Abstract
Unlike Bacillus subtilis and Escherichia coli, the gram-positive lactic acid bacterium Lactococcus lactis does not possess the SecDF protein, a component of the secretion (Sec) machinery involved in late secretion stages and required for the high-capacity protein secretion in B. subtilis. In this study, we complemented the L. lactis Sec machinery with SecDF from B. subtilis and evaluated the effect on the secretion of two forms of staphylococcal nuclease, NucB and NucT, which are efficiently and poorly secreted, respectively. The B. subtilis SecDF-encoding gene was tested in L. lactis at different levels. Increased quantities of the precursor and mature forms were observed only at low levels of SecDF and at high NucT production levels. This SecDF secretion enhancement was observed at the optimal growth temperature (30°C) and was even greater at 15°C. Furthermore, the introduction of B. subtilis SecDF into L. lactis was shown to have a positive effect on a secreted form of Brucella abortus L7/L12 antigen.
The genome sequence of the gram-positive lactic acid bacterium Lactococcus lactis IL1403 (3) allowed identification of the so-called secretome, comprising the secretion (Sec) machinery components (involved in active protein translocation through the plasma membrane) and their substrates, the exported and secreted proteins. Comparison of the protein database of L. lactis IL1403 with those of the extensively studied Escherichia coli and Bacillus subtilis secretomes (30, 31) revealed that the essential Sec components of the translocation machinery (SecA and SecYEG) are present in L. lactis. For early secretion stages, lactococcal secretion-dedicated chaperones (SRP system components) are present and seem to be the only route taken by secretory proteins to reach the extracellular medium. This is strengthened by the absence of an E. coli SecB homologue and a B. subtilis CsaA homologue (3). A unique nonlipoprotein signal peptidase, SipL, and a unique housekeeping extracellular protease, HtrA (23), fulfill late secretion stages in L. lactis. The most striking difference observed is the lack of any sequence homologous to E. coli SecD and SecF (SecD/F) (22) or to the B. subtilis Siamese twin polypeptide SecDF (2). In E. coli, cells lacking SecD/F are severely defective and hardly viable (22). In B. subtilis, SecDF is required for efficient translocation of the Bacillus amyloliquefaciens α-amylase (AmyQ) precursor only under conditions of hyperproduction (2). Inactivation of secDF does not affect growth at 37°C but leads to a cryosensitive growth phenotype at 15°C, which is exacerbated by a secretion stress such as pre-AmyQ hyperproduction (2). At any temperature, inactivation of secDF results in an accumulation of the precursor pre-AmyQ inside the cell and in a slower precursor processing, suggesting the possibility of a role of SecDF in late secretion steps and its requirement for efficient secretion in B. subtilis. Although the exact function of SecD/F in the secretion process remains unclear, it might stimulate Sec machinery assembly, couple proton motive force and SecA cycles for efficient and directional polypeptide translocation (9), or clean up translocation channels from prematurely folded precursor or defective signal peptides which could hamper the Sec machinery (2).
In the last decade, protein secretion in L. lactis has been extensively studied in order to develop new uses of this food-grade bacterium. Due to its extraordinary safety profile, L. lactis is indeed a good candidate to deliver proteins of therapeutic interest in vivo (for a review, see reference 21). Although gene expression can now be tightly controlled and protein production can reach satisfactory yields in L. lactis, secretion efficiency (SE; i.e., the proportion of secreted mature protein versus that of the intracellular precursor) remains low even though the secretion is driven by lactococcal secretion signals (5, 25). The development of L. lactis strains producing high quantities of heterologous proteins is a challenge for both biotechnology processes and pharmaceutical purposes. Various expression and delivery systems have already been developed to produce and secrete heterologous proteins, as well as to target them to specific cellular locations in L. lactis (for a review, see reference 21). Heterologous protein secretion in L. lactis has already been improved by the use of lactococcal signal peptides and synthetic propeptides (18, 19). To our knowledge, the L. lactis secretion machinery has never been considered a potent target for reaching higher heterologous protein secretion rates.
In this work, we hypothesized that the lactococcal Sec machinery is naturally hampered by the lack of a SecDF homologue. To test this hypothesis, we introduced B. subtilis SecDF into L. lactis, expressed the protein at different levels, and analyzed its effect on Staphylococcus aureus nuclease (Nuc) (17, 27) production and secretion. Two forms of Nuc were used, NucB and NucT, which are efficiently and poorly secreted, respectively (19). Several combinations of both SecDF and NucB/T expressed at a low and/or high level were tested. The impact of SecDF complementation on secretion capacities at a low temperature of growth was analyzed. This study was extended to the Brucella abortus L7/L12 protein, whose SE was reported to be low in L. lactis (25).
Bacterial strains, plasmids, and methods used.
The bacterial strains and plasmids used in this work are listed in Table 1. Lactococcus lactis strains were grown in M17 (Difco) (29) supplemented with 0.5% glucose (GM17) at 30°C without agitation. E. coli was grown in LB (26) at 37°C with agitation. Unless otherwise indicated, plasmid constructions were first established in E. coli TG1 and then transferred into L. lactis by electrotransformation (15). Transformants were selected in L. lactis with 5 μg/ml erythromycin or 10 μg/ml chloramphenicol or with 2.5 μg/ml erythromycin and 5 μg/ml chloramphenicol when used together; plasmids were selected in E. coli with 150 μg/ml erythromycin. Plasmid DNA isolation and general DNA manipulation procedures were performed as previously described (26). PCR (Perkin Elmer Cetus apparatus; Norwalk, Conn.) was performed using Taq DNA polymerase (Q-Biogen). Induction of the PnisA promoter (7) was performed using nisin (Sigma) for a 1-h period as previously described (1) or added at the beginning of the culture to maintain continuous expression. Protein sample preparation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Western blotting experiments, and immunodetection with anti-Nuc antibodies were performed as previously described (18). Protein samples were standardized to the culture's optical density (OD) at 600 nm to load equal amounts of total protein on SDS-PAGE gels for direct comparison by immunodetection. Secreted Nuc was measured using a spectrophotometric assay (24).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Replicon | Genotype, plasmid characteristic(s), and/or cloned cassette characteristic(s) | Reference or source |
|---|---|---|---|
| Strains | |||
| E. coli TG1 | supE hsd Δ5 thi Δ(lac-proAB, F′ (traD36 proAB-lacZΔM15) | 13 | |
| L. lactis MG1363 | Wild type, plasmid free | 12 | |
| L. lactis NZ9000 | MG1363 (nisRK genes into the chromosome), plasmid free | 14 | |
| Plasmids | |||
| pBS SK+II | ColE1 | Apr | Stratagene |
| pIL252 | pAMβ1 | EmR, low copy number | 28 |
| pNucori1 | ColE1 and pAMβ1 | Apr/Emr; high copy number; promoterless nuc gene | This work |
| pNucori2 | ColE1 and pAMβ1 | Apr/Emr; low-copy number; promoterless nuc gene | This work |
| pSEC:NucT | pWV01 | Cmr; gene encodes SPUsp-NucT precursor expressed under PnisA | 19 |
| pSecDF | ColE1 | Apr; carries the B. subtilis secDF gene | 2 |
| pSecDFh | ColE1 and pAMβ1 | Apr/Emr; high copy number | This work |
| pSecDF1 | ColE1 and pAMβ1 | Apr/Emr; low copy number | This work |
| pSEC-L7/L12 | pWV01 | Cmr; gene expressed under PnisA encodes a SPUsp-L7/L12 precursor | 25 |
| pVE3537 | ColE1 | ApR; carries promoterless nuc gene | 19 |
| pVE3556 | PAMβ1 | Emr, derivative of high-copy-number plasmid pIL253 | 28 |
| pVE3844 | ColE1 and pAMβ1 | Apr/Emr; low copy number, encodes SecDF-cMyc expressed under P59 | This work |
| pVE5529 | ColE1 and pAMβ1 | Apr/Emr; low copy number, encodes NucA expressed under P59 | 8 |
Strategy for low-level expression of heterologous genes in Lactococcus lactis.
In order to obtain low-level expression of heterologous genes, a cloning strategy based on the lactococcal high-copy-number plasmid pVE3556 (17) and derivatives of pBluescript (pBS SK+II) plasmid carrying a promoterless gene was used. This system was first established with E. coli TG1 using the reporter nuc gene. Plasmid pVE3537 (pBS SK+II derivative containing a promoterless nuc gene) and pVE3556 were cut with XbaI and ligated together to obtain pNucori1 and pNucori2. In pNucori1, nuc and the replication gene of pVE3556 (rep) are divergent and the copy number is the same as that of its parental plasmid, pVE3556 (around 80 copies per cell) (17), whereas they are convergent in pNucori2, whose copy number was estimated to be 10-fold lower (not shown). Plasmids of both orientations were obtained and transferred into MG1363. MG1363(pNucori1) and MG1363(pNucori2) possess Nuc activity, as determined by an activity assay on petri plates as previously described (17). This Nuc activity confirmed the presence of a leaky transcription on pVE3556, upstream of the promoterless nuc, in both orientations (not shown). The lower Nuc activity detected for MG1363(pNucori2) than for MG1363(pNucori1) correlates the plasmid copy number with the Nuc production and secretion level.
Cloning of the B. subtilis secDF gene in L. lactis.
In E. coli, SecD/F proteins are present only at about 30 molecules per cell, compared to 100 to 200 translocon units (22, 32). To avoid (i) the deleterious effects of an imbalanced stoichiometry between SecDF and the other components of the L. lactis Sec machinery and (ii) potential toxicity induced by high-level expression of the large SecDF protein that contains 12 transmembrane segments, secDF was first expressed at a low level by use of the strategy described above. pSecDF (pBS II KS+ carrying a promoterless secDF fragment and its own ribosome binding site; kindly provided by J. M. van Dijl, University of Groningen, Groningen, The Netherlands) and pVE3556 were cut with XbaI and ligated. As described above for pNucori1 and pNucori2, plasmids of both orientations (hereafter referred to as pSecDFh and pSecDFl) were obtained and introduced into L. lactis. Transcripts of secDF were detected in L. lactis containing pSecDFh or pSecDFl by reverse transcription-PCR (using oligonucleotides 5′secDFRT, 5′-CCCTCGAGGTCGACGGTATCG, and 3′ secDFRT, 5′-AGCACCTCAAATCCGCCTTGC; data not shown). This confirms that this strategy based on leak expression enables B. subtilis secDF expression in L. lactis.
Low-level expression of secDF in L. lactis has no effect on NucB and NucT secretion when produced at low levels.
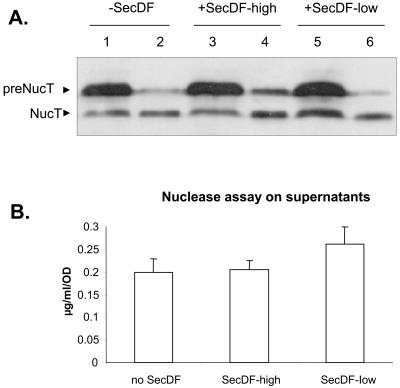
We first evaluated the effect of the weakly expressed secDF gene on two chromosomally encoded forms of Nuc, NucB and NucT, expressed under the control of usp45 transcriptional and translational signals: (i) the precursor SPUsp-NucB (pre-NucB), encoded by the usp-nucB cassette and secreted with a high SE (∼95%), and (ii) the precursor SPUsp-NucT (pre-NucT), encoded by the usp-nucT cassette and poorly secreted (SE, ∼30%) (19). After translocation, signal peptide of pre-NucB is cleaved and the NucB form is secreted. In S. aureus, NucB is processed to the NucA form by an N-terminal cleavage (27). L. lactis MG1363 usp-nucB (16) and L. lactis MG1363 usp-nucT (20) are two derivatives of L. lactis subsp. lactis MG1363 containing chromosomal usp-nucB and usp-nucT expression cassettes inserted in the histidine biosynthesis locus (6). To evaluate the effect of SecDF on the secretion efficiency of NucT, a poorly secreted form of Nuc, plasmids pSecDFh and pSecDFl were introduced into MG1363 usp-nucT, resulting in MG1363 usp-nucT(pSecDFh) and MG1363 usp-nucT(pSecDFl), respectively, and these strains were compared to the control strain MG1363 usp-nucT(pVE3556). Introduction of the two pSecDF plasmids was not deleterious for L. lactis growth, as no differences in generation time and final biomass were observed between secDF and secDF+ strains (not shown). The protein contents of cellular and supernatant fractions determined for both exponential (OD600, 0.5)- and stationary (OD600, >2)-phase cultures were analyzed by Western blotting using Nuc immunodetection as previously described (19), and the Nuc activity in the secreted fraction was quantified. Western blot analysis did not reveal significant differences in the levels of pre-NucT and SE in the three strains (Fig. 1A). These results were confirmed by Nuc activity assays (Fig. 1B). Some mature NucT was found associated with the cell fractions (Fig. 1A, lanes 1, 3, and 5). This is likely due to electrostatic interactions with the cell surface, as previously reported (19, 20), and is not affected here by the presence of SecDF. Some pre-NucT forms were detected in the supernatant fraction of the three strains. However, the levels of pre-NucT are higher in MG1363 usp-nucT(pSecDFh), where secDF is highly expressed (Fig. 1A, lanes 3 and 4). This suggests that cell lysis occurred in the three strains but was higher in MG1363 usp-nucT(pSecDFh). This might be due to a deleterious combination between the highly produced SecDF and the poorly secreted NucT, whose precursor form accumulates within the cell. The effect of SecDF complementation was tested on NucB secretion. Plasmids pSecDFh and pSecDFl were introduced into MG1363 usp-nucB, resulting in MG1363 usp-nucB(pSecDFh) and MG1363 usp-nucB(pSecDFl), respectively. The control strain is MG1363 usp-nucB(pVE3556). Secretion analysis was performed under the same conditions as for NucT. As for NucT, no significant difference was observed in NucB secretion levels independently of the SecDF expression levels (data not shown).
FIG. 1.
Effect of B. subtilis SecDF protein on secretion of NucB and NucT produced at low levels in L. lactis. (A) Protein samples present in cell (lanes 1, 3, and 5) and supernatant (lanes 2, 4, and 6) fractions of stationary-phase cultures were prepared, analyzed by SDS-PAGE, and immunodetected with anti-Nuc antibodies. −SecDF, MG1363 usp-nucT(pVE3556) (lanes 1 and 2); +SecDF-high, MG1363 usp-nucT(pSecDFh) (lanes 3 and 4); +SecDF-low, MG1363 usp-nucT(pSecDFl) (lanes 5 and 6); preNucT, precursor; NucT, mature form. (B) Quantification of secreted NucT mature form in the culture medium.
Together, these results show that B. subtilis SecDF does not enhance secretion yields of chromosomally encoded NucB/T forms, even for the inefficiently secreted NucT form. The absence of effect does not seem to be due to the secDF expression level and might rather be due to the low production levels of the heterologous reporter protein used. In B. subtilis, SecDF is indeed reportedly necessary to maintain good secretion levels at high production levels (2). We then investigated the potential effect of SecDF on NucT and NucB at high expression levels. We focused on the pSecDFl plasmid, as we noticed that low-level SecDF expression did not induce cell lysis (leading to release of pre-NucT in the supernatant), in contrast to the high-level SecDF expression obtained with pSecDFh.
Low-level expression of Bacillus subtilis secDF enhances secretion of overproduced NucT in L. lactis.
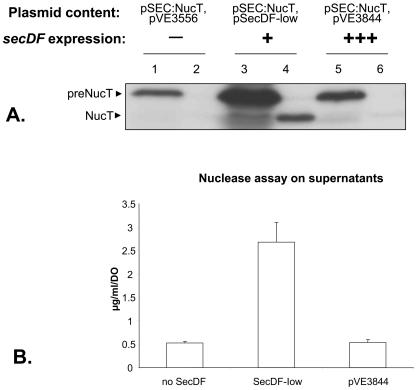
To test the effect of SecDF on the secretion of a highly produced protein, NucT was overproduced in NZ9000 (harboring nisRK regulator genes [7, 14]). The low-copy-number plasmid pSecDFl and pSEC:NucT (containing the usp-nucT cassette under the control of the nisin-inducible promoter PnisA) (19) were cointroduced into NZ9000 to obtain NZ9000(pSecDFl, pSEC:NucT). The secDF strain, NZ9000(pVE3556, pSEC:NucT), was used as a control. NucT production was induced with 1 ng/ml of nisin for 1 h on exponentially growing cultures, and protein samples were analyzed by Western blotting and anti-Nuc immunodetection. Introduction of SecDF has a strong effect on both production and secretion of NucT (Fig. 2A, lanes 1 to 4). The overall amount of pre-NucT was increased up to 10-fold in the secDF+ strain on the basis of Western blots. Moreover, mature NucT was undetectable in NZ9000(pVE3556, pSEC:NucT) supernatant at this autoradiography exposure intensity, whereas it was detected in NZ9000(pSecDFl, pSEC:NucT) supernatant. Using a Nuc activity assay, we determined that NucT is present in the culture medium and that introduction of SecDF led to a fivefold increase in the quantity of secreted mature NucT (Fig. 2B). Under these conditions, although NucT secretion was largely improved, secretion enhancement did not result in a better SE because of the parallel increase of pre-NucT and mature NucT in the presence of SecDF. This pre-NucT accumulation might result either from higher production or from increased stabilization of pre-NucT in the presence of SecDF. Such an increased production and/or precursor stabilization was previously reported by insertion of the secretion-enhancing LEISSTCDA synthetic propeptide in several heterologous precursors (18, 19). In these cases, stabilized precursor might escape intracellular degradation, leading to its accumulation within the cell and consequently to more-efficient secretion.
FIG. 2.
Effect of low and high levels of the B. subtilis SecDF protein on production and secretion of NucT in L. lactis. (A) Protein samples present in cell (lanes 1, 3, and 5) and supernatant fractions (lanes 2, 4, and 6) of exponentially growing cultures were analyzed by SDS-PAGE and anti-Nuc immunodetection. All strains contain pSEC:NucT and a second plasmid, indicated as follows: pVE3556 for NZ9000(pSEC:NucT, pVE3556) (control), pSecDFl for NZ9000(pSEC:NucT, pSecDFl) (low secDF expression), and pVE3844 for NZ9000(pSEC:NucT, pVE3844) (high secDF-c-Myc expression). preNucT, precursor; NucT, mature. (B) Quantification of secreted NucT released in the culture medium.
To further characterize the secretion improvement obtained by SecDF complementation, we determined the total amounts of NucT released in the culture medium in stationary-phase cultures with NucT expression induced from the beginning of growth. Cultures were diluted 1:100 in GM17 containing 1 ng of nisin/ml for continuous induction and grown until the culture entered the stationary phase, and secreted NucT was then quantified by a spectrophotometric assay (24). NZ9000(pSecDFl, pSEC:NucT) secretes an ∼10-fold-higher level of secreted NucT (3.1 μg of Nuc/ml/OD unit) than NZ9000(pVE3556, pSEC:NucT) (0.3 μg of Nuc/ml/OD unit).
Overproduction of the efficiently secreted NucB was analyzed, using a NZ9000(pSecDFl, pSEC:NucB) strain, where the usp-nucB cassette is placed under the control of PnisA (19). The secDF strain, NZ9000(pVE3556, pSEC:NucB), was used as a control. Protein samples were prepared on exponential-phase cultures as described above. Introduction of SecDF has no effect on the secretion of the NucB form (data not shown).
Taken together, these results demonstrate that introduction of SecDF has a positive effect on the secretion of the poorly secreted NucT in both exponential- and stationary-phase cultures, restricted to hyperproduction conditions. In contrast, no effect was observed for the efficiently secreted NucB. This can be compared to what was observed with B. subtilis, where SecDF was required only under secretion stress conditions induced by AmyQ overproduction (2). Until now, the direct cause of this higher level of mature NucT in the culture medium is not known. It could be a higher production level and/or higher stabilization of precursor pre-NucT. The fact that this positive SecDF effect was not observed with the efficiently secreted NucB could suggest that this is not merely a matter of precursor production level, regardless of the secretion process. Moreover, our results show that a high expression level of secDF did not have any positive effect on the NucT production level and do not suggest a dose-dependent effect. Comparing the dynamics of pre-NucT degradation in NZ9000(pVE3556, pSEC:NucT) to that in NZ9000(pSecDFl, pSEC:NucT) by pulse-chase experiments could help to evaluate these two hypotheses (higher production versus stabilization). However, the mechanism of the SecDF effect will be definitively clarified only when the precursor location (cytoplasmic or membrane associated) is determined, which is still a technical challenge with L. lactis.
High-level expression of secDF in Lactococcus lactis.
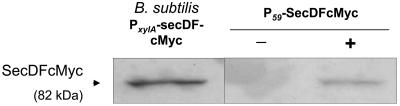
To further evaluate the effect of the expression level of SecDF on NucT secretion, secDF was cloned under the control of the constitutive P59 lactococcal promoter (33). This was done to confirm whether a high secDF expression level is deleterious for secretion or can further enhance secretion when associated with a high heterologous protein production rate. The secDF gene was amplified using oligonucleotides 5′-GGGTCGACAAAAAAGGACGCTTGATTGCGTTTTTC and 5′-CGATATCTCAATTTAAATCTTCTTCTGAAATTAATTTTTGTTCTTGCGCCGAATCTTTTTT by introduction of SalI and EcoRV (underlined). To confirm the presence of SecDF in L. lactis, the 3′ end of the secDF gene cloned under the control of P59 was extended by 11 codons specifying the human c-Myc epitope (EQKLISEEDLN) (10). To clone secDF-c-Myc under the control of P59, pVE5529 (producing a cytoplasmic form of Nuc) (8) was cut by SalI and EcoRV, and the Nuc-encoding fragment was replaced by the SecDF-c-Myc-encoding fragment to obtain pVE3844. The plasmid pSEC:NucT was cointroduced with pVE3844 into NZ9000, to obtain the NZ9000(pSEC:NucT, pVE3844) (secDF+) strain. To confirm production of SecDF-c-Myc, cell fraction proteins were analyzed by Western blot and anti-c-Myc immunodetection (Cell Signaling Technology). B. subtilis XDF-Myc (carrying a chromosomal secDF-c-Myc gene under the control of PxylA promoter, induced as previously described [2]) was used as control strain for SecDF-c-Myc production. As shown in Fig. 3, a SecDF-c-Myc fusion protein was produced in L. lactis NZ9000(pSEC:NucT, pVE3844).
FIG. 3.
Production of the B. subtilis SecDF-c-Myc in L. lactis. Cell fraction proteins prepared from stationary-phase cultures of three strains, B. subtilis XDF-Myc, L. lactis NZ9000(pSEC:NucT, pIL252) (secDF-c-Myc) and L. lactis NZ9000(pSEC:NucT, pVE3844) (secDF-c-Myc+), were analyzed by Western blotting and anti-c-Myc immunodetection.
We determined that NucT secretion was improved to a higher extent by pSecDFl (low copy) than by pSecDFh (high copy) under conditions of high NucT production. To test whether high-level secDF expression might better improve secretion under NucT overproduction conditions, high-level NucT production was induced (1 ng/ml of nisin for 1 h at OD600 of 0.5) in the presence of either weakly expressed secDF (as already shown in Fig. 2A, lanes 1 to 4) or highly expressed secDF-c-Myc. No growth difference was observed between the tested strains. As shown in Fig. 2A (lanes 5 and 6), high secDF expression led to a slight increase in pre-NucT in the cell fraction but did not modify the quantity of mature NucT released into the culture medium (Fig. 2B). A direct comparison (Fig. 2A) of the three strains described above confirms that pSecDFl enhanced more significantly the released quantities of cell-associated and mature NucT than did pVE3844.
Taken together, these results show that under high NucT production conditions, only weakly expressed SecDF significantly improved secretion. This might result from a better stoichiometry between the B. subtilis SecDF and the endogenous L. lactis Sec machinery components, as a deleterious effect of an excess of SecDF molecules per cell might counteract the secretion-positive role of SecDF observed when expressed at low levels.
SecDF restores NucT secretion at a low temperature.
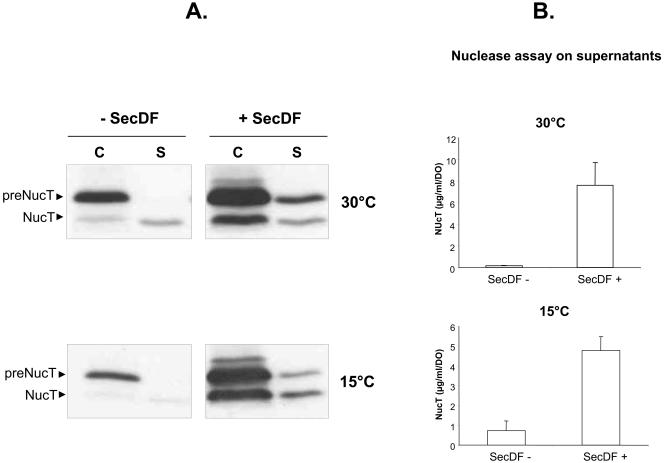
In B. subtilis, SecDF is not essential for cell viability at 37°C, but the secDF mutant shifted to 15°C stopped growing and this phenotype was exacerbated by a secretion stress imposed by AmyQ overproduction (2). Although naturally deprived of SecDF, the L. lactis wild type is able to grow at 15°C. To determine whether SecDF could influence L. lactis growth at 15°C combined with NucT secretion stress, strains NZ9000(pVE3556, pSEC:NucT) and NZ9000(pSecDFl, pSEC:NucT) were grown at 15°C with or without constitutive NucT production (1 ng/ml of nisin added from the beginning of the culture). Both strains presented similar growth rates and final biomasses with NucT production, in the presence or absence of SecDF [final OD600 values, 1.484 and 1.260 for NZ9000(pVE3556, pSEC:NucT) and NZ9000(pSecDFl, pSEC:NucT), respectively]. This confirmed the absence of any direct deleterious effect of SecDF at 15°C and that this NucT production rate did not constitute a lethal secretion stress. The impact of SecDF on NucT secretion at a low temperature was then analyzed. Strains NZ9000(pVE3556, pSEC:NucT) and NZ9000(pSecDFl, pSEC:NucT) were diluted 1:100 in GM17 with or without nisin at 1 ng/ml and grown at 30°C or 15°C until the stationary growth phase. In absence of SecDF, the levels of both pre-NucT and NucT detected were slightly lower at 15°C than at 30°C (Fig. 4A, left panels). However, the NucT SE was unmodified by the growth temperature, as a temperature reduction led to parallel reductions for pre-NucT and NucT. This suggests that the translocation and maturation steps of the secretion process are not cold sensitive in L. lactis under these conditions. Activity assays performed with the supernatant fractions of induced cultures of the two strains revealed a slight increase of NucT secreted at 15°C, probably due to the lower growth rate at this temperature which allows longer NucT production and accumulation in the culture medium. This is in contrast with results obtained for B. subtilis secDF mutants where secretion was severely impaired at low temperature (2). In the presence of SecDF, an enhanced secretion phenotype was observed at 30°C in both exponential (Fig. 2A, lanes 3 and 4) and stationary (Fig. 4A, upper right panel) growth phases. Remarkably, this effect was also conserved at 15°C (Fig. 4A, lower right panel). At both temperatures, the majority of NucT was detected as the precursor form in the cell fraction, and the majority of mature NucT remained associated with the cell fraction of the SecDF-containing strain, probably due to electrostatic interactions between mature NucT and cell walls of stationary-phase cells. In addition, pre-NucT was found in the culture medium revealing some cell lysis. This is likely due to the long induction time with nisin, since a shorter induction time did not lead to pre-NucT release in the supernatant (Fig. 2, lane 4). This phenomenon, due to the activity of lactococcal autolysins in the stationary growth phase (4), is slightly reduced at 15°C. Quantification of secreted NucT confirmed the secretion-stimulatory effect of SecDF at both temperatures (Fig. 4B). Taken together, these data demonstrate that the secretion capacities of L. lactis (production, translocation, and maturation of NucT) are maintained at low temperature and notably that introduction of SecDF can also improve L. lactis secretion at low temperature.
FIG. 4.
SecDF enhances NucT secretion at 30°C and 15°C. (A) Western blot analysis by anti-Nuc immunodetection of NucT distribution in stationary-phase culture induced by 1 ng of nisin/ml from the beginning of growth. C, cell fractions; S, culture medium fractions; −SecDF, NZ9000(pSEC:NucT, pVE3556); +SecDF, NZ9000(pSEC:NucT, pSecDFl); preNucT, precursor; NucT, mature. (B) Quantification of secreted NucT released in the culture medium.
Effect of SecDF introduction on Brucella abortus L7/L12 secretion.
To examine whether SecDF introduction into L. lactis may improve secretion of another heterologous protein, we combined pSecDFl with pSEC:L7/L12 (encoding a secreted form of Brucella abortus L7/L12 protein) (Table 1). Increased amounts of L7/L12 were detected in the SecDF+ strain cell fraction (Fig. 5). This signal corresponds to the pre-L7/L12 form that is poorly secreted in L. lactis, as previously observed (25). L7/L12 SE was unmodified, as no mature L7/L12 was detected in the culture medium. However, the presence of B. subtilis SecDF increases the amount of pre-L7/L12 that accumulates in the cell fraction (Fig. 5). This can be correlated to the similar accumulation of pre-NucT and strengthens the hypothesis that SecDF might have a role in precursor stabilization at the early secretion stages, before its translocation.
FIG. 5.
Effect of SecDF on Brucella abortus L7/L12 protein. Western blot analysis by anti-L7/L12 immunodetection with specific antibodies. L7/L12 expression was induced with 1 ng of nisin/ml from the beginning of growth, and protein samples were prepared on stationary-phase cultures. C, cell fraction; S, culture medium fraction; −SecDF, NZ9000(pSEC:L7/L12; pVE3556); +SecDF, NZ9000(pSEC:L7/L12; pSecDFl).
SecDF of B. subtilis can complement the L. lactis secretome.
The absence of a SecDF homologue in L. lactis is the unique characteristic of Sec components involved in late secretion stages. Of all sequenced bacterial genomes available in public databases, only streptococci, L. lactis, Enterococcus faecalis, and Lactobacillus plantarum share this feature. We hypothesized that the L. lactis Sec machinery could be considered naturally incomplete and that its secretion capacity could therefore be enhanced by interspecies complementation. For this purpose, B. subtilis SecDF was introduced into L. lactis, and we investigated the effect of this complementation on secretion of two forms of Nuc. We observed that secretion improvement was not proportional to the secDF expression level, as most significant improvements were observed at very low secDF expression levels. While L. lactis is naturally deprived of SecDF, this suggests that a stoichiometric balance between SecDF and the other L. lactis Sec components must be maintained to some extent. In E. coli, about 30 copies of SecD/F are present per cell, compared to 100 to 200 translocon units (22, 32). This lack of balance in favor of translocation units might explain why the strong secretion phenotype observed at a very low level of secDF expression is reduced at a high level of secDF expression. An alternative hypothesis could be based on differences in codon usage between L. lactis and B. subtilis. secDF of B. subtilis contains codons that are rare in L. lactis, especially in highly expressed genes (11). For instance, we have calculated that about 50% of phenylalanine, leucine, and arginine codons and 68% of isoleucine codons present in secDF are of the very rare type in L. lactis and thus might impair translation. If we hypothesize that translational efficiency and codon usage are tightly coordinated in L. lactis, then the problem of codon bias would not show up in low-level experiments but only in high-level experiments when the scarcity of some tRNAs becomes a bottleneck. This bottleneck could explain the observed absence of NucT secretion improvement at expected high SecDF expression levels. The effect of SecDF on protein secretion in L. lactis can thus potentially be enhanced by codon optimization in secDF.
The positive effect of SecDF observed at 30°C (the optimal temperature for growth) was also conserved at 15°C: production and secretion levels were impaired at low temperature for the SecDF− strain, whereas the presence of SecDF allowed efficient production and secretion of Nuc at low temperature. The property maintaining secretion at low temperature can be of major importance. Heterologous protein production at low temperature minimizes extracellular degradation and facilitates correct folding.
The presence of SecDF did not improve the secretion efficiency of L7/L12, as no mature form was detected in the culture medium. However, the increased amounts of precursor form detected in the cell fraction suggest that SecDF might improve very early secretion stages (such as the docking of the SRP complex to the Sec machinery), improve precursor stabilization, or facilitate translocation. In this last case, signal peptidase might provide leverage to force secretion if the maturation step is indeed a limiting factor for L7/L12 secretion.
Over the last decade, an increasing number of studies have focused on developing new uses for L. lactis, such as for live vaccine vectors or for the production of heterologous proteins with therapeutic purposes, taking advantage of the extraordinary safety profile of this bacterium (21). The present study shows that secretion capacities can be increased by interspecies complementation of secretion-dedicated components. The complementation of L. lactis secretion machinery developed in this study can be extended to other components involved in late secretion steps, such as heterologous signal peptidases, to improve the precursor maturation step or complementation with extracellular folding catalysts, absent in lactococci and present in other gram-positive bacteria.
Acknowledgments
S. Nouaille and E. Morello contributed equally to this work.
S. Nouaille is a recipient of a MENRT grant from the French government, and E. Morello is a recipient of a CIFRE grant between Agence Nationale de la Recherche and the GTP-Technology Society (Toulouse, France).
We thank Harold Tjalsma and Jan Maarten van Dijl for plasmid pSecDF and the Bacillus subtilis MID strain. We thank Peter Ravn for the spectrophotometric Nuc assay, Luis Bermudez for his critical reading of the manuscript, and the other members of URLGA for their constructive discussions and support. We also thank Stanislas Dusko Ehrlich, who first proposed the complementation strategy of the L. lactis Sec machinery.
REFERENCES
- 1.Bermudez-Humaran, L. G., P. Langella, J. Commissaire, S. Gilbert, Y. Le Loir, R. L'Haridon, and G. Corthier. 2003. Controlled intra- or extracellular production of staphylococcal nuclease and ovine omega interferon in Lactococcus lactis. FEMS Microbiol. Lett. 224:307-313. [DOI] [PubMed] [Google Scholar]
- 2.Bolhuis, A., C. P. Broekhuizen, A. Sorokin, M. L. van Roosmalen, G. Venema, S. Bron, W. J. Quax, and J. M. van Dijl. 1998. SecDF of Bacillus subtilis, a molecular Siamese twin required for the efficient secretion of proteins. J. Biol. Chem. 273:21217-21224. [DOI] [PubMed] [Google Scholar]
- 3.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buist, G., J. Kok, K. J. Leenhouts, M. Dabrowska, G. Venema, and A. J. Haandrikman. 1995. Molecular cloning and nucleotide sequence of the gene encoding the major peptidoglycan hydrolase of Lactococcus lactis, a muramidase needed for cell separation. J. Bacteriol. 177:1554-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatel, J. M., P. Langella, K. Adel-Patient, J. Commissaire, J. M. Wal, and G. Corthier. 2001. Induction of mucosal immune response after intranasal or oral inoculation of mice with Lactococcus lactis producing bovine beta-lactoglobulin. Clin. Diagn. Lab. Immunol. 8:545-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delorme, C., J.-J. Godon, S. D. Ehrlich, and P. Renault. 1993. Gene inactivation in Lactococcus lactis: histidine biosynthesis. J. Bacteriol. 175:4391-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dieye, Y., S. Usai, F. Clier, A. Gruss, and J. C. Piard. 2001. Design of a protein-targeting system for lactic acid bacteria. J. Bacteriol. 183:4157-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Driessen, A. J. 1992. Precursor protein translocation by the Escherichia coli translocase is directed by the protonmotive force. EMBO J. 11:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evan, G. I., G. K. Lewis, G. Ramsay, and J. M. Bishop. 1985. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol. Cell. Biol. 5:3610-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuglsang, A. 2003. Lactic acid bacteria as prime candidates for codon optimization. Biochem. Biophys. Res. Commun. 312:285-291. [DOI] [PubMed] [Google Scholar]
- 12.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gibson, T. J. 1984. Ph.D. thesis. University of Cambridge, Cambridge, England.
- 14.Kuipers, O. P., P. G. de Ruyters, M. Kleerebezem, and W. M. de Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 15.Langella, P., Y. Le Loir, S. D. Ehrlich, and A. Gruss. 1993. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J. Bacteriol. 175:5806-5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langella, P., S. Nouaille, J. Commissaire, A. Bolotin, A. Gruss, and Y. Le Loir. 2001. Caractérisation des facteurs d'hôtes affectant la sécrétion de protéines hétérologues chez Lactococcus lactis. Lait 81:19-28. [Google Scholar]
- 17.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1994. Direct screening of recombinants in gram-positive bacteria using the secreted staphylococcal nuclease as a reporter. J. Bacteriol. 176:5135-5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Loir, Y., A. Gruss, S. D. Ehrlich, and P. Langella. 1998. A nine-residue synthetic propeptide enhances secretion efficiency of heterologous proteins in Lactococcus lactis. J. Bacteriol. 180:1895-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Loir, Y., S. Nouaille, J. Commissaire, L. Bretigny, A. Gruss, and P. Langella. 2001. Signal peptide and propeptide optimization for heterologous protein secretion in Lactococcus lactis. Appl. Environ. Microbiol. 67:4119-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nouaille, S., J. Commissaire, J. J. Gratadoux, P. Ravn, A. Bolotin, A. Gruss, Y. Le Loir, and P. Langella. 2004. Influence of lipoteichoic acid d-alanylation on protein secretion in Lactococcus lactis as revealed by random mutagenesis. Appl. Environ. Microbiol. 70:1600-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nouaille, S., A. L. Ribeiro, A. Miyoshi, D. Pontes, Y. Le Loir, S. Costa Oliveira, P. Langella, and V. Azevedo. 2003. Heterologous protein production and delivery systems for Lactococcus lactis. Genet. Mol. Res. 2:102-111. [PubMed] [Google Scholar]
- 22.Pogliano, J. A., and J. Beckwith. 1994. SecD and SecF facilitate protein export in Escherichia coli. EMBO J. 13:554-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poquet, I., V. Saint, E. Seznec, N. Simoes, A. Bolotin, and A. Gruss. 2000. HtrA is the unique surface housekeeping protease in Lactococcus lactis and is required for natural protein processing. Mol. Microbiol. 35:1042-1051. [DOI] [PubMed] [Google Scholar]
- 24.Ravn, P., J. Arnau, S. M. Madsen, A. Vrang, and H. Israelsen. 2003. Optimization of signal peptide SP310 for heterologous protein production in Lactococcus lactis. Microbiology 149:2193-2201. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro, L. A., V. Azevedo, Y. Le Loir, S. C. Oliveira, Y. Dieye, J. C. Piard, A. Gruss, and P. Langella. 2002. Production and targeting of the Brucella abortus antigen L7/L12 in Lactococcus lactis: a first step towards food-grade live vaccines against brucellosis. Appl. Environ. Microbiol. 68:910-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Shortle, D. 1983. A genetic system for analysis of staphylococcal nuclease. Gene 22:181-189. [DOI] [PubMed] [Google Scholar]
- 28.Simon, D., and A. Chopin. 1988. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie 70:559-566. [DOI] [PubMed] [Google Scholar]
- 29.Terzaghi, B. E, and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tjalsma, H., H. Antelmann, J. D. H. Jongbloed, P. G. Braun, E. Darmon, R. Dorenbos, J.-Y. F. Dubois, H. Westers, G. Zanen, W. J. Quax, O. P. Kuipers, S. Bron, M. Hecker, and J. M. van Dijl. 2004. Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol. Mol. Biol Rev. 68:207-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tjalsma, H., A. Bolhuis, J. D. H. Jongbloed, S. Bron, and J. M. van Dijl. 2000. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol. Mol. Biol Rev. 64:515-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbanus, M. L., L. Froderberg, D. Drew, P. Bjork, J. W. de Gier, J. Brunner, B. Oudega, and J. Luirink. 2002. Targeting, insertion, and localization of Escherichia coli YidC. J. Biol. Chem. 277:12718-12723. [DOI] [PubMed] [Google Scholar]
- 33.van der Vossen, J. M., D. van der Lelie, and G. Venema. 1987. Isolation and characterization of Streptococcus cremoris Wg2-specific promoters. Appl. Environ. Microbiol. 53:2452-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]