chaffeensis, Ehrlichia muris, A. marginale, A. centrale, A. platys, and A. phagocytophilum) were reported (10, 11, 22, 24, 25, 27, 38, 59, 61). Human ehrlichiosis caused by trematode-borne Neorickettsia sennetsu in the family Anaplasmataceae has been reported in Japan and Malaysia (49), and serologic evidence of human infection with A. phagocytophilum and E. chaffeensis was reported from Korea (20, 43).
Wild mammals are the primary reservoirs of Ehrlichia and Anaplasma species infection. In the United States, wild deer are the primary reservoirs of E. chaffeensis and E. ewingii (3, 31, 60, 63, 64), and small rodents and deer are reservoirs of A. phagocytophilum (6, 31, 33, 56). In addition, an Anaplasma sp. called the white-tailed deer (WTD) agent (3, 31) infects wild deer. In Europe, A. phagocytophilum DNA has been detected in deer and varieties of other wild animal species (14, 40, 45, 47). In Asia, wild mammalian reservoirs have been identified for Ehrlichia muris and a newly characterized ‘Candidatus Neoehrlichia mikurensis’ in small wild rodents (23-25). Although several studies have reported the presence of A. phagocytophilum, E. chaffeensis, the HF strain closely related to E. chaffeensis, and Anaplasma bovis in ticks in Asia (10, 11, 27, 44, 52, 62), the natural mammalian reservoir of these species has not been identified in Asia. In the present study, we sought to determine whether and what types of Ehrlichia and Anaplasma species infect deer in Japan using multiple PCR, followed by sequencing of the PCR products. Here, we present the first evidence of Ehrlichia and Anaplasma species among naturally infected wild deer in Asia. These findings identify four species of Ehrlichia and Anaplasma, including a novel Ehrlichia species and novel A. phagocytophilum strains. Analysis of specimens from Hemaphysalis longicornis ticks associated with deer revealed the presence of three of these four species, implying the established deer-tick enzootic cycle.
MATERIALS AND METHODS
Deer and tick specimens.
Sera were collected from 126 wild deer for examination: samples from 79 deer (Cervus nippon yesoensis) from Hokkaido Island, northern Japan, collected during 1975, 1989, and 1991; and samples from 47 deer (Cervus nippon nippon) from Shimane Prefecture on Honshu Island, southwestern Japan, collected during 2001 and 2002. Both locations were within the Piedmont physiographic region and were approximately 1,500 km apart. Sixty-eight Hemaphysalis longicornis ticks were collected by flagging in Shimane Prefecture, Japan, in 1999. Twenty-five H. longicornis ticks were removed from deer (Cervus nippon nippon) in Nara Prefecture, Honshu Island, Japan, in 2004.
PCR.
DNA from the deer sera and homogenates of ticks was extracted using the QIAamp DNA Blood Mini Kit (QIAGEN, Valencia, CA). Extracted DNA was used as the template for nested PCR amplification of 16S rRNA genes. The primer pair EC9 and EC12A (Table 1) used for the first PCR amplifies all known Anaplasma and Ehrlichia species. DNA from E. muris strain AS145 and A. phagocytophilum strain HZ were used as positive controls, and doubly distilled H2O was used as the negative control. The primers for the second-round PCR were specific for E. muris, Ehrlichia strain HF, ‘Candidatus Neoehrlichia mikurensis,’ A. phagocytophilum, A. centrale, and A. bovis (Table 1). The A. phagocytophilum p44 gene was amplified by nested or single-step PCR using primer pairs p3709-p4257 and p3761-p4183 (Table 1) (30). The initial amplification consisted of 40 cycles, each cycle consisting of 30 s at 94°C, 30 s at 52°C, and 1 min at 72°C. For the nested PCR amplification, 1 μl of the product from the first amplification was used for amplification with specific primers in a 25-μl reaction mixture; the amplification consisted of 40 cycles, each of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C.
TABLE 1.
Primers
| Specificity | Primer name | Sequence | PCR product size (bp) |
|---|---|---|---|
| 16S rRNA; all members | EC9 | 5′-TACCTTGTTACGACTT> | 1,462 |
| EC12A | 5′-TGATCCTGGCTCAGAACGAACG | ||
| 16S rRNA E. muris | EM87f | 5′-GGTTCGCTATTAGTGGCAGA | 517 |
| EM584r | 5′-CAGTATTAAAAGCCGCTCCA | ||
| 16S rRNA Ehrlichia HF strain | HF51f | 5′-AAGTCGAACGGACAATTACC | 923 |
| HF954r | 5′-GTTAGGGGGATACGACCTTC | ||
| 16S rRNA N. mikurensis | IS58-62f | 5′-GGAATAGCTGTTAGAAATGACA | 488 |
| IS58-594r | 5′-CTATCCTCTCTCGATCTCTAGTTT | ||
| 16S rRNA A. phagocytophilum | SSAP2f | 5′-GCTGAATGTGGGGATAATTTAT | 641 |
| SSAP2r | 5′-ATGGCTGCTTCCTTTCGGTTA | ||
| 16S rRNA A. centrale | AC1f | 5′-CTGCTTTTAATACTGCAGGACTA | 426 |
| AC1r | 5′-ATGCAGCACCTGTGTGAGGT | ||
| 16S rRNA A. bovis | AB1f | 5′-CTCGTAGCTTGCTATGAGAAC | 551 |
| AB1r | 5′-TCTCCCGGACTCCAGTCTG | ||
| p44 | p3709 | 5′-GCTAAGGAGTTAGCTTATGA | ∼400 |
| p4257 | 5′-AGAAGATCATAACAAGCATTG | ||
| p44 | p3761 | 5′-CTGCTCTKGCCAARACCTC | ∼300 |
| p4183 | 5′-CAATAGTYTTAGCTAGTAACC |
DNA sequencing and data analysis.
Sequences of every PCR amplicon of 16S rRNA genes were determined. All sequences shown in this study had the primer region sequences removed before analysis. Longer (>1,300-bp) 16S rRNA gene sequences were determined for seven representative sequences (ES34P-L from deer on Hokkaido; SS15E-L, SS24B-L, SS33P-L, and SS40C-L from deer in Shimane; TS37 from a tick in Shimane; and NR07 from a tick in Nara) and were compared to sequences of known Anaplasma and Ehrlichia spp. CLUSTAL W (version 1.6.5) and TreeView (version 1.65) on the DDBJ website and GENETYX-WIN (version 7.0.6; Software Development Co., Tokyo, Japan) were used for the sequence analysis and phylogram construction.
Deduced amino acid sequences of 16 newly obtained p44 sequences from Japanese deer were aligned with those from all A. phagocytophilum sequences available at GenBank (all sequences listed below are accession numbers from GenBank). To simplify the comparison, eighteen p44 genes of A. phagocytophilum strain HZ isolated from a human patient in the United States were used to obtain the consensus amino acid sequence designated the P44 USA HZ consensus: P44-1 (ORF00017), P44-2 (ORF01470), P44-3 (ORF01414), P44-4 (ORF01447), P44-5 (ORF00136), P44-6 (ORF01412), P44-7 (ORF01405), P44-11 (ORF00218), P44-12 (ORF00076), P44-14 (ORF00039), P44-15 (ORF00054), P44-16 (ORF00095), P44-18E (ORF00047), P44-23 (ORF00083), P44-24 (ORF00135), P44-32 (ORF00113), P44-36 (ORF01462), and P44-45 (ORF00413). Fifteen p44 genes of the United Kingdom strains described by Casey et al. (12) were used to obtain the consensus amino acid sequence designated the P44 UK consensus: FGA1 (AY176532), FGA4 (AY176534), FGB11 (AY176564), Lep1 (AY176565), Leph4 (AY176567), NW2 (AY176568), NWB2 (AY176581), NWT1 (AY176582), AB1 (AY176512), Cairn1 (AY176513), Cairn2 (AY176514), Cairn3 (AY176515), Cow1 (AY176529), Cow2 (AY176530), and Cow3 (AY176531). Sequence alignments and analyses of p44 genes were performed using MegAlign and MapDraw programs (DNAStar, Inc., Madison, WI).
The groESL sequence (about 1,300 bp) of TS37 was obtained by a nested PCR with primers HS1a (5′-AITGGGCTGGTAITGAAAT) and HS6a (5′-CCICCIGGIACIAIACCTTC) in the first PCR, primers HS43 (5′-ATWGCWAARGAAGCATAGTC) and HSVR (5′-CTCAACAGCAGCTCTAGTAGC), and primers based on the sequencing result of the PCR product amplified with primer pair HS43-HSVR in the nested reactions (32).
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences of ES34P-L, SS33P-L, SS40C-L, SS24B-L, SS15E-L, TS37, and NR07 have been deposited in the GenBank data library under accession numbers AB196720, AB196721, AB211164, AB211163, AB211162, AB074459, and AB196475, respectively. The groESL sequence of TS37 has been deposited in the GenBank data library under accession number AB074462. Sixteen different p44 sequences have been deposited in the GenBank data library under accession numbers DQ020144 to DQ020159.
RESULTS
Deer from two Japanese islands were tested for infection with Anaplasma and Ehrlichia species by PCR-based analysis of 16S rRNA genes. PCR products were sequenced to determine the pathogen identity. As shown in Table 2, 26% (33/126) of deer tested were infected with Anaplasma and/or Ehrlichia species: 14% (11/79) of deer (Cervus nippon yesoensis) from Hokkaido Island and 47% (22/47) of deer (Cervus nippon nippon) from Shimane, Honshu Island. Nineteen percent (24/126) of the deer were infected with A. phagocytophilum. Twelve percent (15/126) of the deer were infected with Anaplasma centrale. Nine percent (11/126) of deer were infected with Anaplasma bovis. Two percent (3/126) of deer were infected with an Ehrlichia sp. E. muris, Ehrlichia HF strain, and ‘Candidatus Neoehrlichia mikurensis’ were not detected in these deer specimens. One of 68 of H. longicornis ticks from the deer in Shimane Prefecture was infected with the Ehrlichia sp. Twelve percent (3/25) of H. longicornis ticks in Nara Prefecture were infected with A. bovis and A. centrale.
TABLE 2.
Detection of 16S rRNA sequences of Anaplasma and Ehrlichia spp. from deer in Japan
| Species or result | No. of specimens
|
Total no. | |
|---|---|---|---|
| Hokkaido | Shimane | ||
| A. phagocytophilium positive | 8 | 16 | 24 |
| A. centrale positive | 1 | 14 | 15 |
| A. bovis positive | 5 | 6 | 11 |
| Ehrlichia sp. (TS37) positive | 0 | 3 | 3 |
| Total positive | 14 | 39 | 53 |
| PCR-positive samplesa | 11 | 22 | 33 |
| No. of deer tested | 79 | 47 | 126 |
Any species, including multiple PCR-positive species.
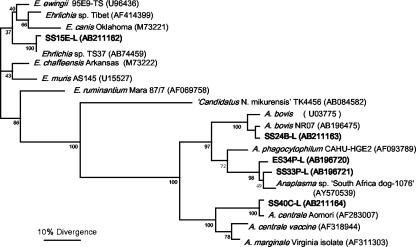
To define the pathogen identity, almost full-length 16S rRNA sequences were determined for seven representative strains from deer and from ticks. The phylogenic analysis used 1,332-bp sequences of all seven new strains determined in this study and previously sequenced Anaplasma and Ehrlichia spp. (Fig. 1). Of the Hokkaido deer, 10% (8/79) were infected with A. phagocytophilum, and the 16S rRNA sequences (579 bp) of the amplicons from all eight infected deer were 99.9 to 100% identical to each other. The longer representative sequence (ES34P-L; 1,338 bp) was most closely related (99.3% identical; 10 bp differed of 1,338 bp compared) to Anaplasma sp. SA1076 from a dog in South Africa (GenBank accession no. AY570539) (21). This sequence was 98.7% identical (1,321 of 1,338 bp compared) to the A. phagocytophilum strain ‘HGE agent’ (where HGE is a designation forhuman granulocytic ehrlichiosis) CAHU-HGE2 from a humanpatient in northern California (GenBank accession no. AF093789). Of the Shimane deer, 34% (16/47) were infected with A. phagocytophilum, and the 16S rRNA sequences (579 bp) of the amplicons from all 16 infected deer were 99.9 to 100% identical to each other. The longer 16S rRNA sequence (SS33P-L; 1,399 bp) from the representative specimen was most closely related (99.4% identical; 1,373 of 1,381 bp compared) to Anaplasma sp. SA1076 from a dog in South Africa (GenBank accession no. AY570539). It also was the next most closely related (98.4% identical; 21 bp differed of 1,402 bp compared) sequence to that of A. phagocytophilum strain LGE from a llama (Llama llama) and llama-associated ticks (Ixodes pacificus) (GenBank accession no. AF241532) (4). The sequence identity between ES34P-L from the Hokkaido deer and SS33P-L from the Shimane deer was 99.6% (Fig. 1; Table 3), implying geographic segregation of strains between the two islands.
FIG. 1.
Phylogram of the five new sequences from deer and other members of the family Anaplasmataceae based on 16S rRNA gene sequence comparison (1,332 bp). GenBank accession numbers are shown in parentheses. Numbers above internal nodes indicate the percentages of 1,000 bootstrap replicates that supported the branch.
TABLE 3.
Anaplasma and Ehrlichia infection of 33 deer in Japan as determined by PCR, followed by sequencing
| Deer identification no.a |
A. phagocytophilum
|
A. centrale | A. bovis | Ehrlichia sp. (TS37) | |
|---|---|---|---|---|---|
| SS33 genotype | ES34 genotype | ||||
| ES11 | + | ||||
| ES28 | + | ||||
| ES34 | + | ||||
| ES35 | + | ||||
| ES40 | + | ||||
| ES43 | + | ||||
| ES51 | + | ||||
| ES55 | + | ||||
| ES56 | + | + | + | ||
| ES57 | + | ||||
| ES60 | + | + | |||
| SS05 | + | ||||
| SS07 | + | ||||
| SS12 | + | + | |||
| SS13 | + | ||||
| SS14 | + | ||||
| SS15 | + | + | + | ||
| SS16 | + | ||||
| SS17 | + | ||||
| SS20 | + | + | |||
| SS23 | + | + | |||
| SS24 | + | + | + | ||
| SS30 | + | + | + | ||
| SS31 | + | + | |||
| SS32 | + | + | + | ||
| SS33 | + | + | |||
| SS37 | + | ||||
| SS38 | + | ||||
| SS39 | + | + | |||
| SS40 | + | + | + | ||
| SS42 | + | ||||
| SS43 | + | ||||
| SS46 | + | + | |||
| Total | 16 | 8 | 15 | 11 | 3 |
ES11 to ES60, Cervus nippon yesoensis from Hokkaido Island; and SS01 to SS46, Cervus nippon nippon from Shimane in Honshu Island. Samples from ES11 was collected in 1991, ES28 to ES60 were collected in 1989, SS05 to SS24 were collected in 2001, and SS30 to SS46 were collected in 2002.
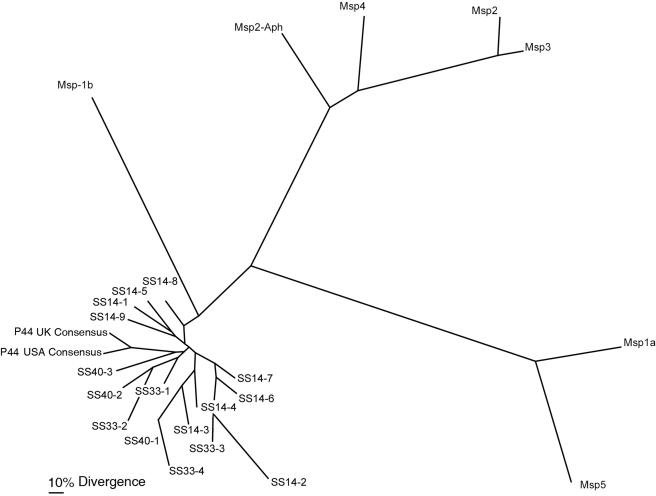
Using the p44-specific primer pairs shown in Table 1 (30) for PCR, followed by sequencing of the amplicons, p44 sequences of approximately 370 bp including primer regions (330 to 410 bp) were obtained from deer blood specimens. We obtained nine, four, and three different p44 paralog sequences from deer SS14, SS33, and SS40, respectively. The sequences of the primer regions were removed, and the deduced P44 amino acid sequences were compared. These sequences were characterized by the central hypervariable regions flanked by N- and C-terminal-conserved regions (30). The P44 sequences from Japanese deer samples contained the identical amino acids C, C, W, and P found in the P44 USA HZ and P44 UK A. phagocytophilum consensus amino acid sequences (Fig. 2) (12, 30). However, all p44 genes of Japanese samples had relatively shorter regions, delineated by two conserved cysteines (∼12 amino acid residues), in the hypervariable region than those of the P44 USA HZ stains available at GenBank and the P44 UK strains (∼25 to 30 amino acid residues). When 16 deduced amino acid sequences from Japanese deer were compared, ∼63% (10/16) of Japanese deer P44 sequences had >50% identity, and 37% (6/16) had 50 to 30% identity. Phylogenetic analyses showed that the 16 sequences clustered with P44 sequences of A. phagocytophilum from the P44 USA HZ and the P44 UK consensus sequences (Fig. 3). These P44 sequences constituted a separate cluster from the Msp2 sequences of A. phagocytophilum and from the Msp1, Msp2, Msp3, and Msp5 sequences of A. marginale (Fig. 3).
FIG. 2.
Comparison between the consensus-deduced amino acid sequences of p44 genes from the 16 Japanese deer (SS) obtained in this study with consensus sequences of p44 paralogs from strains from the United States (USA HZ) and the United Kingdom (UK) (11, 27). Underlined letters indicate the absolutely conserved amino acids from Japan, the United States, and the United Kingdom within the hypervariable region. Dashes show sequence gaps. The region delineated by two cysteines (underlined with dots) is shorter in Japanese strains than in strains from the United States and the United Kingdom.
FIG. 3.
Phylogram of deduced amino acid sequences of p44 genes from three deer specimens compared to consensus sequences of p44 paralogs from strains from the United States and the United Kingdom and sequences of Msp2 A. phagocytophilum (Msp2-Aph; GenBank no. AY541007 for strain NY-31) and sequences of St. Maries strains of Anaplasma marginale. GenBank accession numbers for sequences determined in this work are SS14-1 (DQ020144), SS14-2 (DQ020145), SS14-3 (DQ020146), SS14-4 (DQ020147), SS14-5 (DQ020148), SS14-6 (DQ020149), SS14-7 (DQ020150), SS14-8 (DQ020151), SS14-9 (DQ020152), SS33-1 (DQ020153), SS33-2 (DQ020154), SS33-3 (DQ020155), SS33-4 (DQ020156), SS40-1 (DQ020157), SS40-2 (DQ020158), and SS40-3 (DQ020159). The GenBank accession number for the whole genome sequence of Anaplasma marginale strain St. Maries is CP000030. The accession numbers for strain St. Maries are Msp1a (AM530), Msp1b (AM133), Msp2 (AM1144), Msp3 (AM1063), Msp4 (AM090), and Msp5 (AM236).
The 16S rRNA sequences from A. centrale (426 bp) from 1 Hokkaido deer, 14 Shimane deer, and two Hemaphysalis longicornis ticks removed from deer in Nara Prefecture on Honshu Island, Japan, in 2004 were 99.9 to 100% identical to each other, despite the geographic separation (two separate islands) and up to 13 years of time span between collections (1989 on Hokkaido and 2001 and 2002 in Shimane). The longer representative sequence (SS40C-L; 1,361 bp) was obtained from one of the Shimane deer; it was 99.9% identical to that of A. centrale (GenBank accession no. AF283007) previously detected in the cattle from Aomori Prefecture on northern Honshu Island, Japan(22).
Furthermore, the 16S rRNA sequences (550 bp) of A. bovis from five Hokkaido deer, six Shimane deer, and one Hemaphysalis longicornis tick removed from deer in Nara Prefecture were 99.9 to 100% identical, despite geographic separation and up to 13 years separating collections (1989 and 1991 on Hokkaido and 2001 and 2002 in Shimane). The longer sequence (SS24B-L; 1,344 bp) from one representative specimen was 99.9% identical to strain NR07 (1,391 bp) that was detected in the H. longicornis tick in Nara Prefecture on Honshu Island, Japan, in 2004 and 99.7% identical (1,448 bp were compared) to that of the A. bovis strain from South Africa (GenBank accession no. U03775; strain name unavailable) (unpublished data).
Of the 22 Anaplasma species-positive specimens, three specimens, all from Shimane Prefecture, were coinfected with a novel Ehrlichia sp. of the identical 16S rRNA gene sequence (475 bp). The almost full-length 16S rRNA gene sequence (1,447 bp) from the Ehrlichia sp. (SS15E-L; 1,365 bp) was 99.9% identical to that of Ehrlichia sp. TS37 (GenBank accession no. AB074459) that was detected in a single tick out of 68 H. longicornis ticks collected in the Shimane Prefecture in 1999 (the TS37 result was presented at the 101st General Meeting of American Society for Microbiology, Orlando, Fla., 20 to 24 May 2001). It was 98.7% identical to that of Ehrlichia sp. strain Tibet from Boophilus microplus ticks (GenBank accession no. AF414399) (62), 98.3% identical to Ehrlichia ewingii strain 95E9-TS (GenBank accession no. U96436) (19), and 98.2% identical to E. chaffeensis strain Arkansas (GenBank accession no. AF416764). To confirm the novel nature of the Ehrlichia sp. found from the deer and one tick, the 1,316-bp groESL sequence of TS37 was obtained. This sequence was 90.2, 90.2, 89.2, 90.6, 89.8, and 88.37% identical to that of E. ewingii, E. chaffeensis, E. canis, Ehrlichia sp. Anan, Ehrlichia sp. HF565, and E. muris, respectively. The deduced GroEL 402-amino-acid sequence of strain TS37 (GenBank accession no. AB074462) was 97.5, 97.3, 97.3, 97.0, 86.6, and 56.7% identical to that of HF565, E. muris, E. ewingii, E. chaffeensis, A. phagocytophilum, and N. sennetsu, respectively. A phylogram based on GroEL amino acid sequences showed that strain TS37 fell between E. ewingii and E. canis. The lengths of the intergenic space between the groES and groEL genes of TS37 were 96 bp, in agreement with the sequence-based phylogram (54).
Of 33 infected deer, 14 (42%) deer had concurrent infections with one or more Ehrlichia and Anaplasma species, where 8 deer were coinfected with two species and 6 deer were coinfected with three different species (Table 3). Thirty-three percent (11/33) of deer had concurrent infections with more thantwo species of Anaplasma and four deer had concurrent infections with three Anaplasma species. The three Ehrlichia-infected deer had concurrent infections with one of three Anaplasma species.
DISCUSSION
Findings from the present study show that the wild deer residing in two islands in Japan are naturally infected with four Anaplasma and Ehrlichia spp. and suggest that they may play a role in the enzootic maintenance of Anaplasma and Ehrlichia spp. in the region. White-tailed deer (Odocoileus virginianus) in the United States are known to be infected with A. phagocytophilum (3, 6, 31). Wild deer (Cervus elaphus, Capreolus capreolus, and Odocoileus virginianus) infected with A. phagocytophilum have also been reported in Europe (8, 33, 40, 45). Thus, Japan is the third geographic region where a high prevalence of infection of wild deer with A. phagocytophilum is found. What is unique about A. phagocytophilum in Japan is that the 16S rRNA gene sequences were divergent from any previously reported A. phagocytophilum sequences from deer or other mammals in Europe or the United States or from ticks from Asia. The 16S rRNA gene sequence of A. phagocytophilum was found in ticks from China and Korea (11, 27). A. phagocytophilum 16S rRNA gene sequences from Hokkaido and Shimane deer were 99.3% and 98.9% identical, respectively, to the Korean tick strain (GenBank accession no. AF470699; 926 bp) and were 98.5% and 98.0% identical, respectively, to the Chinese tick strain (GenBank accession no. AY079425; 919 bp). Surprisingly, the bacterium closest to A. phagocytophilum from Japanese deer, as determined by 16S rRNA gene sequence comparison, was Anaplasma sp. SA1076 from a dog in South Africa (21). Therefore, it is possible that this A. phagocytophilum strain infects domestic dogs in Japan.
The p44 gene of A. phagocytophilum encodes the immunodominant major outer membrane protein P44 (66), and multiple p44 homologs have been found in every A. phagocytophilum strain in the United States and England examined so far. Although the hypervariable regions are quite diverse, phylogenetic analyses of these p44 sequences are possible (12, 30, 66). To further define Japanese A. phagocytophilum strains, p44 gene sequences were determined. Sequences of 16 distinct p44 genes were obtained from three infected Japanese deer. The p44 locus consists of a central hypervariable region and 5′ and 3′ conserved regions (30, 66). The Japanese deer p44 genes were quite unique compared to all known p44 sequences, but retained the conserved p44 group-specific deduced amino acids observed within the hypervariable region of all sequenced p44 genes. Thus, the P44 major surface protein structure of the A. phagocytophilum strain from Japanese deer had a unique feature compared with those of A. phagocytophilum strains found in the United States and England. This p44 sequence difference also may explain why serological testing using the recombinant P44 protein of an A. phagocytophilum strain from the United States (55) could not detect most of the infected deer in Japan (data not shown).
In Japan, A. phagocytophilum has not been detected in ticks. However, A. phagocytophilum was found in Ixodes persulcatus from China (11) and in Hemaphysalis longicornis ticks from Korea (27). I. persulcatus is present on Hokkaido but has not been found in Shimane Prefecture (65). H. longicornis ticks are found on Honshu Island in Japan (65), but this tick has not been noted on Hokkaido. Thus, on Hokkaido and Honshu Islands, different species of I. persulcatus and H. longicornis ticks, respectively, may serve as vectors for A. phagocytophilum transmission between mammals.
To our knowledge, the present work is the first to document infection of deer with A. centrale or A. bovis, although the seroprevalence of Anaplasma marginale among deer in the United States and Mexico has been previously reported (26, 35); infection with an A. marginale-A. ovis-like agent in roe deer (Capreolus capreolus) in Spain has been previously reported as well (14, 40). The sequence of 16S rRNA from deer in Shimane Prefecture was 99.9% identical to that of A. centrale from cattle in Aomori, Japan (22); 98.6% identical to that of A. marginale from cattle in the United States (strain Virginia; GenBank accession no. AF309866) (unpublished data); and 98.5% identical to that of A. centrale from cattle in Europe (GenBank accession no. AF318944) (5). Thus, wild deer may serve as the reservoir for economically important anaplasmosis of cattle caused by several Anaplasma species inthe United States, Europe, and Japan. The Rhipicephalus simus tick is considered to be a vector of A. centrale in Africa (48), but in other geographic regions, a vector tick species has not been identified. Hokkaido and Shimane regions are not known to be inhabited by Rhipicephalus sp. ticks (65). In the present study, we found that the H. longicornis tick is the potential vector of A. bovis and A. centrale. Furthermore, A. bovis from an H. longicornis tick collected in Korea was reported (27).
White-tailed deer (Odocoileus virginianus) in the United States are known to be infected with Ehrlichia chaffeensis, the agent of human monocytic ehrlichiosis, and with Ehrlichia ewingii, the agent of human granulocytic ehrlichiosis (3, 31, 63). In the present study, the sequence (SS15E-L; 1,332 bp) detected in the deer from Shimane Prefecture was 99.9% identical to that of Ehrlichia sp. strain TS37 from H. longicornis ticks from Shimane Prefecture. This result suggests that H. longicornis ticks serve as vectors for the mammalian transmission of Ehrlichia sp. strain TS37.
Both 16S rRNA and groEL sequences of TS37 were distinct from any known Ehrlichia species and phylogenetically close to those of E. muris, E. ewingii, and E. chaffeensis. We propose to name this Ehrlichia species ‘Candidatus Ehrlichia shimanensis.’
All three Ehrlichia-positive deer were coinfected with Anaplasma species. Similar coinfections of deer with Ehrlichia and Anaplasma species, including E. ewingii, E. chaffeensis, or the WTD agent (an Anaplasma sp.) in Missouri or E. chaffeensis, A. phagocytophilum, or the WTD agent in Georgia were previously reported (3, 31). The WTD agent was not detected in Japanese deer in the present study. The 16S rRNA gene sequences from Japanese deer had only very low levels of identity (97%) to those of the WTD agent.
Several deer were infected with three Anaplasma species, namely A. centrale, A. phagocytophilum, and A. bovis; these agents infect erythrocytes, granulocytes, and monocytes, respectively (Table 3). To our knowledge, this is the first report of concurrent infection of any animal with three Anaplasma species. The results suggest the absence of cross protection among these Anaplasma species, illustrate the potential difficulty in diagnosing the deer infection by stained blood smear and/or serological test, and support the usefulness of molecular diagnosis of Anaplasma and Ehrlichia infection.
Serum specimens are a more convenient source than whole-blood specimens for retrospective analyses of infection, since many well-preserved archival and clinical specimens are available. Although less DNA from obligatory intracellular bacteria can be recovered from serum than from whole blood, previous studies have indicated the utility of human and deer serum specimens for the nested-PCR amplification of A. phagocytophilum DNA (36). The present study showed that serum from deer blood is a good source for DNA from Ehrlichia and Anaplasma species.
This present study suggests that enzootic cycles of several Ehrlichia and Anaplasma species between ticks and wild deer are established in Japan.
Acknowledgments
This research was partially supported by grant R01AI47407 from the National Institutes of Health.
REFERENCES
- 1.Aguirre, E., A. Sainz, S. Dunner, I. Amusategui, L. Lopez, F. Rodriguez-Franco, I. Luaces, O. Cortes, and M. A. Tesouro. 2004. First isolation and molecular characterization of Ehrlichia canis in Spain. Vet. Parasitol. 125:365-372. [DOI] [PubMed] [Google Scholar]
- 2.Allsopp, M. T., H. Van Heerden, H. C. Steyn, and B. A. Allsopp. 2003. Phylogenetic relationships among Ehrlichia ruminantium isolates. Ann. N. Y. Acad. Sci. 990:685-691. [DOI] [PubMed] [Google Scholar]
- 3.Arens, M. Q., A. M. Liddell, G. Buening, M. Gaudreault-Keener, J. W. Sumner, J. A. Comer, R. S. Buller, and G. A. Storch. 2003. Detection of Ehrlichia spp. in the blood of wild white-tailed deer in Missouri by PCR assay and serologic analysis. J. Clin. Microbiol. 41:1263-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barlough, J. E., J. E. Madigan, D. R. Turoff, J. R. Clover, S. M. Shelly, and J. S. Dumler. 1997. An. Ehrlichia strain from a llama (Llama llama) and llama-associated ticks (Ixodes pacificus). J. Clin. Microbiol. 35:1005-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekker, C. P. J., S. de Vos, A. Taoufik, O. A. E. Sparagano, and F. Jongejan. 2002. Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet. Microbiol. 89:223-238. [DOI] [PubMed] [Google Scholar]
- 6.Belongia, E. A., K. D. Reed, P. D. Mitchell, C. P. Kolbert, D. H. Persing, J. S. Gill, and J. J. Kazmierczak. 1997. Prevalence of granulocytic Ehrlichia infection among white-tailed deer in Wisconsin. J. Clin. Microbiol. 35:1465-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezuidenhout, J. D., and C. J. Jacobsz. 1986. Proof of transovarial transmission of Cowdria ruminantium by Amblyomma hebraeum. Onderstepoort J. Vet. Res. 53:31-34. [PubMed] [Google Scholar]
- 8.Blanco, J. R., and J. A. Oteo. 2002. Human granulocytic ehrlichiosis in Europe. Clin. Microbiol. Infect. 8:763-772. [DOI] [PubMed] [Google Scholar]
- 9.Buller, R. S., M. Arens, S. P. Hmiel, C. Paddock, J. W. Sumner, Y. Rikihisa, A. Unver, M. Graudreauls-Keener, F. A. Marinian, A. M. Liddell, N. Schmulewitz, and G. Storch. 1999. Ehrlichia ewingii, a newly recognized agent of human ehrlichiosis. N. Engl. J. Med. 341:148-155. [DOI] [PubMed] [Google Scholar]
- 10.Cao, W. C., Y. M. Gao, P. H. Zhang, X. T. Zhang, O. H. Dai, J. S. Dumler, L. Q. Fang, and H. Yang. 2000. Identification of Ehrlichia chaffeensis by nested PCR in ticks from southern China. J. Clin. Microbiol. 38:2778-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao, W. C., Q. M. Zhao, P. H. Zhang, J. S. Dumler, X. T. Zhang, L. Q. Fang, and H. Yang. 2000. Granulocytic Ehrlichiae in Ixodes persulcatus ticks from an area in China where Lyme disease is endemic. J. Clin. Microbiol. 38:4208-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey, A. N., R. J. Birtles, A. D. Radford, K. J. Bown, N. P. French, Z. Woldehiwet, and N. H. Ogden. 2004. Groupings of highly similar major surface protein (p44)-encoding paralogues: a potential index of genetic diversity amongst isolates of Anaplasma phagocytophilum. Microbiology 150:727-734. [DOI] [PubMed] [Google Scholar]
- 13.Davoust, B., J. L. Marie, S. Mercier, M. Boni, A. Vandeweghe, D. Parzy, and F. Beugnet. 2003. Assay of fipronil efficacy to prevent canine monocytic ehrlichiosis in endemic areas. Vet. Parasitol. 112:91-100. [DOI] [PubMed] [Google Scholar]
- 14.De La Fuente, J., J. Vicente, U. Hofle, F. Ruiz-Fons, I. G. Fernandez De Mera, R. A. Van Den Bussche, K. M. Kocan, and C. Gortazar. 2004. Anaplasma infection in free-ranging Iberian red deer in the region of Castilla-La Mancha, Spain. Vet. Microbiol. 100:163-173. [DOI] [PubMed] [Google Scholar]
- 15.Deutz, A., K. Fuchs, N. Nowotny, H. Auer, W. Schuller, D. Stunzner, H. Aspock, U. Kerbl, and J. Kofer. 2003. Sero-epidemiological studies of zoonotic infections in hunters—comparative analysis with veterinarians, farmers, and abattoir workers. Wien. Klin. Wochenschr. 115:61-67. [PubMed] [Google Scholar]
- 16.Dreher, U. M., R. Hofmann-Lehmann, M. L. Meli, G. Regula, A. Y. Cagienard, K. D. Stark, M. G. Doherr, F. Filli, M. Hassig, U. Braun, K. M. Kocan, and H. Lutz. 2005. Seroprevalence of anaplasmosis among cattle in Switzerland in 1998 and 2003: no evidence of an emerging disease. Vet. Microbiol. 107:71-79. [DOI] [PubMed] [Google Scholar]
- 17.Dumler, J. S., A. F. Barbet, C. P. Bekker, G. A. Dasch, G. H. Palmer, S. C. Ray, Y. Rikihisa, and F. R. Rurangirwa. 2001. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 51:2145-2165. [DOI] [PubMed] [Google Scholar]
- 18.Georges, K., G. R. Loria, S. Riili, A. Greco, S. Caracappa, F. Jongejan, and O. Sparagano. 2001. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 99:273-286. [DOI] [PubMed] [Google Scholar]
- 19.Goldman, E. E., E. B. Breitschwerdt, C. B. Grindem, B. C. Hegarty, J. J. Walls, and J. S. Dumler. 1998. Granulocytic ehrlichiosis in dogs from North Carolina and Virginia. J. Vet. Intern. Med. 12:61-70. [DOI] [PubMed] [Google Scholar]
- 20.Heo, E. J., J. H. Park, J. R. Koo, M. S. Park, M. Y. Park, J. S. Dumler, and J. S. Chae. 2002. Serologic and molecular detection of Ehrlichia chaffeensis and Anaplasma phagocytophila (human granulocytic ehrlichiosis agent) in Korean patients. J. Clin. Microbiol. 40:3082-3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inokuma, H., M. Oyamada, P. J. Kelly, L. A. Jacobson, P.-E. Fournier, K. Itamoto, M. Okuda, and P. Brouqui. 2005. Molecular detection of a new Anaplasma species closely related to Anaplasma phagocytophilum in canine blood from South Africa. J. Clin. Microbiol. 43:2934-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inokuma, H., Y. Terada, T. Kamio, D. Raoult, and P. Brouqi. 2001. Analysis of the 16S rRNA gene sequence of Anaplasma centrale and its phylogenetic relatedness to other Ehrlichiae. Clin. Diagn. Lab. Immunol. 8:241-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawahara, M., Y. Rikihisa, E. Isogai, M. Takahashi, H. Misumi, C. Suto, S. Shibata, C. Zhang, and M. Tsuji. 2004. Ultrastructure and phylogenetic analysis of ‘Candidatus Neoehrlichia mikurensis’ in the family Anaplasmataceae isolated from wild rats and found in Ixodes ovatus ticks. Int. J. Syst. Evol. Microbiol. 54:1837-1843. [DOI] [PubMed] [Google Scholar]
- 24.Kawahara, M., C. Suto, Y. Rikihisa, S. Yamamoto, and Y. Tsuboi. 1993. Characterization of ehrlichial organisms isolated from a wild mouse. J. Clin. Microbiol. 31:89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawahara, M., I. Tadahiko, C. Suto, S. Shibata, Y. Rikihisa, K. Hata, and K. Hirai. 1999. Comparison of Ehrlichia muris strains isolated from wild mice and ticks and serologic survey of humans and animals with E. muris as antigen. J. Clin. Microbiol. 37:1123-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keel, M. K., W. I. Goff, and W. R. Davidson. 1995. An assessment of the role of white-tailed deer in the epizootiology of anaplasmosis in the southeastern United States. J. Wildl. Dis. 31:378-385. [DOI] [PubMed] [Google Scholar]
- 27.Kim, C. M., M. S. Kim, M. S. Park, J. H. Park, and J. S. Chae. 2003. Identification of Ehrlichia chaffeensis, Anaplasma phagocytophilum, and A.bovis in Haemaphysalis longicornis and Ixodes persulcatus ticks from Korea. Vector Borne Zoonotic Dis. 3:17-26. [DOI] [PubMed] [Google Scholar]
- 28.Kocan, K. M., J. de la Fuente, A. A. Guglielmone, and R. D. Melendez. 2003. Antigens and alternatives for control of Anaplasma marginale infection in cattle. Clin. Microbiol. Rev. 16:698-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lew, A. E., K. R. Gale, C. M. Minchin, V. Shkap, and D. T. de Waal. 2003. Phylogenetic analysis of the erythrocytic Anaplasma species based on 16S rDNA and GroEL (HSP60) sequences of A. marginale, A. centrale, and A. ovis and the specific detection of A. centrale vaccine strain. Vet. Microbiol. 92:145-160. [DOI] [PubMed] [Google Scholar]
- 30.Lin, Q., N. Zhi, N. Ohashi, H. W. Horowitz, M. E. Aguero-Rosenfeld, J. Ruffalli, G. P. Wormser, and Y. Rikihisa. 2002. Analysis of sequences and loci of p44 homologs expressed by Anaplasma phagocytophila in acutely infected patients. J. Clin. Microbiol. 40:2981-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little, S. E., D. E. Stallknecht, J. M. Lockhart, J. E. Dawson, and W. R. Davidson. 1998. Natural coinfection of a white-tailed deer (Odocoileus virginianus) population with three Ehrlichia spp. J. Parasitol. 84:897-901. [PubMed] [Google Scholar]
- 32.Liz, J. S., L. Anderes, J. W. Sumner, R. F. Massung, L. Gern, B. Rutti, and M. Brossard. 2000. PCR detection of granulocytic ehrlichiae in Ixodes ricinus ticks and wild small mammals in western Switzerland. J. Clin. Microbiol. 38:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liz, J. S., J. W. Sumner, K. Pfister, and M. Brossard. 2002. PCR detection and serological evidence of granulocytic ehrlichial infection in roe deer (Capreolus capreolus) and chamois (Rupicapra rupicapra). J. Clin. Microbiol. 40:892-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long, S, W., X. Zhang, J. Zhang, R. P. Ruble, P. Teel, and X. J. Yu. 2003. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J. Med. Entomol. 40:1000-1004. [DOI] [PubMed] [Google Scholar]
- 35.Martinez, A., A. Salinas, F. Martinez, A. Cantu, and D. K. Miller. 1999. Serosurvey for selected disease agents in white-tailed deer from Mexico. J. Wildl. Dis. 35:799-803. [DOI] [PubMed] [Google Scholar]
- 36.Massung, R. F., K. Slater, J. H. Owens, W. L. Nicholson, T. N. Mather, V. B. Solberg, and J. G. Olson. 1998. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 36:1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molad, T., K. A. Brayton, G. H. Palmer, S. Michaeli, and V. Shkap. 2004. Molecular conservation of MSP4 and MSP5 in Anaplasma marginale and A. centrale vaccine strain. Vet. Microbiol. 100:55-64. [DOI] [PubMed] [Google Scholar]
- 38.Motoi, Y., H. Satoh, H. Inokuma, T. Kiyuuna, Y. Muramatsu, H. Ueno, and C. Morita. 2001. First detection of Ehrlichia platys in dogs and ticks in Okinawa, Japan. Microbiol. Immunol. 45:89-91. [DOI] [PubMed] [Google Scholar]
- 39.Mylonakis, M. E., A. F. Koutinas, E. B. Breitschwerdt, B. C. Hegarty, C. D. Billinis, L. S. Leontides, and V. S. Kontos. 2004. Chronic canine ehrlichiosis (Ehrlichia canis): a retrospective study of 19 natural cases. J. Am. Anim. Hosp. Assoc. 40:174-184. [DOI] [PubMed] [Google Scholar]
- 40.Oporto, B., H. Gil, M. Barral, A. Hurtado, R. A. Juste, and A. L. Garcia-Perez. 2003. A survey on Anaplasma phagocytophila in wild small mammals and roe deer (Capreolus capreolus) in northern Spain. Ann. N. Y. Acad. Sci. 990:98-102. [DOI] [PubMed] [Google Scholar]
- 41.Oura, C. A., R. P. Bishop, E. M. Wampande, G. W. Lubega, and A. Tait. 2004. Application of a reverse line blot assay to the study of haemoparasites in cattle in Uganda. Int. J. Parasitol. 34:603-613. [DOI] [PubMed] [Google Scholar]
- 42.Paddock, C. D., and J. E. Childs. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16:37-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park, J. H., E. J. Heo, K. S. Choi, J. S. Dumler, and J. S. Chae. 2003. Detection of antibodies to Anaplasma phagocytophilum and Ehrlichia chaffeensis antigens in sera of Korean patients by Western immunoblotting and indirect immunofluorescence assays. Clin. Diagn. Lab. Immunol. 10:1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parola, P., J. P. Cornet, Y. O. Sanogo, R. S. Miller, H. V. Thien, J. P. Gonzalez, D. Raoult, S. R. Telford III, and C. Wongsrichanalai. 2003. Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and other eubacteria in ticks from the Thai-Myanmar border and Vietnam. J. Clin. Microbiol. 41:1600-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrovec, M., A. Bidovec, J. W. Sumner, W. L. Nicholson, J. E. Childs, and T. Avsic-Zupanc. 2002. Infection with Anaplasma phagocytophila in cervids from Slovenia: evidence of two genotypic lineages. Wien. Klin. Wochenschr. 31:641-647. [PubMed] [Google Scholar]
- 46.Pierard, D., E. Levtchenko, J. E. Dawson, and S. Lauwers. 1995. Ehrlichiosis in Belgium. Lancet 346:1233-1234. [DOI] [PubMed] [Google Scholar]
- 47.Polin, H., P. Hufnagl, R. Haunschmid, F. Gruber, and G. Ladurner. 2004. Molecular evidence of Anaplasma phagocytophilum in Ixodes ricinus ticks and wild animals in Austria. J. Clin. Microbiol. 42:2285-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Potgieter, F. T., and L. van Rensburg. 1987. Tick transmission of Anaplasma centrale. Onderstepoort J. Vet. Res. 54:5-7. [PubMed] [Google Scholar]
- 49.Rikihisa, Y. 1991. The tribe Ehrlichieae and ehrlichial diseases. Clin. Microbiol. Rev. 4:286-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarih, M., Y. M'Ghirbi, A. Bouattour, L. Gern, G. Baranton, and D. Postic. 2005. Detection and identification of Ehrlichia spp. in ticks collected in Tunisia and Morocco. J. Clin. Microbiol. 43:1127-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scoles, G. A., M. W. Ueti, and G. H. Palmer. 2005. Variation among geographically separated populations of Dermacentor andersoni (Acari: Ixodidae) in midgut susceptibility to Anaplasma marginale (Rickettsiales: Anaplasmataceae). J. Med. Entomol. 42:153-162. [DOI] [PubMed] [Google Scholar]
- 52.Shibata, S., M. Kawahara, Y. Rikihisa, H. Fujita, Y. Watanabe, C. Suto, and T. Ito. 2000. New Ehrlichia species closely related to Ehrlichia chaffeensis isolated from Ixodes ovatus ticks in Japan. J. Clin. Microbiol. 38:1331-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sparagano, O. A., A. P. de Vos, B. Paoletti, C. Camma, P. de Santis, D. Otranto, and A. Giangaspero. 2003. Molecular detection of Anaplasma platys in dogs using polymerase chain reaction and reverse line blot hybridization. J. Vet. Diagn. Investig. 15:527-534. [DOI] [PubMed] [Google Scholar]
- 54.Sumner, J. W., W. L. Nicholson, and R. F. Massung. 1997. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J. Clin. Microbiol. 35:2087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tajima, T., N. Zhi, Q. Lin, Y. Rikihisa, H. W. Horowitz, J. Ralfalli, G. P. Wormser, and K. F. Hechemy. 2000. Comparison of two recombinant major outer membrane proteins of the human granulocytic ehrlichiosis agent for use in an enzyme-linked immunosorbent assay. Clin. Diagn. Lab Immunol. 7:652-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Telford, S. R., III, J. E. Dawson, P. Katavolos, C. K. Warner, C. P. Kolbert, and D. H. Persing. 1996. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc. Natl. Acad. Sci. USA 93:6209-6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Topolovec, J., D. Puntaric, A. Antolovic-Pozgain, D. Vukovic, Z. Topolovec, J. Milas, V. Drusko-Barisic, and M. Venus. 2003. Serologically detected “new” tick-borne zoonoses in eastern Croatia. Croat. Med. J. 44:626-629. [PubMed] [Google Scholar]
- 58.Uhaa, I. J., J. D. MacLean, C. R. Greene, and D. B. Fishbein. 1992. A case of human ehrlichiosis acquired in Mali: clinical and laboratory findings. Am. J. Trop. Med. Hyg. 46:161-164. [DOI] [PubMed] [Google Scholar]
- 59.Unver, A., Y. Rikihisa, M. Kawahara, and S. Yamamoto. 2003. Analysis of 16S rRNA gene sequences of Ehrlichia canis, Anaplasma platys, and Wolbachia species from canine blood in Japan. Ann. N. Y. Acad. Sci. 990:692-698. [DOI] [PubMed] [Google Scholar]
- 60.Varela, A. S., D. E. Stallknecht, M. J. Yabsley, V. A. Moore IV, E. W. Howerth, W. R. Davidson, and S. E. Little. 2005. Primary and secondary infection with Ehrlichia chaffeensis in white-tailed deer (Odocoileus virginianus). Vector Borne Zoonotic Dis. 5:48-57. [DOI] [PubMed] [Google Scholar]
- 61.Wen, B., W. Cao, and H. Pan. 2003. Ehrlichiae and ehrlichial diseases in China. Ann. N. Y. Acad. Sci. 990:45-53. [DOI] [PubMed] [Google Scholar]
- 62.Wen, B., R. Jian, Y. Zhang, and R. Chen. 2002. Simultaneous detection of Anaplasma marginale and a new Ehrlichia species closely related to Ehrlichia chaffeensis by sequence analyses of 16S ribosomal DNA in Boophilus microplus ticks from Tibet. J. Clin. Microbiol. 40:3286-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yabsley, M. J., V. G. Dugan, D. E. Stallknecht, S. E. Little, J. M. Lockhart, J. E. Dawson, and W. R. Davidson. 2003. Evaluation of a prototype Ehrlichia chaffeensis surveillance system using white-tailed deer (Odocoileus virginianus) as natural sentinels. Vector Borne Zoonotic Dis. 3:195-207. [DOI] [PubMed] [Google Scholar]
- 64.Yabsley, M., J., A. S. Varela, C. M. Tate, V. G. Dugan, D. E. Stallknecht, S. E. Little, and W. R. Davidson. 2002. Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus). Emerg. Infect. Dis. 8:668-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamaguchi, N., and S. Kitaoka. 1980. Key to the Japanese ticks of Ixodoidea Neumann. p. 451-471. In S. Ehara (ed.), Illustrations of the mites and ticks of Japan. Zenkoku Noson-kyoiku Kyokai, Tokyo, Japan. (In Japanese.)
- 66.Zhi, N., N. Ohashi, and Y. Rikihisa. 1999. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J. Biol. Chem. 274:17828-17836. [DOI] [PubMed] [Google Scholar]