Abstract
COPII-coated vesicles, first identified in yeast and later characterized in mammalian cells, mediate protein export from the endoplasmic reticulum (ER) to the Golgi apparatus within the secretory pathway. In these organisms, the mechanism of vesicle formation is well understood, but the process of soluble cargo sorting has yet to be resolved. In plants, functional complements of the COPII-dependent protein traffic machinery were identified almost a decade ago, but the selectivity of the ER export process has been subject to considerable debate. To study the selectivity of COPII-dependent protein traffic in plants, we have developed an in vivo assay in which COPII vesicle transport is disrupted at two distinct steps in the pathway. First, overexpression of the Sar1p-specific guanosine nucleotide exchange factor Sec12p was shown to result in the titration of the GTPase Sar1p, which is essential for COPII-coated vesicle formation. A second method to disrupt COPII transport at a later step in the pathway was based on coexpression of a dominant negative mutant of Sar1p (H74L), which is thought to interfere with the uncoating and subsequent membrane fusion of the vesicles because of the lack of GTPase activity. A quantitative assay to measure ER export under these conditions was achieved using the natural secretory protein barley α-amylase and a modified version carrying an ER retention motif. Most importantly, the manipulation of COPII transport in vivo using either of the two approaches allowed us to demonstrate that export of the ER resident protein calreticulin or the bulk flow marker phosphinothricin acetyl transferase is COPII dependent and occurs at a much higher rate than estimated previously. We also show that the instability of these proteins in post-ER compartments prevents the detection of the true rate of bulk flow using a standard secretion assay. The differences between the data on COPII transport obtained from these in vivo experiments and in vitro experiments conducted previously using yeast components are discussed.
INTRODUCTION
In yeast and mammalian cells, the export of proteins from the endoplasmic reticulum (ER) occurs in COPII-coated vesicles. The process of COPII vesicle formation is well understood and can be reconstituted using purified yeast components (Barlowe et al., 1994). Vesicle formation in vitro depends solely on the ER donor membrane, the GTPase Sar1p, the Sar1p-specific guanosine nucleotide exchange factor Sec12p, the cytosolic COPII coat components, and GTP (Barlowe et al., 1994). Considerably less is known about the sorting of soluble cargo molecules during ER export in eukaryotes (Klumperman, 2000), and conflicting reports from the plant field have contributed to the ongoing discussions (Crofts et al., 1999; Gomord and Faye, 2000; Pagny et al., 2000; Pimpl and Denecke, 2000).
To support protein synthesis and folding, the ER lumen maintains high levels of soluble residents such as the lumenal binding protein (BiP), protein disulfide isomerase, or calreticulin. The concentration of nonresidents in the ER lumen that are in transit to other compartments, such as the vacuole or the extracellular matrix, usually is much lower (Macer and Koch, 1988). Despite this fact, anterograde transport of nonresidents is efficient, whereas the cells are able to restrict the secretion of the far more abundant ER residents to a minimum (Pelham, 1995).
The fact that different secreted proteins are secreted at various rates in mammalian cells led to the postulation of ER export signals, which would exhibit different affinities for a common ER export receptor (Fitting and Kabat, 1982; Lodish et al., 1983). A few years later, an alternative concept emerged, which implied that sorting signals act essentially as retention signals that deviate proteins from a default route that leads to the cell surface (Munro and Pelham, 1987; Wieland et al., 1987). The default pathway or “bulk flow” model has been popular in the plant field as a result of very convincing results obtained with neutral passenger molecules that normally reside in the cytosol of bacteria or plants but are secreted when introduced into the ER lumen (Denecke et al., 1990; Hunt and Chrispeels, 1991; reviewed by Vitale and Denecke, 1999).
In addition, ER resident and secretory proteins were found to have the same mobility in the ER lumen of Xenopus oocytes (Ceriotti and Colman, 1988), which suggests that both types of proteins can diffuse freely into and out of nascent anterograde transport vesicles. This result led to the suggestion that ER resident proteins must be retrieved from a post-ER compartment, a notion that was supported by the fact that soluble ER proteins were more abundant than any ER membrane protein that could act as a receptor. Evidence for such a recycling mechanism has been obtained by allowing a typical mammalian ER retention motif (KDEL) to compete with a lysosomal sorting signal on the same hybrid protein. This protein accumulated in the ER lumen but had received modifications of the mannose-6 phosphate group, which are inherent to the cis-Golgi apparatus (Pelham, 1988).
Further evidence for recycling came from the discovery of the transmembrane receptor ERD2, which binds to the C-terminal sorting motifs of ER residents (mainly HDEL, KDEL, and RDEL) in a sequence- and pH-dependent manner (Lewis et al., 1990; Wilson et al., 1993). Conclusive proof for Golgi-to-ER recycling arose by demonstrating that the ligand induced redistribution of ERD2 from the cis-Golgi to the ER (Lewis and Pelham, 1992). Yeast ERD2 and mammalian ERD2 homologs are now among the most well characterized sorting receptors of the secretory pathway (Scheel and Pelham, 1998). An Arabidopsis ERD2 homolog was cloned (Lee et al., 1993), and evidence for Golgi-to-ER recycling of this protein in tobacco was demonstrated with the help of green fluorescent protein fusion proteins (Boevink et al., 1999).
One remaining inconsistency of the bulk flow model is that efficient recycling of large numbers of ligands by a limited number of receptors would operate satisfactorily only if the receptor were exported to the Golgi more rapidly than the ER residents. This would suggest that at least membrane proteins could be exported selectively at a rate that surpasses bulk flow. Interestingly, COPII vesicles generated in vitro, which perform anterograde transport between the ER and the Golgi, were found to be enriched for secretory cargo but lacked the ER resident BiP (Barlowe et al., 1994). This indicated that secretory cargo is selected and concentrated into COPII vesicles during budding so that ER residents are excluded. Further evidence for the concentration of secretory cargo during export was provided by the identification of a diacidic ER export signal (Asp-X-Glu, where X is any amino acid) in the cytosolic tail of vesicular stomatitis virus glycoprotein (Balch et al., 1994; Nishimura and Balch, 1997). These results suggest the presence of an export receptor, which was originally proposed almost two decades ago (Fitting and Kabat, 1982; Lodish et al., 1983).
The diacidic ER export signal requires a cytosolic receptor molecule, but selective export of soluble proteins would depend on the presence of a receptor in the lumen. ERGIC-53 has been suggested to act as the lumenal sorting receptor for glycoproteins (Appenzeller et al., 1999), whereas p24 is considered to be the receptor for glycosyl phosphatidyl inositol-anchored proteins (Muñiz et al., 2000). However, the p24 group does not appear to be essential for the secretion of soluble proteins such as invertase (Springer et al., 2000), and the identification of an export signal on these proteins remains elusive. Furthermore, concentration of soluble cargo is likely to occur through exclusion from selective retrograde transport, and thus after (rather than during) the ER export step in the COPII vesicles (Martinez-Menarguez et al., 1999). In summary, evidence for specific export receptors is emerging (Hauri et al., 2000), but evidence for bulk flow also has been obtained, particularly for soluble proteins (Vitale and Denecke, 1999; Klumperman, 2000).
Inefficient ER export of some ER residents could occur through competition or steric hindrance, thus providing an alternative explanation for the enrichment of cargo molecules in transport vesicles without a requirement for active sorting. The notion that ER residents are present in large complexes (Tatu and Helenius, 1997; Crofts and Denecke, 1998; Crofts et al., 1998) could mean that their diffusion by bulk flow would be minimal (Pagny et al., 2000). This would explain the lack of BiP in anterograde COPII vesicles (Barlowe et al., 1994). However, this does not rule out the transport of secretory proteins by bulk flow, and at least the results from Ceriotti and Colman (1988) suggest that ER residents also can diffuse freely in the ER of Xenopus oocytes. The fact that deletion of HDEL from calreticulin results in secretion of the protein (Crofts et al., 1999) supports the idea that some ER export is a result of bulk flow. In addition, the often cited case of BiP has now been shown to represent a special situation, because truncated BiP devoid of its ER retention motif is retained via interaction with calreticulin (Crofts et al., 1999). Hence, the data currently available certainly provide sufficient evidence for the existence of anterograde bulk flow, but it remains to be shown how efficient this type of transport is in vivo and which route it takes. In addition, it is not well established whether HDEL-mediated recycling occurs mainly from the cis-Golgi or from more distal compartments in plants (Crofts et al., 1999; Frigerio et al., 1999, 2001; Gomord and Faye, 2000; Pagny et al., 2000; Pimpl and Denecke, 2000).
The studies described here have determined how the ER resident protein calreticulin, the secretory protein α-amylase, and a bulk flow marker exit the ER in vivo and from which compartment HDEL proteins are retrieved. The results suggest that bulk flow of soluble proteins is COPII dependent and occurs at a much higher rate than perceived previously.
RESULTS
Retrieval of HDEL Proteins Occurs Mainly from the cis-Golgi
Overexpression of the soluble ER resident protein calreticulin results in saturation of the HDEL receptor in tobacco cells but does not lead to recycling from post-cis-Golgi compartments (Crofts et al., 1999). This saturation is not restricted to calreticulin but also leads to the secretion of other ER residents, including BiP. However, the secretion of ER residents is very low, and the true level of ER export is masked by degradation in a post-ER compartment (Denecke et al., 1992; Crofts et al., 1999). To overcome this problem, we used barley α-amylase as a soluble cargo molecule. This enzyme can be detected using a sensitive and quantitative colorimetric assay and should exit the ER at a high rate because it is a natural secretory protein. The ER retention motif HDEL, fused to the C terminus of this cargo molecule, creates a ligand for the HDEL receptor and does not affect the stability and enzymatic properties of the enzyme (Crofts et al., 1999). Moreover, if ER export of α-amylase and its HDEL-tagged derivative is more efficient than the export of ER residents, much lower levels should suffice to saturate the HDEL receptor.
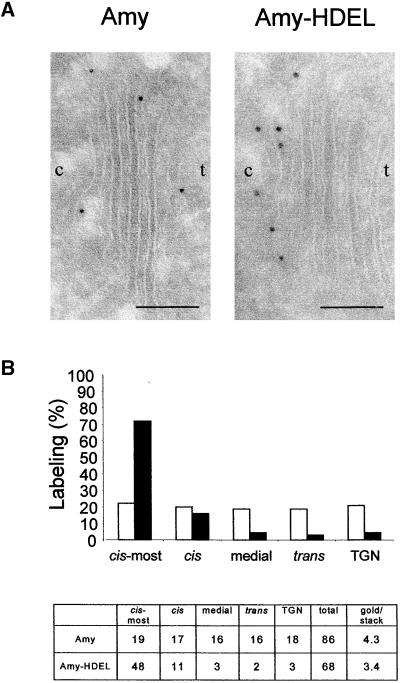
Figure 1A shows an electron micrograph of the Golgi apparatus from plants producing either α-amylase or α-amylase-HDEL. Labeling using antibodies to α-amylase revealed that the secreted molecule was distributed equally over all of the cisternae of the Golgi apparatus. However, tagging α-amylase with HDEL led to predominant labeling of the cis- and cis-most cisternae. This is particularly clear from the statistical data from 20 Golgi stacks (Figure 1B). Occasional labeling of the compartments distal to the cis-Golgi in α-amylase-HDEL–producing plants indicated that saturation of ER retention may have occurred. In addition, both α-amylase and α-amylase-HDEL were detected in the cell wall with equal density (data not shown), confirming that ER retention via HDEL tagging was incomplete.
Figure 1.
Accumulation of HDEL Ligands in the cis-Golgi of Plants.
(A) Cryosections of roots from transgenic tobacco plants expressing either α-amylase (Amy) or α-amylase-HDEL (Amy-HDEL). Immunogold labeling (10 nm) was performed with α-amylase antibodies. The figures show a typical Golgi stack, which contains five cisternae. The orientation of the Golgi is indicated (c, cis; t, trans). Bars = 1 μm.
(B) Statistical analysis of the distribution of α-amylase (white bars) and α-amylase-HDEL (black bars) over 20 counted Golgi stacks. The table indicates the total number of gold particles counted for each Golgi cisterna. The last two columns indicate the total number of gold particles counted and the average labeling density per Golgi stack. Note that most of the Amy-HDEL labeling was found in the cis-most Golgi cisternae and very little labeling was found in the medial and trans-Golgi cisternae or the trans-Golgi network (TGN).
Partial Secretion of α-Amylase-HDEL Is Attributable to Saturation of the ER Retention Machinery
The presence of α-amylase-HDEL in the cell wall could be attributable to incomplete functionality of the HDEL motif in the context of the α-amylase C terminus. Indeed, it has been shown that ER retention tetrapeptide motifs are not completely independent of their context (Denecke et al., 1992). Alternatively, partial retention could be caused by saturation of the HDEL receptor, possibly as a result of the high rate at which the cargo molecule is transported out of the ER. To distinguish between these two possibilities, a time course expression experiment with tobacco protoplasts was conducted to compare the transport of α-amylase and α-amylase-HDEL. At different times after transfection, the culture medium and cells were harvested and α-amylase activity was measured.
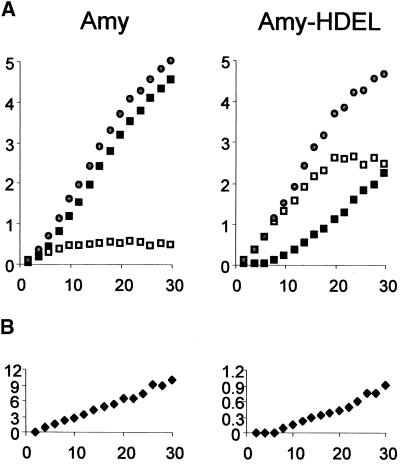
Figure 2A shows that α-amylase was secreted rapidly as judged by the low intracellular steady state level reached as a result of an equilibrium between de novo synthesis and export. Extracellular α-amylase activity was detected soon after transfection and increased linearly after 6 hr of expression. In contrast, α-amylase-HDEL accumulated to high levels within the cells and only reached an intracellular steady state level 20 hr after transfection. Secretion of α-amylase-HDEL into the medium became apparent after 10 hr of expression. At later times, the secreted levels of the HDEL-tagged enzyme increased, and the slope of the curve approached that of α-amylase. Thus, at the end of the time course, the secretion of both enzymes was comparable.
Figure 2.
α-Amylase-HDEL Secretion Is Caused by Saturation of the HDEL Receptor.
(A) Transient expression in tobacco protoplasts transfected with plasmid DNA encoding α-amylase (Amy) or the HDEL-tagged derivative (Amy-HDEL). For each construct, 10 standard transfections were pooled and redistributed over 15 samples to achieve minimal variation. A time course is shown with α-amylase activity in cells (open squares), in the culture medium (closed squares), and the total activity (open circles). The time after transfection is given in hours. α-Amylase activity is given in change in OD per milliliter of culture suspension per minute.
(B) Ratio between the extracellular and intracellular α-amylase activities, termed the secretion index. Note the 10-fold difference in the scales between the α-amylase and the α-amylase-HDEL panels.
Figure 2 shows that retention was complete as long as the intracellular levels did not reach a certain threshold. If partial secretion were caused by incomplete presentation of the HDEL motif at the C terminus of α-amylase, secretion would have occurred immediately after synthesis. The results demonstrated that the secretion of α-amylase-HDEL was attributable to high intracellular levels of this protein, probably causing saturation of the HDEL receptor.
It should be noted that the total activity obtained was independent of the presence of the HDEL motif. This finding shows that the enzyme was stable and enzymatically active regardless of its location in the secretory pathway and confirms previous observations that HDEL tagging has no deleterious effect on the activity of α-amylase (Crofts et al., 1999). In contrast, the ratio of extracellular activity to intracellular activity, termed the secretion index (Denecke et al., 1990), was decreased dramatically by tagging with the retention motif, as shown in Figure 2B. The secretion index increased linearly over time for both cargo molecules but generally was 1 order of magnitude lower for the HDEL-tagged protein, as illustrated by the scale of the y axis. Because of the linear nature of these curves, the secretion rate of the two different cargo molecules can be compared simply via measurement of the secretion index at a single time, conveniently after 24 hr of incubation.
Saturation of ER Export by α-Amylase Overproduction
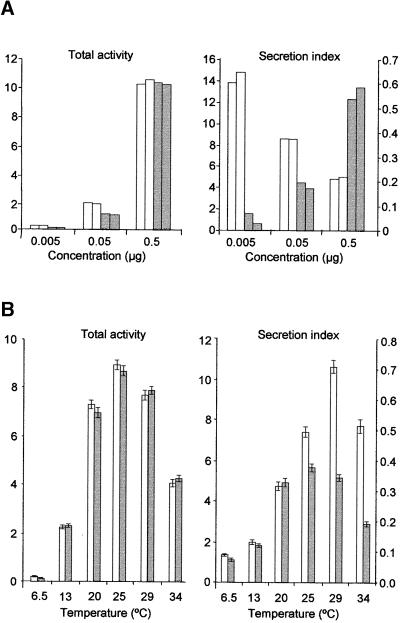
Although it appears that the secretion of HDEL ligands was cargo dosage dependent, a change in the physiology of the cells during prolonged incubation could provide an alternative explanation. To test this possibility, a concentration series of plasmids was used for transfections and cells were incubated for a constant period of 24 hr. Figure 3A shows that the secretion index for α-amylase-HDEL (gray bars) increased with higher plasmid concentration. Because the incubation time was identical in all cases, the data are consistent with the saturation model.
Figure 3.
Effect of Cargo Dosage and Temperature on Secretion and Retention.
Transient expression in tobacco protoplasts transfected with plasmid DNA encoding either α-amylase or the HDEL-tagged derivative.
(A) Total activity and secretion index of α-amylase (white bars) compared with α-amylase-HDEL (gray bars) measured 24 hr after transfection of protoplasts. The units of α-amylase activity are the same as in Figure 2. The concentration of the plasmids used for transfection is indicated on the x axis. The secretion index of α-amylase is given on the left y axis, and the secretion index of α-amylase-HDEL is given on the right y axis. Data from two independent experiments are shown.
(B) Influence of temperature on protein synthesis (total activity) and the secretion index of α-amylase (white bars) compared with α-amylase-HDEL (gray bars). For each construct, four standard transfections were pooled and redistributed over six samples to achieve minimal variation. The secretion index of α-amylase is given on the left y axis, and the secretion index of α-amylase-HDEL is given on the right y axis. Data shown are averages from three independent experiments, and error bars indicate ±sd.
Interestingly, the control experiment using different concentrations of the plasmid encoding α-amylase (white bars) gave rise to an unexpected result. High dosage of the cargo molecule resulted in a reduction of the secretion index, suggesting that saturation of anterograde transport also had occurred. This is in contrast to the results obtained previously with bulk flow markers, which appear to be secreted more efficiently when higher levels are synthesized (Denecke et al., 1990).
The fact that secretion can be saturated by overexpression suggests that there is a limiting factor that can be saturated. The data for α-amylase-HDEL (Figure 3A) thus could be the result of superimposing two counteracting effects, the saturation of anterograde transport and the saturation of the HDEL receptor. The latter effect appears to be much stronger, hence the net increase in the secretion index for α-amylase-HDEL.
Differential Effect of Temperature on Anterograde and Retrograde Transport
To further optimize our transport assay, we tested the influence of temperature on the transport of these two cargo molecules. Whereas α-amylase would follow the anterograde transport route, the HDEL-tagged version would engage in both anterograde and retrograde transport between the ER and the Golgi. Figure 3B reveals that the temperature had a strong effect on the total amount of enzyme activity obtained, but this effect was identical for both cargo molecules. In contrast, the secretion index was influenced in a differential manner that was dependent on the presence or absence of the HDEL motif.
Considering the effect of the expression levels on the secretion index of α-amylase-HDEL shown in Figure 3A, the temperature-dependent increase in the secretion index of this cargo molecule could be explained by the expression levels alone (cf. left and right panels). This suggests that temperature has no influence on the secretion index of this cargo molecule. However, this is not the case for α-amylase, because correction for expression levels revealed an even steeper increase in α-amylase secretion, particularly in the low temperature range. This gradual increase is in sharp contrast to findings from mammalian cells. The differential effect of the temperature on these two molecules shows that anterograde and retrograde transport are different cellular processes supported by different molecular machinery. All further transient expression experiments were performed at 25°C, because that appeared to be the optimum temperature for both synthesis and transport.
Sec12p Dosage-Dependent Inhibition of α-Amylase and α-Amylase-HDEL Export
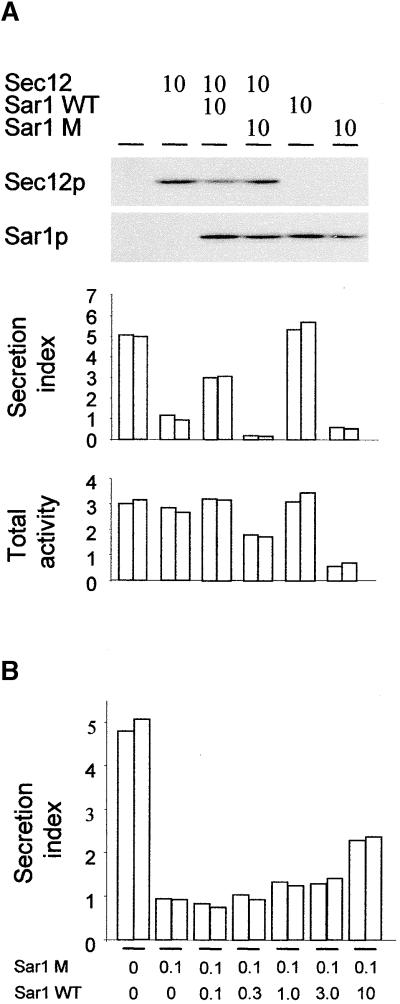
To show that α-amylase and its HDEL-tagged derivative exit the ER in COPII vesicles, we took advantage of the fact that overproduction of Sec12p reduces ER export (d'Enfert et al., 1991a, 1991b; Hardwick et al., 1992; Barlowe and Schekman, 1993; Barlowe et al., 1993; Nishikawa et al., 1994), presumably via the titration of Sar1p, which is essential for COPII vesicle budding (Barlowe and Schekman, 1993; Barlowe et al., 1993, 1994). We used the Arabidopsis homolog of Sec12p, which was shown to complement the corresponding yeast mutant (d'Enfert et al., 1992).
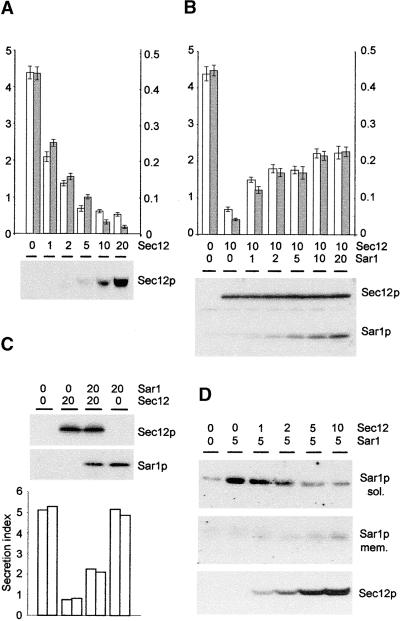
Figure 4A shows that Sec12p overproduction resulted in a clear dosage-dependent inhibition of α-amylase secretion. In the case of α-amylase-HDEL, inhibition of ER export via Sec12p overproduction would be expected to alleviate the saturation of the HDEL receptor because smaller amounts of the ligand reach the cis-Golgi. Indeed, Figure 4A shows that at high levels of Sec12p a steeper inhibition of secretion for α-amylase-HDEL was observed, providing additional support for the saturation model.
Figure 4.
In Vivo Manipulation of COPII-Dependent ER Export.
Transient expression in tobacco protoplasts transfected with plasmid DNA encoding α-amylase, the HDEL-tagged derivative, a Sec12p overexpression construct, or a Sar1p overexpression construct.
(A) Dosage-dependent inhibition of secretion of α-amylase and α-amylase-HDEL by Sec12p coexpression measured 24 hr after transfection. The amount of Sec12p-encoding plasmid is given in micrograms, and a constant amount of α-amylase–encoding (white bars) or α-amylase-HDEL–encoding (gray bars) plasmids (2 μg) was used in each lane. The secretion index of α-amylase is given on the left y axis, and the secretion index of α-amylase-HDEL is given on the right y axis. Averages of three independent experiments are shown, and error bars indicate ±sd. The protein gel blot shows the expression of Sec12p in each lane from one representative experiment.
(B) Partial reconstitution of Sec12p-mediated inhibition of secretion by coexpression of Sar1p. Annotations are as in (A). Averages of three independent experiments are shown, and error bars indicate ±sd. The protein gel blots illustrate the constant levels of Sec12p and the increased expression of Sar1p (concentrations of Sar1p-encoding plasmid given in micrograms) and are from one representative experiment.
(C) Demonstration that Sar1p overexpression alone does not influence secretion. Annotations are as in (B), but only the secretion of α-amylase was tested with maximum levels of Sec12p and Sar1p. Quantities of plasmids are given in micrograms. Two independent experiments are shown for each lane, and the protein gel blots are from one representative experiment.
(D) Membrane recruitment of Sar1p by increasing the levels of Sec12p. Protoplasts were extracted by osmotic shock to yield the cytoplasmic fraction (sol.) and the membrane fraction (mem.). Sec12p was present only in the membrane fraction. Quantities of plasmids are given in micrograms.
Sec12p Overexpression Inhibits COPII Transport via Titration of the GTPase Sar1p
It has been suggested that the inhibition of ER export via Sec12p overexpression is the result of titration of the low molecular weight GTPase Sar1p (d'Enfert et al., 1991a, 1991b; Hardwick et al., 1992; Barlowe and Schekman, 1993; Barlowe et al., 1993; Nishikawa et al., 1994), thus leading to an inhibition of COPII vesicle budding that is dependent on Sar1p (Barlowe and Schekman, 1993; Barlowe et al., 1993, 1994). To confirm that Sar1p was limiting during Sec12p overexpression, we attempted to rescue the secretion of α-amylase via coexpression of increasing levels of Sar1p superimposed onto a constant inhibitory level of Sec12p. Figure 4B shows that a partial reconstitution of α-amylase secretion did occur upon coexpression of Sar1p.
To exclude the possibility that this recovery was caused by an independent effect of Sar1p overexpression on secretion, increasing levels of Sar1p were tested in the absence of Sec12p overexpression. In this case, α-amylase secretion was unaffected (Figure 4C). This result shows that the recovery observed in Figure 4B must have been the result of a suppression of the Sec12p effect. This finding provides further proof of the titration hypothesis (d'Enfert et al., 1991a, 1991b; Hardwick et al., 1992; Barlowe and Schekman, 1993; Barlowe et al., 1993; Nishikawa et al., 1994).
To confirm that Sec12p can interact physically with Sar1p or alter its cellular location, we coexpressed an increasing amount of Sec12p with a constant amount of Sar1p. Figure 4D shows that Sar1p is mostly cytosolic when overproduced but that increasing levels of Sec12p result in recruitment of the GTPase to the membrane. The four experiments whose results are shown in Figure 4 clearly establish that Sec12p overproduction can be used to inhibit COPII transport in vivo.
Inhibition of COPII Transport via Coexpression of Mutant GTP-Trapped Sar1p
To establish an alternative method to manipulate COPII transport in our system, we took advantage of the known trans-dominant negative effect on COPII vesicle transport by a GTP-trapped mutant of Sar1p (Saito et al., 1998; Takeuchi et al., 1998, 2000). This mutant is less sensitive to the GTPase-activating activity of Sec23, and it traps vesicles in a coated configuration so that they are unable to fuse with the target membrane. Figure 5A shows that, in contrast to wild-type Sar1p, mutant Sar1p inhibits α-amylase secretion when coexpressed. In addition, coexpression of Sec12p with mutant Sar1p causes a further reduction in secretion compared with coexpression of either molecule alone. In contrast, wild-type Sar1p alleviates the effect of Sec12p overexpression. Both wild-type and mutant Sar1p were detected at comparable levels using protein gel blotting, thus ruling out any artifact resulting from differences in the expression levels.
Figure 5.
Inhibition of ER-to-Golgi Transport Using Mutant Sar1p.
Transient expression in tobacco protoplasts transfected with plasmid DNA encoding α-amylase with a variety of cotransfected constructs.
(A) Transport assay to detect the influence of the dominant negative GTP-trapped mutant of Sar1p (H74L) after 24 hr of coexpression. The numbers above the lanes refer to the amount in micrograms of plasmids encoding Sec12p, Sar1p wild type (Sar1p WT), or mutant Sar1p (Sar1p M). The top panel shows a protein gel blot to detect Sec12p and the two different forms of Sar1p. The bottom panel shows the secretion index and the total activity. Data from two independent experiments are shown, lanes are as in the top panel, and the protein gel blots are from one representative experiment. Note the strong reduction in the secretion index when both Sec12p and mutant Sar1p are coexpressed and that the total activity is higher than with individually coexpressed mutant Sar1p (last pair of bars).
(B) Transport assay to monitor the effect of coexpressed wild-type Sar1p on a constant level of mutant Sar1p. The secretion index is shown, and the lanes indicate the quantities of the corresponding plasmids transfected in micrograms. Data from two independent experiments are shown. Note that only a 100-fold excess of wild-type Sar1p rescues some of the secretion inhibited by the mutant GTPase.
Interestingly, the Sar1p mutant caused a reduction in the total amount of the secretory marker (Figure 5A). This is in contrast to Sec12p, which inhibits secretion without reducing the levels of this enzyme. Second, further inhibition of secretion via the combined effect of the Sar1p mutant and Sec12p overexpression resulted in a recovery of total α-amylase. This means that the negative effect of the GTPase mutant on total α-amylase levels was not attributable to the inhibition of the secretion process itself and that Sec12p coexpression actually prevented the loss of marker protein.
These data suggest that Sec12p overexpression and Sar1p mutant coexpression inhibit secretion in different manners. Thus, we tried to establish whether the GTPase mutant inhibits COPII transport through a displacement of the wild-type molecule. Figure 5B shows that coexpression of the wild-type protein at stoichiometric levels did not restore secretion to any extent. Unless 100-fold overexpression of the wild-type protein relative to the mutant was implemented, hardly any effect was observed. These results are in contrast to those shown in Figure 4B, in which recovery was immediately apparent with the lowest levels of Sar1p. It should be noted that 100-fold lower levels of mutant Sar1p were used compared with the levels used in Figure 5A, yet an efficient inhibition of α-amylase secretion was seen.
Together, these results suggest that the GTPase mutant inhibits the COPII transport mechanism at a different level compared with Sec12p, which acts through a depletion of Sar1p. It is likely that Sec12p overexpression inhibits the recruitment of the COPII coat, which is dependent on Sar1p, and thus acts at the earliest possible position in the pathway. In contrast, the GTPase mutant prevents uncoating of the vesicles and thus acts at a later stage. Thus, both methods are complementary approaches to manipulate COPII transport in vivo.
ER Export of Calreticulin and a Bulk Flow Marker Occurs in a COPII-Dependent Manner
COPII vesicles from yeast generated in vitro have been shown to contain anterograde cargo molecules such as yeast α-factor but not ER residents such as BiP (Barlowe et al., 1994). However, bulk flow to the cell surface has been shown repeatedly to occur for a number of soluble passenger molecules in plant cells (Vitale and Denecke, 1999). This suggests either differences in the early secretory pathway between yeast and plants or that, in addition to COPII vesicles, other transport mechanisms exist to carry bulk flow out of the ER. To distinguish between these possibilities, it was necessary to determine whether the secretion of bulk flow markers (Denecke et al., 1990) or ER residents (Crofts et al., 1999) is COPII dependent.
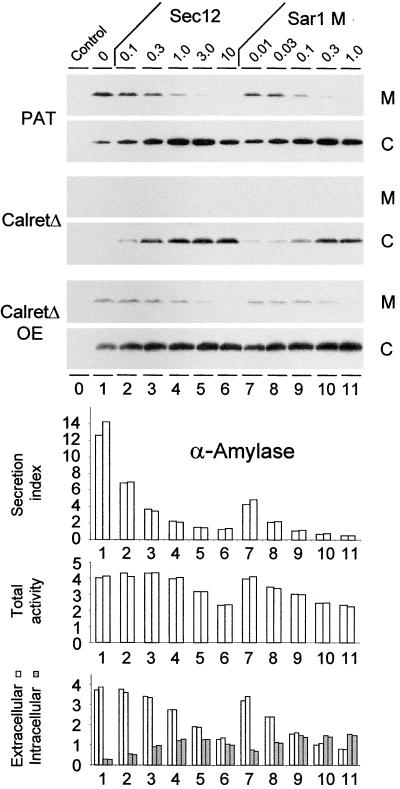
Figure 6 shows that secretion of the bulk flow marker phosphinothricin acetyl transferase (PAT) (Denecke et al., 1990, 1992) is inhibited by Sec12p overexpression as well as mutant Sar1p coexpression, demonstrating that PAT was transported in a COPII-dependent manner. In contrast to α-amylase, the increase in intracellular PAT levels exceeded the reduction in extracellular PAT. This confirms an observation reported previously that a portion of PAT is degraded when allowed to proceed further than the ER in the secretory pathway (Denecke et al., 1992; Höfte and Chrispeels, 1992).
Figure 6.
Secretion of ER Residents and Bulk Flow Markers Is COPII Dependent.
Transient expression in tobacco protoplasts. The culture medium (M) and the cells (C) of protoplast suspensions were recovered 48 hr after transfection. Cargo molecules were detected either by protein gel blotting (top) or via enzymatic analysis (bottom). The numbers above the lanes refer to the amount of Sec12p-encoding plasmid (Sec12) or mutant Sar1p-encoding plasmid (Sar1 M) in micrograms. As cargo molecules, the bulk flow marker PAT or calreticulinΔHDEL (CalretΔ) were coexpressed in constant amounts (2 μg). CalreticulinΔHDEL was also 10-fold overexpressed (CalretΔ OE) in the bottom panel (20 μg). The control lane is devoid of any cargo molecules. To detect PAT, anti-PAT serum was used (Denecke et al., 1992), whereas introduced calreticulinΔHDEL was detected with c-myc antibodies to allow distinction from endogenous tobacco calreticulin. Note that calreticulinΔHDEL secretion is seen only when the protein is overexpressed. The bottom panel shows the effect of the various inhibitory levels of Sec12p and mutant Sar1p on the transport of the secretory marker α-amylase coexpressed with calreticulinΔHDEL at equal levels (2 μg). The secretion index, the total activity, and the activity in the cells (grays bars) and the culture medium (white bars) are indicated, and the lanes correspond to the lanes at top. Data for two independent experiments are shown. Note that the total α-amylase activity is reduced slightly with increasing levels of either transport inhibitor. Note also that the intracellular accumulation of α-amylase is not higher than that of calreticulinΔHDEL indicated above (CalretΔ).
To determine if ER residents also are transported in a COPII-dependent fashion, a truncated form of calreticulin lacking its HDEL signal was tagged with a c-myc epitope to distinguish it from the endogenous calreticulin. This hybrid protein shows transport properties identical to those of untagged calreticulinΔHDEL in previous experiments (Crofts et al., 1999). To avoid lengthy names, we will refer to the myc-tagged calreticulin as calreticulinΔHDEL. Figure 6 shows that the secretion of calreticulinΔHDEL is detected only when the molecule is overexpressed, confirming earlier observations (Crofts et al., 1999). However, gradual inhibition of COPII-dependent ER export via increasing Sec12p levels led to a strong recovery of intracellular calreticulinΔHDEL. This is particularly clear at low expression levels, where no protein is detected in the absence of Sec12p.
In contrast to Figure 4, the experiment shown in Figure 6 was performed using a 48-hr incubation to maximize the effect of the inhibitors. To compare the data with those of Figure 4, we conducted the same experiment with the secretory marker α-amylase (Figure 6, bottom). Under these conditions, all secretion indices are approximately twice as high as in Figure 4A, as predicted from the linear relationship of the secretion index seen in Figure 2. The longer incubation period allowed more time for Sec12p overproduction or mutant Sar1p production. This increases the sensitivity of the assay, allowing for the use of smaller quantities of Sec12p- or mutant Sar1p-encoding plasmids. In addition, the GTP-trapped mutant also can exhibit a dosage-dependent effect and reduces the total yield to a much lesser extent compared with the data shown in Figure 5, where much higher plasmid concentrations were used. These results illustrate the quantitative nature of our transport assays and the fact that coexpression of the secretory marker can be used as a routine method in conjunction with Sec12p or the Sar1p mutant to document the inhibition of ER export.
The data shown in Figure 6 allow a direct comparison of the intracellular accumulation of the two test molecules PAT and calreticulinΔHDEL with that of the secretory marker α-amylase. The intracellular levels of α-amylase increase by approximately a factor of 4. Intracellular accumulation of PAT and calreticulinΔHDEL appeared to be similar, if not higher, particularly when the latter was coexpressed at low levels. This means that the three cargo molecules exit the ER in COPII vesicles at a comparable rate.
Because neither inhibition strategy has led to an increase in the total yield of the secretory marker, it can be concluded that the capacity of the ER to synthesize proteins was not higher. Hence, the increase in the total levels of PAT and particularly calreticulinΔHDEL is unlikely to be caused by a higher synthesis rate or an indirect effect on the 35S promoter, which controls the transcription of these three cargo molecules.
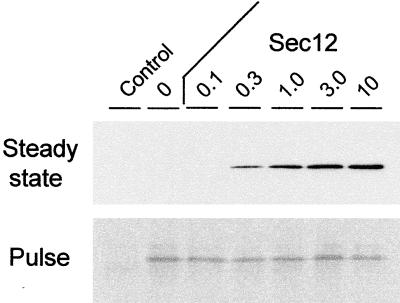
One further control experiment was conducted to rule out an increased synthesis rate of calreticulinΔHDEL as a result of the inhibition of ER export. At 24 hr, we compared the steady state protein levels with the protein synthesis rate. To estimate the latter, we conducted a pulse labeling of the cells for 30 min at the 24-hr time point. Subsequent quantitative immunoprecipitation and SDS-PAGE revealed the amount of protein synthesized during this short time interval and provided an indication of the synthesis rate. In contrast, the total protein levels detected by protein gel blotting had accumulated over the course of the 24-hr period and depended on the rate of synthesis as well as the rate of degradation, resulting in a steady state level.
Figure 7 clearly shows that, although the steady state protein levels are very much increased by the presence of Sec12p, the amount synthesized during the 30-min interval (pulse) was almost identical in all cases. This finding indicates once more that Sec12p overexpression had no effect on the synthesis of calreticulinΔHDEL and that the increase in the steady state level must result from preventing turnover. The inhibition of ER export effectively rescues calreticulinΔHDEL from degradation.
Figure 7.
Comparison of Steady State Protein Levels and the de Novo Synthesis Rate.
Results of a transient expression experiment in which tobacco protoplasts were harvested 24 hr after transfection and equal portions were analyzed for protein levels (Steady state) or for the ability to synthesize protein de novo during a 30-min pulse-labeling procedure (Pulse). The steady state levels of calreticulinΔHDEL were assessed by protein gel blotting using anti-c-myc antibodies, whereas pulse-labeled calreticulinΔHDEL was detected via quantitative immunoprecipitation using anti-c-myc antibodies, subsequent SDS-PAGE, and autoradiography. The numbers above the lanes refer to the amount of coelectroporated Sec12p-encoding plasmid (Sec 12) in micrograms. CalreticulinΔHDEL was coexpressed in a low amount (2 μg) as in Figure 6, which does not allow the detection of protein in the medium. Note that the levels detected using pulse labeling were constant, in sharp contrast to the steady state protein levels.
Comparison of Figures 6 and 7 reveals that at 24 hr, the lowest level of Sec12p has no stabilizing effect on calreticulinΔHDEL, whereas at 48 hr, a weak stabilization is observed compared with the control. This again shows that longer incubations provide a more sensitive ER export inhibition assay, as could be predicted from the fact that it requires time to accumulate Sec12p to sufficient levels to titrate Sar1p.
We conclude that PAT and calreticulinΔHDEL are exported efficiently from the ER via COPII-mediated transport and then are degraded primarily in a post-ER compartment. This degradation prevents a significant proportion of these molecules from reaching the culture medium. This finding explains previous results showing poor secretion of ER residents when devoid of their ER retention signal or during saturation of the HDEL pathway (Crofts et al., 1999). When overexpressed, some calreticulinΔHDEL escapes degradation and can be detected in the culture medium, but this still does not adequately represent the amount of ER export that takes place.
DISCUSSION
A Model System for Anterograde and Retrograde ER-to-Golgi Transport in Plants
Using the secretory protein α-amylase, we have created an artificial ligand for the HDEL receptor and obtained a quantitative transport assay for either anterograde transport alone (α-amylase) or a combination of anterograde and retrograde transport (α-amylase-HDEL). Together with the properties of homogeneous protoplast suspensions and the ease with which cell extracts and medium can be analyzed, this system has proven to be very suitable for studying ER-to-Golgi transport in vivo.
The first result (Figure 1) confirms earlier observations suggesting that in plants the cis-Golgi apparatus is the main compartment from which ERD2-mediated retrieval occurs (Pedrazzini et al., 1997; Crofts et al., 1999; Frigerio et al., 1999, 2001; Eliot Herman, personal communication). Whereas α-amylase was distributed evenly among the Golgi stacks, α-amylase-HDEL was enriched specifically in the cis-most Golgi and to a lesser extent in the cis-Golgi (Figure 1). The strong statistical analysis shown in Figure 1 adds further weight to previous results on the distribution of the auxin binding protein (a KDEL protein), which is localized predominantly in the cis-Golgi (Bauly et al., 2000). This was observed as well when KDEL was replaced by HDEL, but not when the signal was rendered nonfunctional (KEQL or KDELGL). Labeling of the other cisternae (medial, trans, and trans-Golgi network) was weak but evenly distributed, suggesting that no significant retrieval occurs once the cis-Golgi has been passed. Indeed, escape from the Golgi may be relatively quick, as indicated by the lack of endo-H–resistant forms of HDEL-truncated calreticulin inside the cells (Crofts et al., 1999). However, we cannot exclude the possibility that under stress situations, such as a viral infection or contact with toxins, retrograde transport from more distal Golgi compartments is possible (Lord and Roberts, 1997).
By using this quantitative transport assay, it was also possible to demonstrate that both ER export of α-amylase and Golgi-derived retrograde transport of α-amylase-HDEL are saturable (Figures 2 and 3). This result provides a possible explanation for the partial retention of KDEL-tagged phytohemagglutinin in the ER (Herman et al., 1990). Most of this recombinant protein appeared to be present in the storage vacuoles, and it stands to reason that rapid ER export and active vacuolar transport led to an effective bypass of a limiting number of HDEL/KDEL receptors. The results shown in Figures 2 and 3 suggest that the HDEL/KDEL receptor appears to be more easily saturated than the components needed for ER export.
It also should be noted that plant cells do not exhibit a defined temperature block of secretion, as is observed in mammalian cells. Previous studies indicated that cold shock inhibits the secretion of green fluorescent protein (Boevink et al., 1999). We have extended these observations and tested several temperatures (Figure 3). The results suggest that plant cells exhibit a more gradual temperature dependence of secretion compared with mammalian cells. This corresponds well with the fact that plants survive and grow under variable temperatures. Even though the effect of the temperature on the total yield is independent of the presence of the HDEL motif, measurement of the secretion index revealed a differential effect that was clearly dependent on this sorting motif. Plant cells may compensate for an increase in anterograde transport by adequate retrograde transport to maintain an equilibrium.
α-Amylase and α-amylase-HDEL provide a quantitative test system, consisting of one cargo molecule that follows anterograde transport only and another cargo molecule that follows both the anterograde and the retrograde transport pathways. This test system will provide a useful tool to study the transport machinery of the early secretory pathway.
Sec12p Overexpression Inhibits COPII Transport through the Depletion of Sar1p
The transport assay also has proven to be very suitable for testing the biological activity of the key components Sec12p and Sar1p in vivo. The results clearly confirm that Sec12p overexpression slows anterograde ER-to-Golgi transport (d'Enfert et al., 1991a, 1991b; Hardwick et al., 1992; Barlowe and Schekman, 1993; Barlowe et al., 1993; Nishikawa et al., 1994), and we have established conclusive experimental proof that this effect is caused by a titration of Sar1p. It is well established that Sar1p is essential for the formation of COPII vesicles (Barlowe and Schekman, 1993; Barlowe et al., 1993, 1994) because it controls the recruitment of the COPII coat and becomes part of the COPII vesicle (Barlowe et al., 1994). Because Sec12p overproduction titrates Sar1p from the available pool, it prevents the formation of COPII vesicles and acts at the earliest possible point in the anterograde transport pathway between the ER and the Golgi.
COPII vesicles have not been visualized in plants using electron microscopy, but there is an abundance of data that predicts their presence. First, the identification of Sec12p and Sar1p in plants and their functional complementation in yeast (d'Enfert et al., 1992) suggests that these components control this pathway. Second, the identification of COPII coat components in plants (Movafeghi et al., 1999) and the identification of COPI vesicles in plants suggest that the principle of non-clathrin-coated vesicle formation has been well documented (Pimpl et al., 2000). Whether COPII transport occurs in a vesicular or a tubular manner is not relevant at this stage, and we do not wish to endorse either model; however, because the term COPII vesicles is used in the yeast and mammalian literature, it seems appropriate to use this term for plants as well until hard evidence against this has been presented.
Stable overexpression of Sec12p in transgenic plants was reported previously (Bar-Peled and Raikhel, 1997), but the authors did not report a negative effect on cell viability or a redistribution of Sar1p. Given our results, it may seem strange that Sec12p-overexpressing plants were viable. However, it is possible that plant cells contain regulatory mechanisms to respond to an imbalance in the Sec12p/Sar1p ratio. Hence, the material generated by Raikhel and co-workers could be a useful source of further information. It is also possible that the generation of transgenic plants led to selection for overproduction of a mutated form of Sec12p that is incapable of titrating Sar1p. Thus, transgenic plants would represent an elegant model for the identification of Sec12p mutants.
In contrast to transgenic plants, transiently expressing protoplasts do not have sufficient time to respond to the imbalance; hence, they exhibit the result of the perturbation in our transport assay. Likewise, the saturation of the HDEL receptor may have been so readily observed in protoplasts (Figures 2 and 3) because the time to respond, for instance by upregulating receptor numbers, is insufficient. Hence, transient expression using protoplasts is a powerful technique with which to study and manipulate COPII transport in vivo. In addition to this, the α-amylase transport assays can now be used for the functional analysis of Sec12p using the inhibition assay or for the analysis of Sar1p in the rescue assay. This should prove valuable as an additional tool in combination with the use of GTPase mutants that exhibit a trans dominant negative effect on secretion (Batoko et al., 2000; Takeuchi et al., 2000) (Figure 5).
Sec12p Overproduction and Mutant Sar1p Inhibit COPII Transport at Different Stages
We have compared our method based on Sec12p overproduction with the established inhibition of COPII transport using a mutant form of Sar1p that is trapped in the GTP-bound form. Interesting differences were noted. First, the effects of Sec12p overproduction and mutant Sar1p production were additive and surpassed those of increased individual Sec12p overproduction or Sar1p mutant coexpression. This suggests that the inhibitory mechanism is different. Second, the Sar1p mutant caused a loss of yield of the secretory marker when coexpressed at high levels. This could be rescued by further inhibition of ER export with superimposed Sec12p overproduction. These results show that loss of yield is not simply the result of loss of secretion.
From the data on in vitro vesicle budding (Barlowe et al., 1994), it can be predicted that incorporation into COPII vesicles of a mutant Sar1p that is unable to respond to the GTPase-activating action of Sec23 (Saito et al., 1998; Takeuchi et al., 1998, 2000) would prevent vesicle uncoating and fusion with the target membrane. Perhaps this leads to an as yet undiscovered mechanism for the disposal of such defective vesicles, which would explain the loss of enzyme yield. Sec12p overexpression is likely to inhibit COPII transport at an earlier stage, the recruitment of the COPII complex to the ER membrane, and thus the formation of the vesicle. This could explain why superimposed Sec12p overexpression restores higher levels of the secretory marker, because it would prevent ER export itself and render ER-retained α-amylase insensitive to any mechanism that disposes of vesicles with an uncoating-deficient coat. Indeed, such a mechanism could be of physiological importance, but further research will be required to provide evidence for this. We used lower concentrations of the GTPase mutant (Figure 6) to overcome this problem.
Interestingly, stoichiometric levels of wild-type Sar1p did not overcome the effect of mutant Sar1p. Approximately 100-fold overexpression of the wild-type protein relative to the mutant was required to detect any recovery of secretion at all. It is not known how many copies of the Sar1p protein are present in one COPII vesicle, but it is possible that uncoating of the entire vesicle can be blocked if only a minority of mutant Sar1p molecules are incapable of hydrolyzing GTP. This is worth investigating further but was beyond the scope of this report.
In conclusion, we propose that the Sec12p overexpression strategy inhibits the first step of COPII transport, the export step itself, and constitutes an ideal method of manipulating this stage of the secretory pathway.
Bulk Flow Occurs in a COPII-Dependent Manner
The demonstration of secretory bulk flow (Wieland et al., 1987; Denecke et al., 1990) is in sharp contrast to the absence of ER residents in purified COPII vesicles induced in vitro (Barlowe et al., 1994). The conflicting data could be explained by differences in the specificity of anterograde cargo recruitment between yeast and higher eukaryotes or by a non-COPII ER export pathway that supports bulk flow. The manipulation of COPII vesicle transport in vivo allowed us to determine if this transport mechanism is involved in the secretion of bulk flow markers or ER residents deprived of the HDEL motif (Denecke et al., 1990; Hunt and Chrispeels, 1991; Crofts et al., 1999).
Sec12p overproduction led to a sharp inhibition in the secretion of the bulk flow marker PAT and the ER resident calreticulin (Figure 6). This finding demonstrates that COPII-dependent ER export does support bulk flow in vivo and contrasts with results from in vitro assays (Barlowe et al., 1994). Similar results were obtained with the Sar1p mutant, confirming COPII dependence. The effects of the mutant GTPase on PAT and calreticulinΔHDEL are indistinguishable from those obtained by Sec12p overexpression as long as the levels of the mutant GTPase used inhibit secretion but do not show a loss of yield, as observed in Figure 5.
Inhibition of COPII Transport Prevents Degradation in a Post-ER Compartment
The fact that the recovery of intracellular PAT or calreticulinΔHDEL through the inhibition of COPII transport was at least as high as that of the secretory marker α-amylase indicates that the rate of ER export is comparable for these three proteins. This means that bulk flow is significant. It was observed as well that intracellular recovery exceeded the loss of secreted proteins, particularly in the case of calreticulinΔHDEL when it was expressed at lower levels. Sec12p is a membrane-spanning protein, and it was necessary to determine if Sec12p overexpression could cause an increase in the ER surface and perhaps in the capacity of the ER to synthesize proteins. Measurement of the secretory marker shows that this capacity was even slightly lower during Sec12p overexpression. Second, because expression of all cargo molecules was driven by the same 35S promoter, a possible stimulatory effect of Sec12p on the promoter could be excluded as well. Finally, the GTPase mutant is not a membrane protein, and it causes a reduction in the yield of the secretory marker, but still it led to an increase in the total amount of PAT and calreticulinΔHDEL.
To exclude the possibility of a specific stimulatory effect of Sec12p on calreticulin synthesis but not α-amylase, we compared the synthesis rate, estimated by pulse labeling, with steady state protein levels (Figure 7). Clearly, the synthesis rate of the cargo molecule is not affected by Sec12p, and it can be concluded that the inhibition of anterograde ER-to-Golgi transport leads to a stabilization of calreticulinΔHDEL. In the case of PAT, it was shown previously that poor secretion is attributable to degradation of the majority of the synthesized protein at some point in the secretory pathway before it reaches the culture medium (Höfte and Chrispeels, 1992). The present data suggest that this must occur in a post-ER compartment.
Poor secretion and low expression levels of truncated ER residents have been reported previously (Munro and Pelham, 1987). In the case of calreticulin, deletion of the HDEL motif led to a 100-fold reduction in the steady state protein levels in transgenic plants, yet only low levels of secreted calreticulinΔHDEL were detected (Crofts et al., 1999). HDEL tagging is known to stabilize recombinant proteins transported by the plant secretory pathway (Denecke et al., 1992; Wandelt et al., 1992). Addition of an ER retention motif could alter the protein itself and cause stabilization. Likewise, removal of an HDEL motif from an ER resident could cause a higher turnover. However, our present findings were not based on altering the cargo molecules themselves but through the inhibition of anterograde transport at two different positions in the pathway. Therefore, the stabilization of the cargo molecules must have occurred indirectly. We conclude that this must be the result of the fact that they no longer reach a lytic compartment in which they are degraded.
Because the retrieval of HDEL proteins occurs mainly via the cis-Golgi (Figure 1), it can be concluded that the proteolytic compartment must reside in a more distal location. Degradation could occur via partial transport to the lytic vacuole, which would indicate that, in addition to secretory bulk flow, some molecules can diffuse nonspecifically to other locations. Alternatively, the proteins could be unstable when in transit to the cell surface, perhaps as a result of a lower pH compared with the neutral pH of the ER in which they usually reside. Further work will be required to determine which of the two possible explanations applies.
Bulk Flow Is Efficient
The results strongly indicate that the rate of secretory bulk flow has been systematically underestimated when ER proteins, lacking a retention motif, were studied (Munro and Pelham, 1987; Crofts et al., 1999). This also would be applicable to some bulk flow markers such as PAT (Denecke et al., 1990), whereas other neutral cargo molecules were more stable and exhibited high transport rates to the cell surface (Hunt and Chrispeels, 1991). Based on our findings, we propose that recycling of ER residents occurs frequently and is crucial for the maintenance of these proteins in the ER lumen. Thus, ER chaperones may serve to rescue folding intermediates from degradation in the vacuole (Hong et al., 1996).
Our findings also suggest that the first experiment to demonstrate secretory bulk flow, based on a short peptide as a bulk flow marker (Wieland et al., 1987), was far more representative of the in vivo situation than is perceived at present (Romisch and Schekman, 1992; Kuehn and Schekman, 1997). Given this notion, it is not surprising that ERD2 mutants with defective ER retention affinity cause drastic transcriptional induction of the KAR2 gene to compensate for the loss of BiP through increased ER export (Semenza et al., 1990). Furthermore, the membrane-bound kinase IRE1 was shown to act as an inducible backup system when ERD2-mediated retrieval fails to operate (Beh and Rose, 1995). The discovery that ERD2 mutants were viable only because some residual activity of the ERD2 mutants was still present and that the complete loss of ERD2 function is lethal (Townsley et al., 1994) strongly suggests that BiP export occurs at a high rate rather than being marginal. Recycling from the Golgi is thus an essential process.
Also, the so-called “BiP bodies” observed when Sec12p is overproduced (Nishikawa et al., 1994) could be explained by the fact that under normal physiological conditions a significant amount of BiP is exported in COPII vesicles, followed by degradation. Prevention of this process causes its accumulation or stabilization. The fact that BiP accumulates in conjunction with vacuolar proteins (Nishikawa et al., 1994) means that these BiP bodies are in fact dilated, globular expansions of the ER that contain soluble proteins and that BiP is simply one of these.
The combined data do not rule out the possibility that specific ER export signals could exist to facilitate very efficient anterograde transport of receptors or other components of the vesicle-budding machinery (Klumperman, 2000). For instance, ERD2 anterograde transport must be more efficient than bulk flow of the far more abundant reticuloplasmins. However, from Figure 6, it does not appear that a naturally secretory protein such as α-amylase is transported out of the ER any faster than a bulk flow marker or an ER resident, confirming earlier observations on the mobility of proteins in the ER lumen of Xenopus oocytes (Ceriotti and Colman, 1988).
The fact that bulk flow does occur in COPII vesicles in vivo requires an explanation for the contrasting results obtained with ER-derived COPII vesicle budding in yeast (Barlowe et al., 1994). The Sec12p-induced BiP bodies (Nishikawa et al., 1994) would argue against the possibility that yeast COPII vesicles possess a more restrictive cargo selection fidelity than their counterparts in plants. The most significant difference between the in vivo and in vitro studies is the fact that in the latter, only one cargo molecule, the α-factor precursor, is introduced into the ER lumen via in vitro translation and translocation (Barlowe et al., 1994). It is possible that under these conditions, overproduction of α-factor precursor displaces ER residents such as BiP from ER export vesicles. When a complex mixture of secretory proteins synthesized de novo are transported, transport of the bulk flow marker and calreticulinΔHDEL showed a similar COPII dependence as the secretory marker α-amylase (Figure 6). We suggest that to test bulk flow using the in vitro COPII vesicle budding assay, two distinct cargo molecules should be introduced into the ER lumen in vitro, one representing secretory cargo such as α-factor and one encoding an ER resident such as BiP or calreticulin. This would give both molecules more comparable chances to enter COPII vesicles.
We conclude that although in vitro reconstitution experiments have been crucial in establishing the minimum number of components required for vesicle budding using biochemical techniques, a more critical evaluation of the cargo selection process could be complemented by in vivo experiments. Our results do not exclude the possibility that ER residents such as BiP can be ER export incompetent under certain conditions. For example, BiP molecules associated physically with the translocation pores or trapped in high molecular weight complexes may not diffuse into COPII vesicles. It is also possible that overexpression in transgenic plants or in transient expression disrupts the stoichiometry of putative high molecular weight chaperone complexes and overrepresents the rate of bulk flow in vivo under natural physiological conditions. However, if calreticulin were mainly retained and not recycled, disruption of the stoichiometry would be more dramatic when overexpression was more severe. The contrary is true in our experiments, in which stabilization of calreticulinΔHDEL was most pronounced when low levels of plasmids were transfected (Figure 6). In other words, calreticulinΔHDEL is exported more rapidly from the ER and degraded when expressed at lower levels.
This result strongly argues against the true retention model based on a putative chaperone matrix (Crofts and Denecke, 1998). Additionally, the majority of calreticulin is not present in high molecular weight complexes, and only a minor portion is associated with BiP (Crofts et al., 1998). More importantly, calreticulin was copurified with COPI vesicles from plants that did not overexpress calreticulin but an unrelated secretory marker fused to HDEL (Pimpl et al., 2000). This should not have disrupted any specific chaperone complex. Finally, the HDEL motif was shown to be crucial for the accumulation of high levels of calreticulin and BiP in tobacco plants (Crofts et al., 1999). If stoichiometry were an important mechanism for retention and HDEL-mediated recycling were just a minor backup system, the presence of the HDEL motif should not have made a 100-fold difference in protein levels (Crofts et al., 1999).
The data that we present here, showing that inhibition of ER export leads to the accumulation of high levels of calreticulinΔHDEL, correspond very well with all of the other findings showing that HDEL-mediated recycling is not marginal (Semenza et al., 1990; Nishikawa et al., 1994; Townsley et al., 1994; Beh and Rose, 1995; Crofts et al., 1999; Pimpl et al., 2000). Therefore, we propose that HDEL-mediated recycling plays a major role in the retention of ER residents such as calreticulin, in contrast to a recent suggestion (Pagny et al., 2000).
METHODS
Plasmid Construction for Transient and Stable Expression
All DNA manipulations were performed according to established procedures. The Escherichia coli MC1061 strain (Casadaban and Cohen, 1980) was used for the amplification of all plasmids. Plasmids encoding for α-amylase and α-amylase-HDEL were as described (Crofts et al., 1999). To allow a distinction between transiently expressed calreticulinΔHDEL (Crofts et al., 1999) and the endogenous wild-type calreticulin, pDE314C (Crofts et al., 1999) was engineered to incorporate a c-myc tag just before the stop codon, resulting in the plasmid pCalmyc. The Sec12p and Sar1p overexpression plasmids were generated through polymerase chain reaction amplification of the corresponding cDNA clones (d'Enfert et al., 1992), resulting in coding regions placed between the 35S promoter of cauliflower mosaic virus and the 3′ untranslated end of the nopaline synthase gene, as in pDE314C (Crofts et al., 1999). Site-directed mutagenesis of the Sar1p coding region was performed to exchange the histidine codon in position 74 with a leucine codon, using the following two oligonucleotides as polymerase chain reaction primers: SARH74L-sense (5′-TTGATTTGGGTGGTCTTCAGATTGCTCGTAG-3′) and SARH74L-antisense (5′-CTACGAGCAATCTGAAGACCACCCAAATCAA-3′). All constructions were verified by sequencing.
Plant Material and Growth Culture Conditions
Tobacco plants (Nicotiana tabacum cv Petit Havana) (Maliga et al., 1973) were grown in Murashige and Skoog (1962) medium and 2% sucrose in a controlled room at 25°C with a 16-hr daylength at the light irradiance of 200 μE · m−2 · sec−1. Tobacco leaf protoplasts were prepared as described (Denecke and Vitale, 1995) except that a new leaf-piercing device was used that displayed 122 stainless steel needles arranged at 1-mm distance from each other, allowing fast preparation of pierced leaves for overnight digestion with cellulase and pectinase. Transfection via electroporation was performed as described (Denecke and Vitale, 1995), and the plasmid concentrations used are given in the figure legends.
Protein Extraction and Transport Assays
Harvesting of cells and culture medium as well as protein extractions were performed as described previously (Denecke and Vitale, 1995; Crofts et al., 1999; Leborgne-Castel et al., 1999). Washed cells from a 2-mL protoplast suspension were extracted in a final volume of 200 μL, and clear culture medium devoid of cells was concentrated 10-fold as described (Crofts et al., 1999). Comparison of cellular and extracellular protein levels was possible simply by analyzing equal volumes of cell extracts or concentrated medium on protein gel blots. Membrane recruitment was assessed by an osmotic shock procedure described previously (Denecke et al., 1990, 1992).
Enzymatic Assays
Protoplasts were extracted in α-amylase extraction buffer (Crofts et al., 1999) via sonication for 5 sec. In all cases, extracts were cleared by 10 min of centrifugation at 25,000g at 4°C, and the supernatant was recovered. Clear culture medium also was spun at 25,000g for 10 min at 4°C to remove small quantities of cell debris, and the supernatant was recovered. α-Amylase activity was determined as described (Crofts et al., 1999) and calculated as change in OD per milliliter of original suspension per minute. For instance, if the cell extract was prepared in a 10-fold smaller volume than the original suspension, the value was divided by 10. The high sensitivity of the α-amylase assay permitted the analysis of nonconcentrated culture medium, thus limiting the number of steps and increasing the accuracy of the assay. The calculation of the secretion index was performed as described previously and represents the ratio between the extracellular and the intracellular activity (Denecke et al., 1990).
Protein Gel Blotting
Samples were loaded after twofold dilution with 2 × SDS-PAGE loading buffer (200 mM Tris-Cl, pH 8.8, 5 mM EDTA, 1 M sucrose, and 0.1% bromphenol blue). Proteins in SDS–polyacrylamide gels were transferred onto a nitrocellulose membrane and then blocked with PBS, 0.5% Tween 20, and 5% milk powder for 1 hr. The filter was then incubated in blocking buffer with primary antibody at a dilution of 1:5000 for anti-phosphinothricin acetyl transferase (PAT) antibodies and anti-c-myc antibodies (Santa Cruz Biotechnology, CA), whereas 1:2500 dilutions were used for anti-Sec12p antibodies (Bar-Peled and Raikhel, 1997) and anti-Sar1p antibodies (Pimpl et al., 2000). All antisera were from rabbits, and incubation of secondary antibodies and further steps were performed as described (Crofts et al., 1999).
Pulse Labeling and Immunoprecipitation
Protoplasts (106) were labeled as described previously (Crofts et al., 1998), except that the concentration of labeled methionine was higher (200 μCi/mL). Immunoprecipitation of myc-tagged calreticulinΔHDEL was performed as described previously (Crofts et al., 1998) but using polyclonal anti-c-myc antibodies (Santa Cruz Biotechnology). The supernatants of the immunoprecipitations were tested for remaining myc-tagged calreticulinΔHDEL by reprecipitation, which did not reveal detectable levels, demonstrating that the antibodies were not limiting in the immunoprecipitations (see Figure 7).
Immunocytochemistry
Preparation of ultrathin cryosections from tobacco root tips was performed as described previously (Pimpl et al., 2000). Immunogold labeling was performed with protein A–Sepharose–purified barley α-amylase antiserum diluted 1:100 (kindly provided by Birte Svensson, Carlsberg Laboratory, Copenhagen, Denmark). Labeled sections were observed in a Philips (Eindhoven, The Netherlands) CM10 electron microscope operating at 80 kV.
Acknowledgments
We thank Karin Sinjorgo (TNO Voeding, Delft, The Netherlands) for introducing us to barley α-amylase as a natural secreted cargo molecule for quantitative analysis and John Rogers (Washington State University, Pullman) for providing the cDNA clone. The authors also thank Birte Svensson of the Carlsberg Laboratory (Carlsberg Laboratory, Copenhagen, Denmark) for providing us with α-amylase antiserum and Christophe d'Enfert (Institut Pasteur, Paris, France) for providing cDNA clones encoding Arabidopsis Sec12p and Sar1p. Alessandro Vitale (Instituto Biosintesi Vegetali, Consiglio Nazionale delle, Milano, Italy) is thanked for communicating unpublished results and for continuous scientific discussion. Bruno Denecke (European Institute for Reference, Materials and Measurements, Mol, Belgium) is thanked for the design of a new leaf-piercing device, which permitted a much faster preparation of protoplasts. J.D. is indebted to Jane Hadlington, who combined her own time course experiment on two phytepsin constructs with that of α-amylase and α-amylase-HDEL and was responsible for the night shift (samples taken at 11 pm, 1 am, 3 am, and 5 am). This work was supported by grants from the Biotechnology and Biological Sciences Research Council, the European Union (Grant CHRX-CT94-0590), and the German Research Council (SFB 523, Teilprojekt A7), which we gratefully acknowledge. A.J.C. is indebted to the Biotechnology and Biological Sciences Research Council for a quota studentship. L.L.P.d.S. is indebted to the British Council for supporting a Link Exchange programme.
References
- Appenzeller, C., Andersson, H., Kappeler, F., and Hauri, H.P. (1999). The lectin ERGIC-53 is a cargo transport receptor for glycoproteins. Nat. Cell Biol. 1 330–334. [DOI] [PubMed] [Google Scholar]
- Balch, W.E., McCaffery, J.M., Plutner, H., and Farquhar, M.G. (1994). Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell 76 841–852. [DOI] [PubMed] [Google Scholar]
- Barlowe, C., and Schekman, R. (1993). SEC12 encodes a guanine-nucleotide exchange factor essential for transport vesicle budding from the ER. Nature 365 347–349. [DOI] [PubMed] [Google Scholar]
- Barlowe, C., d'Enfert, C., and Schekman, R. (1993). Purification and characterisation of Sar1p, a small GTP-binding protein required for transport vesicle formation from the endoplasmic reticulum. J. Biol. Chem. 268 873–879. [PubMed] [Google Scholar]
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M.F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77 895–907. [DOI] [PubMed] [Google Scholar]
- Bar-Peled, M., and Raikhel, N.V. (1997). Characterization of AtSEC12 and AtSAR1: Proteins likely involved in endoplasmic reticulum and Golgi transport. Plant Physiol. 114 315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batoko, H., Zheng, H.-Q., Hawes, C.R., and Moore, I. (2000). AtRAB1b is required for transport between the endoplasmic reticulum and Golgi apparatus and for normal Golgi movement in plant cells. Plant Cell 12 2201–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauly, J.M., Sealy, I.M., Macdonald, H., Brearley, J., Droge, S., Hillmer, S., Robinson, D.G., Venis, M.A., Blatt, M.R., Lazarus, C.M., and Napier, R.M. (2000). Overexpression of auxin-binding protein enhances the sensitivity of guard cells to auxin. Plant Physiol. 124 1229–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beh, C.T., and Rose, M.D. (1995). Two redundant systems maintain levels of resident proteins within the yeast endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 92 9820–9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boevink, P., Martin, B., Oparka, K., Cruz, S.S., and Hawes, C. (1999). Transport of virally expressed green fluorescent protein through the secretory pathway in tobacco leaves is inhibited by cold-shock and brefeldin A. Planta 208 392–400. [Google Scholar]
- Casadaban, M.J., and Cohen, S. (1980). Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138 179–207. [DOI] [PubMed] [Google Scholar]
- Ceriotti, A., and Colman, A. (1988). Binding to membrane proteins within the endoplasmic reticulum cannot explain the retention of the glucose-regulated protein GRP78 in Xenopus oocytes. EMBO J. 7 633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts, A.J., and Denecke, J. (1998). Calreticulin and calnexin in plants. Trends Plant Sci. 3 396–399. [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Pesca, M., Vitale, A., and Denecke, J. (1998). BiP and calreticulin form an abundant complex that is independent of endoplasmic reticulum stress. Plant Cell 10 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Hillmer, S., Robinson, D.G., Phillipson, B., Carlsson, L.E., Ashford, D.A., and Denecke, J. (1999). Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell 11 2233–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., and Vitale, A. (1995). The use of plant protoplasts to study protein synthesis, quality control, protein modification and transport through the plant endomembrane system. Methods Cell Biol. 50 335–348. [DOI] [PubMed] [Google Scholar]
- Denecke, J., Botterman, J., and Deblaere, R. (1990). Protein secretion in plant cells can occur via a default pathway. Plant Cell 2 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., De Rycke, R., and Botterman, J. (1992). Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 11 2345–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert, C., Wuestehube, L.J., Lila, T., and Schekman, R. (1991. a). Sec12p-dependent membrane binding of the small GTP-binding protein Sar1p promotes formation of transport vesicles from the ER. J. Cell Biol. 114 663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert, C., Barlowe, C., Nishikawa, S., Nakano, A., and Schekman, R. (1991. b). Structural and functional dissection of a membrane glycoprotein required for vesicle budding from the endoplasmic reticulum. Mol. Cell. Biol. 11 5727–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d'Enfert, C., Gensse, M., and Gaillardin, C. (1992). Fission yeast and a plant have functional homologs of the Sar1 and Sec12 proteins involved in ER to Golgi traffic in budding yeast. EMBO J. 11 4205–4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting, T., and Kabat, D. (1982). Evidence for a glycoprotein “signal” involved in transport between subcellular organelles: Two membrane glycoproteins encoded by murine leukemia virus reach the cell surface at different rates. J. Biol. Chem. 257 4011–4017. [PubMed] [Google Scholar]
- Frigerio, L., Pastres, A., Prada, A., and Vitale, A. (1999). KDEL acts before the Golgi-mediated formation of complex asparagine-linked glycans. Annual Meeting of the American Society of Plant Physiologists; July 24–28, 1999; Baltimore, MD.
- Frigerio, L., Pastres, A., Prada, A., and Vitale, A. (2001). Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: Selective use of an alternative route to vacuoles. Plant Cell 13 1109–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomord, V., and Faye, L. (2000). Reply. Glycobiology and the plant cell: A world of information. Plant Cell 12 1519–1521. [Google Scholar]
- Hardwick, K.G., Boothroyd, J.C., Rudner, A.D., and Pelham, H.R.B. (1992). Genes that allow yeast cells to grow in the absence of the HDEL receptor. EMBO J. 11 4187–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri, H.P., Kappeler, F., Andersson, H., and Appenzeller, C. (2000). ERGIC-53 and traffic in the secretory pathway. J. Cell Sci. 113 587–596. [DOI] [PubMed] [Google Scholar]
- Herman, E.M., Tague, B.W., Hoffman, L.M., Kjemtrup, S.E., and Chrispeels, M.J. (1990). Retention of phytohemagglutinin with carboxy-terminal tetrapeptide KDEL in the nuclear envelope and the endoplasmic reticulum. Planta 182 305–312. [DOI] [PubMed] [Google Scholar]
- Höfte, H., and Chrispeels, M.J. (1992). Protein sorting to the vacuolar membrane. Plant Cell 4 995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, E., Davidson, A.R., and Kaiser, C.A. (1996). A pathway for targeting soluble misfolded proteins to the yeast vacuole. J. Cell Biol. 135 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, D.C., and Chrispeels, M.J. (1991). The signal peptide of a vacuolar protein is necessary and sufficient for the efficient secretion of a cytosolic protein. Plant Physiol. 96 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman, J. (2000). Transport between ER and Golgi. Curr. Opin. Cell Biol. 12 445–449. [DOI] [PubMed] [Google Scholar]
- Kuehn, M.J., and Schekman, R. (1997). COPII and secretory cargo capture into transport vesicles. Curr. Opin. Cell Biol. 9 477–483. [DOI] [PubMed] [Google Scholar]
- Leborgne-Castel, N., Jelitto-Van Dooren, E.P.W.M., Crofts, A.J., and Denecke, J. (1999). Overexpression of BiP in tobacco alleviates endoplasmic reticulum stress. Plant Cell 11 459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H.I., Gal, S., Newman, T.C., and Raikhel, N.V. (1993). The Arabidopsis endoplasmic reticulum retention receptor functions in yeast. Proc. Natl. Acad. Sci. USA 90 11433–11437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, M.J., and Pelham, H.R.B. (1992). Ligand-induced redistribution of a human KDEL receptor from the Golgi complex to the endoplasmic reticulum. Cell 68 353–364. [DOI] [PubMed] [Google Scholar]
- Lewis, M.J., Sweet, D.J., and Pelham, H.R.B. (1990). The ERD2 gene determines the specificity of the luminal ER protein retention system. Cell 61 1359–1363. [DOI] [PubMed] [Google Scholar]
- Lodish, H.F., Kong, N., Snider, M., and Storus, G.J.A.M. (1983). Hepatoma secretory proteins migrate from rough endoplasmic reticulum to Golgi at characteristic rates. Nature 304 80–83. [DOI] [PubMed] [Google Scholar]
- Lord, J.M., and Roberts, L.M. (1997). Retrograde transport: Going against the flow. Curr. Biol. 8 R56–R58. [DOI] [PubMed] [Google Scholar]
- Macer, D.R.J., and Koch, G.L.E. (1988). Identification of a set of calcium binding proteins in reticuloplasm, the luminal content of the endoplasmic reticulum. J. Cell Sci. 92 61–70. [DOI] [PubMed] [Google Scholar]
- Maliga, P., Breznowitz, A., and Marton, L. (1973). Streptomycin-resistant plants from callus culture of haploid tobacco. Nature 244 29–30. [DOI] [PubMed] [Google Scholar]
- Martinez-Menarguez, J.A., Geuze, H.J., Slot, J.W., and Klumperman, J. (1999). Vesicular tubular clusters between the ER and Golgi mediate concentration of soluble secretory proteins by exclusion from COPI-coated vesicles. Cell 98 81–90. [DOI] [PubMed] [Google Scholar]
- Movafeghi, A., Happel, N., Pimpl, P., Tai, G.H., and Robinson, D.G. (1999). Arabidopsis Sec21p and Sec23p homologs: Probable coat proteins of plant COP-coated vesicles. Plant Physiol. 119 1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñiz, M., Nuoffer, C., Hauri, H.P., and Riezman, H. (2000). The Emp24 complex recruits a specific cargo molecule into endoplasmic reticulum–derived vesicles. J. Cell Biol. 148 925–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro, S., and Pelham, H.R.B. (1987). A C-terminal signal prevents secretion of luminal ER proteins. Cell 48 899–907. [DOI] [PubMed] [Google Scholar]
- Murashige, R., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15 473–497. [Google Scholar]
- Nishikawa, S., Hirata, A., and Nakano, A. (1994). Inhibition of endoplasmic reticulum (ER)-to-Golgi transport induces relocalization of binding protein (BiP) within the ER to form the BiP bodies. Mol. Biol. Cell 5 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, N., and Balch, W.E. (1997). A di-acidic signal required for selective export from the endoplasmic reticulum. Science 277 556–558. [DOI] [PubMed] [Google Scholar]
- Pagny, S., Cabanes-Macheteau, M., Gillikin, J.W., Leborgne-Castel, N., Lerouge, P., Boston, R.S., Faye, L., and Gomord, V. (2000). Protein recycling from the Golgi apparatus to the endoplasmic reticulum in plants and its minor contribution to calreticulin retention. Plant Cell 12 739–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedrazzini, E., Giovinazzo, G., Bielli, A., de Virgilio, M., Frigerio, L., Pesca, M., Faoro, F., Bollini, R., Ceriotti, A., and Vitale, A. (1997). Protein quality control along the route to the plant vacuole. Plant Cell 9 1869–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, H.R.B. (1988). Evidence that luminal ER proteins are sorted from secreted proteins in a post-ER compartment. EMBO J. 7 913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham, H.R.B. (1995). Sorting and retrieval between the endoplasmic reticulum and Golgi apparatus. Curr. Opin. Cell Biol. 7 530–535. [DOI] [PubMed] [Google Scholar]
- Pimpl, P., and Denecke, J. (2000). ER retention of soluble proteins: Retrieval, retention, or both? Plant Cell 12 1517–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpl, P., Movafeghi, A., Coughlan, S., Denecke, J., Hillmer, S., and Robinson, D.G. (2000). In situ localization and in vitro induction of plant COPI-coated vesicles. Plant Cell 12 2219–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romisch, K., and Schekman, R. (1992). Distinct processes mediate glycoprotein and glycopeptide export from the endoplasmic reticulum in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 89 7227–7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, Y., Kimura, K., Oka, T., and Nakano, A. (1998). Activities of mutant Sar1 proteins in guanine nucleotide binding, GTP hydrolysis, and cell-free transport from the endoplasmic reticulum to the Golgi apparatus. J. Biochem. 124 816–823. [DOI] [PubMed] [Google Scholar]
- Scheel, A.A., and Pelham, H.R.B. (1998). Identification of amino acids in the binding pocket of the human KDEL receptor. J. Biol. Chem. 273 2467–2472. [DOI] [PubMed] [Google Scholar]
- Semenza, J.C., Hardwick, K.G., Dean, N., and Pelham, H.R.B. (1990). ERD2, a yeast gene required for the receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Cell 61 1349–1357. [DOI] [PubMed] [Google Scholar]
- Springer, S., Chen, E., Duden, R., Marzioch, M., Rowley, A., Hamamoto, S., Merchant, S., and Schekman, R. (2000). The p24 proteins are not essential for vesicular transport in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 97 4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, M., Tada, M., Saito, C., Yashiroda, H., and Nakano, A. (1998). Isolation of a tobacco cDNA encoding Sar1 GTPase and analysis of its dominant mutations in vesicular traffic using a yeast complementation system. Plant Cell Physiol. 39 590–599. [DOI] [PubMed] [Google Scholar]
- Takeuchi, M., Sato, K., Abe, H., Nagata, T., and Nakano, A. (2000). A dominant negative mutant of Sar1 GTPase inhibits protein transport from the endoplasmic reticulum to the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 23 517–525. [DOI] [PubMed] [Google Scholar]
- Tatu, U., and Helenius, A. (1997). Interactions between newly synthesized glycoproteins, calnexin and a network of resident chaperones in the endoplasmic reticulum. J. Cell Biol. 136 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley, F.M., Frigerio, G., and Pelham, H.R. (1994). Retrieval of HDEL proteins is required for growth of yeast cells. J. Cell Biol. 127 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., and Denecke, J. (1999). The endoplasmic reticulum: Gateway of the secretory pathway. Plant Cell 11 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandelt, C.I., Khan, M.R.I., Craig, S., Schroeder, H.E., Spencer, D., and Higgins, T.J.V. (1992). Vicilin with carboxy-terminal KDEL is retained in the endoplasmic reticulum and accumulates to high levels in the leaves of transgenic plants. Plant J. 2 181–192. [DOI] [PubMed] [Google Scholar]
- Wieland, F.T., Gleason, M.L., Serafini, T.A., and Rothman, J.E. (1987). The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell 50 289–300. [DOI] [PubMed] [Google Scholar]
- Wilson, D.W., Lewis, M.J., and Pelham, H.R.B. (1993). pH-dependent binding of KDEL to its receptor in vitro. J. Biol. Chem. 268 7465–7468. [PubMed] [Google Scholar]