Abstract
Protein sorting to plant vacuoles is known to be dependent on a considerable variety of protein motifs recognized by a family of sorting receptors. This can involve either traffic from the endoplasmic reticulum (ER) through the Golgi apparatus or direct ER-to-vacuole transport. Barley aspartic protease (Phytepsin) was shown previously to reach the vacuole via trafficking through the Golgi apparatus. Here we show that Phytepsin normally exits the ER in a COPII-mediated manner, because the Phytepsin precursor accumulates in the ER upon specific inhibition of the formation of COPII vesicles in vivo. Phytepsin differs from its yeast and mammalian counterparts by the presence of a saposin-like plant-specific insert (PSI). Deletion of this domain comprising 104 amino acids causes efficient secretion of the truncated molecule (PhytepsinΔPSI) without affecting the enzymatic activity of the enzyme. Interestingly, deletion of the PSI also changes the way in which Phytepsin exits the ER. Inhibition of COPII vesicle formation causes accumulation of the Phytepsin precursor in the ER but has no effect on the secretion of PhytepsinΔPSI. This suggests either that vacuolar sorting commences at the ER export step and involves recruitment into COPII vesicles or that the PSI domain carries two signals, one for COPII-dependent export from the ER and one for vacuolar delivery from the Golgi. The relevance of these observations with respect to the bulk flow model of secretory protein synthesis is discussed.
INTRODUCTION
Plant cells contain at least two functionally distinct vacuolar compartments: the central lytic vacuole, which is related to the mammalian lysosome, and the so-called storage vacuoles, which appear to be unique to the plant kingdom (Marty, 1999). Whereas the lytic vacuole is typical for vegetative cells, storage vacuoles are found mostly in reserve tissues of seeds. Exceptions to this are the vegetative storage vacuoles that are formed during stress conditions and that could be related to a neutral vacuolar compartment recently discovered in tobacco protoplasts (Di Sansebastiano et al., 1998). It was first believed that the various vacuolar compartments share a common origin but change appearance and contents according to physiological conditions or tissue type. However, the simultaneous presence of storage vacuoles and lytic vacuoles within the same cell, as determined using specific membrane markers (Hoh et al., 1995; Paris et al., 1996; Jauh et al., 1998), argues against this notion. In addition, the great variety of vacuolar sorting signals described in plants (Matsuoka and Neuhaus, 1999) also suggests the possibility that the various types of vacuoles have different origins and are supported by different protein transport pathways (Chrispeels and Herman, 2000).
The first evidence for distinct transport routes arose from the use of pharmacological agents that exhibit a differential effect on the vacuolar sorting of a variety of cargo molecules (Gomez and Chrispeels, 1993; Matsuoka et al., 1995). Furthermore, the tonoplast intrinsic membrane protein α-TIP was found to segregate specifically into Golgi-derived dense vesicles en route to the storage vacuoles, whereas the vacuolar sorting receptor BP80 was detected in clathrin-coated vesicles that are thought to deliver proteins to the prevacuolar compartment (Hinz et al., 1999). In addition to this deviation from the Golgi apparatus, there is evidence for the existence of a Golgi-independent route to storage vacuoles directly from the endoplasmic reticulum (ER) (Levanony et al., 1992; Hara-Nishimura et al., 1998; Chrispeels and Herman, 2000; Frigerio et al., 2001). Indirect evidence for transport routes diverging as early as the ER has arisen from the discovery that transcripts encoding vacuolar proteins segregate on the rough ER surface to distinct subdomains via specific mRNA targeting mechanisms (Li et al., 1993; Choi et al., 2000).
The general route for proteins to exit from the ER was thought to involve vesicles coated with a non-clathrin-type coat, termed COPII (Barlowe et al., 1994). So far, evidence has been presented that ER export can occur via bulk flow or via COPII-mediated cargo selection (Klumperman, 2000). Although it was proposed that both mechanisms could operate in plants (Vitale and Denecke, 1999), bulk flow of neutral passengers as well as ER residents was shown to be efficient and to occur in a COPII-dependent manner (Phillipson et al., 2001), in contrast to previous findings in yeast (Barlowe et al., 1994). However, the unusual transport of vacuolar cysteine proteinases in large ER-derived vesicles may represent an example of a COPII-independent ER export pathway (Chrispeels and Herman, 2000; Toyooka et al., 2000). This class of vacuolar proteinases is characterized by the presence of the C-terminal KDEL motif and appears to be present exclusively in the plant kingdom. Similarly, precursor-accumulating vesicles (Hara-Nishimura et al., 1998) also may represent COPII-independent export structures, but no direct evidence has been reported to solidify this hypothesis. It is clear that many unknown factors involved in ER export and vacuolar sorting are still to be identified, and these may reveal additional unique features of the plant secretory pathway (Hadlington and Denecke, 2000).
Barley Phytepsin is a vacuolar protein that has been detected in two different types of vacuoles (Paris et al., 1996). Like most other plant aspartic proteinases, it differs from its mammalian and microbial counterparts by the presence of a plant-specific insert (PSI) of 104 amino acids (Runeberg-Roos et al., 1991). After cleavage of the signal peptide in the ER, the N-terminal propeptide as well as the internal PSI are removed proteolytically (Runeberg-Roos et al., 1991; Sarkkinen et al., 1992; Glathe et al., 1998), possibly upon arrival in the vacuoles. The protein also is glycosylated and acquires complex modifications (Costa et al., 1997), demonstrating that the protein passes through the Golgi apparatus on the way to the vacuole.
According to the three-dimensional structure of the Phytepsin precursor, the PSI is expected to form an external loop on the surface of the protein (Kervinen et al., 1999). This insert shows no homology with mammalian, yeast, or viral aspartic proteinases, but it is similar to mammalian saposins (Guruprasad et al., 1994; Ponting and Russell, 1995). Saposins are lysosomal sphingolipid-activating proteins that appear to be required for the hydrolysis of sphingolipids by specific lysosomal hydrolases (O'Brien and Kishimoto, 1991; Kishimoto et al., 1992). It also has been suggested that the association of saposin C with cathepsin D may be responsible for the mannose-6-phosphate (M6P)-independent targeting of the latter to the mammalian lysosome (Glickman and Kornfeld, 1993; Zhu and Conner, 1994).
We have attempted to determine if the saposin-like PSI domain of barley Phytepsin has a role in the intracellular targeting of this protease. The results suggest that in addition to vacuolar sorting, the PSI influences the route Phytepsin takes to leave the ER. This finding supports the notion that protein sorting is not restricted to a defined point in the secretory pathway such as the Golgi apparatus.
RESULTS
Wild-Type Phytepsin Exits the ER in COPII Vesicles and Is Delivered Correctly to Tobacco Vacuoles
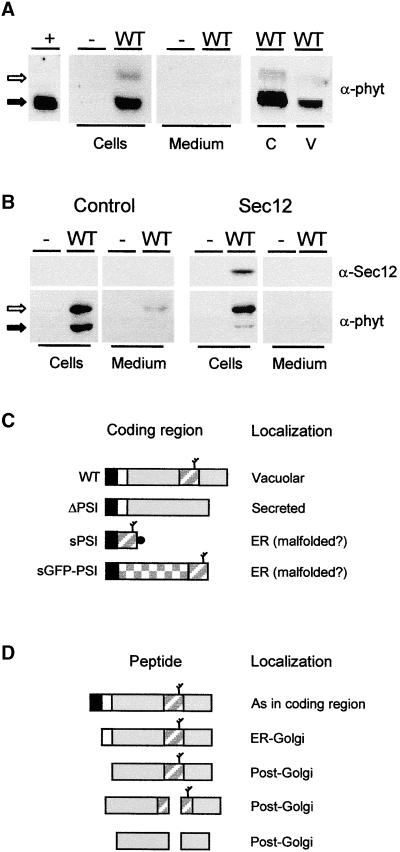
To determine if tobacco protoplasts could be used to identify the vacuolar sorting signal of Phytepsin, we wanted to establish whether barley Phytepsin is targeted correctly to the vacuoles of tobacco protoplasts. Figure 1A clearly shows that transiently expressed wild-type Phytepsin is retained in the cells and that the cellular protein is recovered in purified vacuoles. It is possible to detect both the higher molecular weight precursor and a processed form of Phytepsin lacking the N-terminal propeptide. Unlike the processed form, the unprocessed form colocalizes with the ER marker BiP in sucrose gradients (data not shown). The unprocessed form also can be detected in the culture medium and is enriched in the cells when saturation of the vacuolar sorting pathway is achieved via overexpression (Figure 1B). This finding suggests that the unprocessed form is a transport intermediate that is localized predominantly in the ER and that N-terminal processing occurs distal to the position where vacuolar proteins segregate from secreted proteins.
Figure 1.
Transport of Barley Phytepsin in Tobacco Protoplasts.
(A) Transient expression of wild-type Phytepsin (WT) in tobacco protoplasts. After transfection and incubation, equal quantities of cells and culture medium were loaded on SDS-PAGE gels for analysis by protein gel blotting using anti-Phytepsin serum (α-phyt). As a negative control, protoplasts were electroporated without DNA (−). As positive control, barley Phytepsin was extracted from barley grains (+). Note that the unprocessed form (open arrow) is not detected in the positive control, suggesting that in the steady state situation most of the Phytepsin is N-terminally processed (closed arrow). Phytepsin is detected in cell extracts, but it is not secreted into the culture medium. Cellular Phytepsin (C) is recovered from purified vacuoles (V). Extracts containing equal amounts of α-mannosidase (a vacuolar marker) were loaded in these two lanes.
(B) Similar to (A), but a threefold greater amount of plasmid carrying the gene encoding Phytepsin was transfected to saturate vacuolar targeting. The antisera used are indicated for Phytepsin (α-phyt) and Sec12p (α-Sec12). The right panel shows data from an experiment in which 10 μg of Sec12p-encoding plasmid was coelectroporated. Coexpression of Sec12p, which inhibits COPII vesicle budding from the ER (Phillipson et al., 2001), prevents the processing of cellular Phytepsin and secretion to the medium.
(C) Scheme of coding regions for Phytepsin and its derivatives. Signal peptides are shown in black, and propeptides are shown in white. The mature portion of Phytepsin is shown in gray, and the GFP is checkered. The PSI is indicated by a diagonally striped rectangle, and a branch indicates the glycan. The black circle indicates the myc epitope present on the PSI construct. WT, wild-type Phytepsin; ΔPSI, Phytepsin lacking the PSI domain; sPSI, soluble PSI; sGFP-PSI, secretory GFP fusion protein containing the PSI domain at the C terminus.
(D) Hypothetical processing pathway of barley Phytepsin expressed in tobacco protoplasts, based on findings past (Glathe et al., 1998) and present. Annotations are as in (C) and correspond only to wild-type Phytepsin. The fragmentation products formed in post-Golgi compartments are of low abundance in the steady state situation and cannot be detected in the exposures appropriate for the higher molecular weight forms.
Because of the complexity of vacuolar transport in plants and the presence of different routes to the vacuole starting from the ER export step (Hara-Nishimura et al., 1998; Toyooka et al., 2000), we wanted to determine if Phytepsin uses COPII-mediated ER export to reach the Golgi apparatus. We took advantage of the inhibitory effect of Sec12p overproduction on COPII vesicle budding (Hardwick et al., 1992; Nishikawa et al., 1994), which occurs through titration of the low molecular weight GTPase Sar1p and results in ER accumulation of secretory proteins (Phillipson et al., 2001). Figure 1B shows that coexpression of Arabidopsis Sec12p leads to a sharp decrease in the levels of the processed form in the cells and inhibits secretion of the precursor. This result clearly establishes that Phytepsin leaves the ER in a COPII-dependent manner and that saturation of vacuolar sorting is prevented when fewer precursors reach the Golgi.
Together, these results show that tobacco cells can be used as host cells for the analysis of Phytepsin and its derivatives. Figure 1C summarizes the various truncated forms of Phytepsin and fusion proteins used in this study, and in Figure 1D, a hypothetical processing scheme is illustrated based on previous reports (Glathe et al., 1998) and our present data. After removal of the signal peptide, processing of the N-terminal propeptide is the first event, which occurs before further fragmentation by cleavage of the PSI domain.
Removal of the PSI Causes Secretion
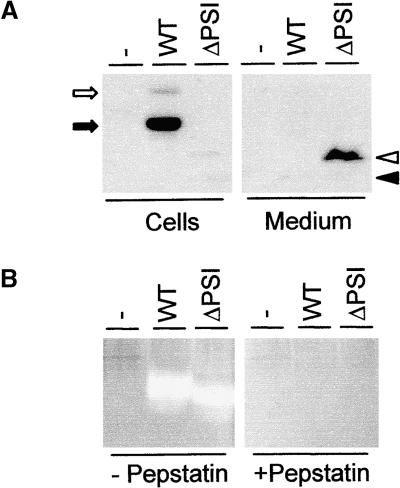
A putative candidate for the vacuolar targeting signal of Phytepsin is the saposin-like PSI, based on the implication of saposin C in the M6P-independent targeting of mammalian cathepsin D to the lysosomes (Glickman and Kornfeld, 1993; Zhu and Conner, 1994). Thus, we deleted the PSI-encoding sequence of Phytepsin to generate a modified aspartic proteinase that would resemble cathepsin D or other nonplant aspartic proteinases. The fate of the truncated polypeptide (PhytepsinΔPSI) was then studied using transient expression experiments. Figure 2A clearly shows that removal of the PSI leads to the secretion of PhytepsinΔPSI into the culture medium. In the cellular portion, the protein is barely detectable, indicating that secretion is very efficient. A minor portion of N-terminally processed PhytepsinΔPSI was detected in the cells, possibly as a result of proteolysis during cell extraction.
Figure 2.
Deletion of PSI Results in Secretion.
(A) Secretion/retention experiment using transient expression of wild-type Phytepsin (WT) and a truncated version lacking PSI (ΔPSI) in tobacco protoplasts. Annotations are as in Figure 1. Arrows indicate unprocessed (open arrow) and N-terminally processed (closed arrow) wild-type Phytepsin, whereas arrowheads indicate unprocessed (open arrowhead) and N-terminally processed (closed arrowhead) PhytepsinΔPSI. Note that the truncated protein, in contrast to the wild-type protein, is recovered mainly in the medium and that it is unprocessed.
(B) Proteinase activity assay showing that the PhytepsinΔPSI is proteolytically active. The presence (+) or absence (−) of pepstatin to specifically inhibit aspartic proteinase activity is indicated. Lanes from left to right are as follows: untransformed tobacco extract (−), extract from transgenic tobacco plant producing wild-type Phytepsin (WT), and extract from transgenic tobacco producing the PhytepsinΔPSI (ΔPSI). Note the strong bands of aspartic proteinase activity in both transgenic plant extracts, as shown by the appearance of nonstained regions.
Deletion of the PSI Does Not Affect the Enzymatic Activity of Phytepsin
Because the truncation involves deletion of 104 amino acids from the center of the Phytepsin polypeptide, we wanted to determine if the resulting protein still exhibited enzymatic activity. Retention of enzymatic activity would be a strong indication of the maintenance of the overall three-dimensional structure of the enzyme. This would provide arguments against malfolding and make it unlikely that the deletion has secondary effects on regions elsewhere in the molecule that could be responsible for vacuolar sorting. For this purpose, transgenic plants were generated to obtain sufficient biological material. Equal amounts of wild-type Phytepsin and PhytepsinΔPSI were analyzed for aspartic protease activity via in situ proteolysis within a native gel. Figure 2B clearly shows that PhytepsinΔPSI retains the enzymatic activity of wild-type Phytepsin. In addition, proteinase activities of both proteins could be inhibited by pepstatin, demonstrating that the activity detected results from aspartic proteinases. Untransformed tobacco did not show detectable amounts of aspartic proteinase activity at these positions, confirming that the activities observed originate from the introduced transgenes.
These results demonstrate that the PSI is not required for the enzymatic properties of barley Phytepsin and correspond well with earlier findings suggesting that the PSI is absent in the fragmented form of Phytepsin (Sarkkinen et al., 1992; Glathe et al., 1998). Most importantly, they suggest that secretion of the truncated Phytepsin cannot be caused by malfolding and masking of a targeting signal present elsewhere on the protein. The truncation thus results in a protein that resembles mammalian or microbial aspartic proteinases that do not contain this PSI domain.
Deletion of the PSI Causes Accumulation of Phytepsin in the Extracellular Space of Transgenic Plants
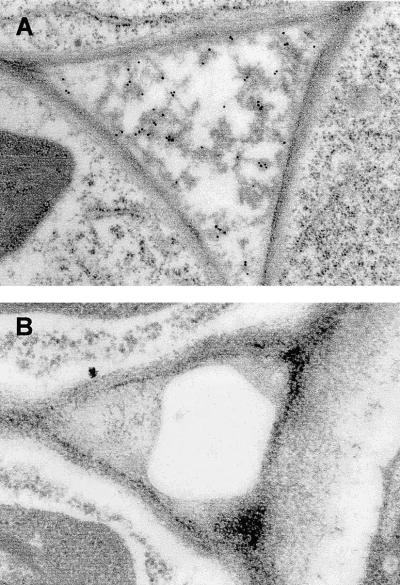
To confirm the results obtained from transient expression, tissue sections of plants producing wild-type Phytepsin and PhytepsinΔPSI were analyzed using immunocytochemistry. Figure 3A shows that the truncated molecule accumulates in the extracellular space. In plants producing the wild-type form of Phytepsin, no secretion of the protein was observed, as indicated by the lack of gold labeling and the absence of electron-dense material in the extracellular space (Figure 3B). However, the same tissue sections showed labeling of the central vacuole, as expected (data not shown).
Figure 3.
Deletion of the PSI Results in the Redirection of Phytepsin to the Apoplast.
In situ localization of PhytepsinΔPSI (A) and wild-type Phytepsin (B) in roots from transgenic tobacco plants using immunogold electron microscopy. Note the apoplastic localization of electron-dense material labeled with Phytepsin antibodies in plants producing PhytepsinΔPSI. The intracellular spaces of wild-type Phytepsin–producing plants contain neither electron-dense material nor gold labeling.
Increased Expression Levels of PhytepsinΔPSI Are Attributable to Apoplastic Stability
Analysis of the transgenic plants expressing wild-type Phytepsin and the truncated mutant revealed that the absence of vacuolar targeting was accompanied by a vast increase in the amount of accumulated protein (Figure 4A). Of 50 independent transgenic lines for each construct, four independent transformants showing the highest transgene product levels were chosen for comparison. Secreted PhytepsinΔPSI accumulated to much higher levels than the other forms that were shown to reach the vacuoles. This effect was much weaker in transient expression experiments, perhaps because a steady state level dependent on the rate of synthesis and degradation is not reached in the short time span of the experiment. In addition, transgenic plants accumulated mainly the processed form of Phytepsin and its derivatives, in contrast to protoplasts in transient expression experiments, in which unprocessed Phytepsin and its derivatives were detected for all proteins. This finding suggests that in transient expression, transport may occur more slowly as a result of a greater amount of proteins synthesized in transfected cells.
Figure 4.
Phytepsin Protein Levels Are Influenced by the Subcellular Localization.
(A) Leaf extracts from untransformed plants (C) or plants expressing wild-type Phytepsin (WT) or PhytepsinΔPSI (ΔPSI). At right, 20-fold dilutions of PhytepsinΔPSI plants were loaded to highlight the drastically increased levels compared with the wild type.
(B) Cellular protein levels detected by pulse labeling and immunoprecipitation. Protoplasts were prepared from transgenic plants used in the experiment described in (A), labeled in vivo, and immunoprecipitated with Phytepsin antiserum. Annotations are as in (A). Note that the levels observed for the truncated proteins are slightly lower than those of the wild-type protein, in sharp contrast to (A).
Processed and unprocessed forms of either molecules are indicated by arrows (WT) or arrowheads (ΔPSI) as in Figures 1 and 2.
To exclude the possibility that the drastically increased levels of PhytepsinΔPSI are attributable to a higher synthesis rate, protoplast suspensions were pulse labeled and the extracts were immunoprecipitated with anti-Phytepsin antibodies. Figure 4B shows that the synthesis rates for the two constructs are much more comparable than the steady state levels seen in protein gel blots. The apparent synthesis rate of the wild-type Phytepsin is even slightly higher than that of PhytepsinΔPSI. This finding shows that the high levels of PhytepsinΔPSI in plant extracts certainly are not caused by a higher synthesis rate but must be attributable to the greater stability of the protein. These data correspond well with reports on truncated phaseolin lacking its C-terminal vacuolar sorting motif, which also showed greater stability (Frigerio et al., 1998a).
Folding of the PSI May Be Dependent on Intrinsic Properties of the Phytepsin Precursor
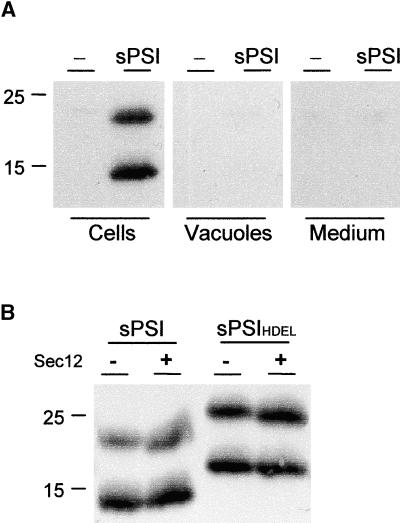
The experiments described so far demonstrated the requirement of the PSI for vacuolar sorting of barley Phytepsin in tobacco protoplasts and in transgenic tobacco plants. To show that the PSI carries all of the sorting information and therefore can be regarded as “sufficient” for vacuolar sorting, we attempted to express the molecule alone after direct fusion to a signal peptide, as described in Figure 1. Two polypeptides were detected, the smaller of which corresponds to the predicted molecular weight of the recombinant molecule. The higher molecular weight form could be a processing intermediate that still contains the signal peptide, but this requires further experimental evidence. Neither of the two polypeptides was transported to the vacuoles or secreted (Figure 5A). In addition, the soluble PSI could not be stabilized by retaining the molecule in the ER by Sec12p overexpression or HDEL tagging (Figure 5B). This indicates that the lack of the soluble PSI in the vacuole was not caused by rapid degradation in a post-ER compartment. The most plausible explanation for this result is that individually expressed PSI probably was folded incorrectly and consequently was unable to exit the ER, although this is merely a working hypothesis.
Figure 5.
Expression and Intracellular Localization of a Soluble PSI.
(A) Tobacco protoplasts expressing the PSI domain fused to a signal peptide containing a C-terminal myc tag before the stop codon for detection (sPSI; see Figure 1). Two polypeptides are detected within the cellular fraction (Cells) but cannot be recovered from the vacuolar fraction (Vacuoles) or the culture medium (Medium). Molecular mass markers are given in kilodaltons and indicated at left.
(B) Coexpression of the soluble PSI (sPSI; left) or the HDEL-tagged soluble PSI (sPSIHDEL; right) with Sec12p (Sec12). Ten micrograms of the Sec12p-encoding plasmids (Phillipson et al., 2001) was coelectroporated to achieve strong inhibition of COPII transport. Note that neither HDEL tagging nor Sec12p-mediated inhibition of COPII transport led to an increase in the intracellular level of either protein.
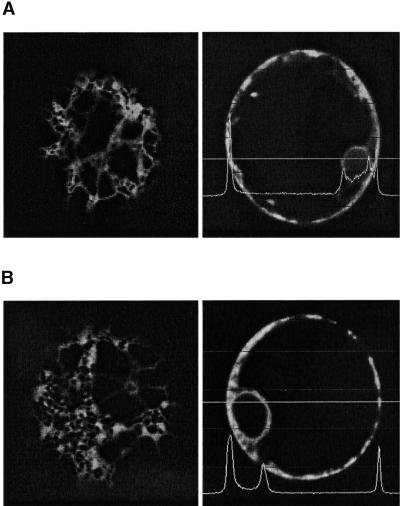
We also expressed the PSI as a C-terminal fusion to a secreted green fluorescent protein (sGFP), in the hope that folding would be facilitated in the context of a GFP C terminus. The resulting GFP fusion protein exhibited a typical ER pattern, as determined by comparison with sGFP-HDEL (Figure 6A), and failed to exhibit any detectable green fluorescence in the central vacuole (Figure 6B). The strong fluorescence of GFP suggests that this portion of the fusion protein is still folded correctly. However, the failure of the GFP fusion protein to leave the ER in the absence of an ER retention motif must be the result of the fused PSI domain, which is probably the same mechanism that prevents soluble PSI from leaving the ER. We suspect that correct folding of PSI depends on its natural environment on the polypeptide precursor; thus, we discontinued this line of investigation.
Figure 6.
A C-Terminally Fused PSI Domain Causes ER Retention of GFP.
(A) Tobacco protoplasts expressing sGFP-HDEL. GFP labels cortical elements of the ER (left) and the nuclear envelope (right). Measurement of fluorescence intensity is restricted to the cell cortex and the nuclear envelope, as indicated by the line measurement. Magnification ×600.
(B) Tobacco protoplasts expressing sGFP-PSI show a similar distribution of fluorescence within the cell.
Deletion of the PSI Causes COPII-Independent ER Export
It has been shown that different vacuolar sorting routes deviate at the ER export site (Gomez and Chrispeels, 1993). This could be explained if vacuolar sorting signals are recognized already in the ER and may serve as ER export signals in addition to vacuolar sorting signals. Indeed, the vacuolar sorting receptor BP80 was shown to bind to its ligands at pH 7.0, even though optimal binding was observed at pH 6.5 (Kirsch et al., 1994). Other putative vacuolar sorting receptors may have a similar pH dependence on ligand binding, which could explain the fact that vacuolar sorting can deviate as early as in the ER (Levanony et al., 1992; Gomez and Chrispeels, 1993; Hara-Nishimura et al., 1998; Toyooka et al., 2000).
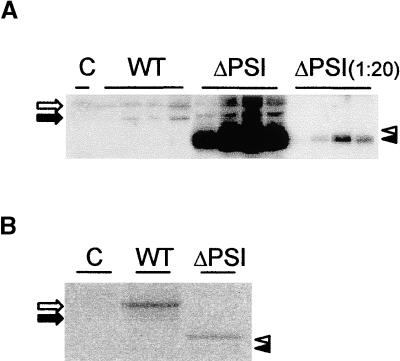
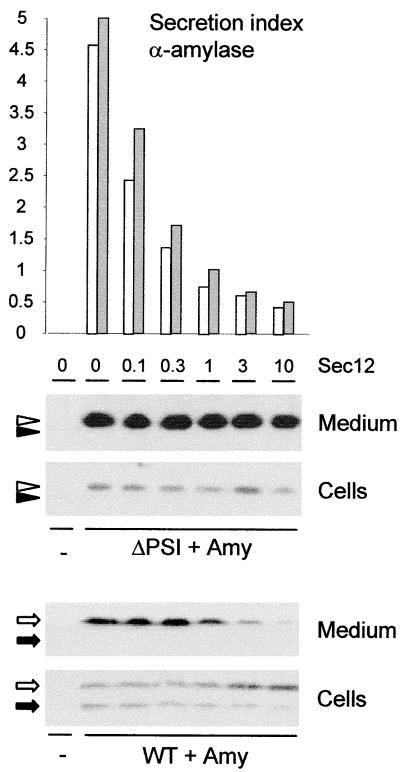
To determine if deletion of the PSI alters the way in which the protein is exported from the ER, we tested if the secretion of PhytepsinΔPSI was still inhibited by Sec12p overproduction. The secretory marker α-amylase was cotransfected to monitor the effect of Sec12 coexpression quantitatively. Figure 7 shows that in contrast to the transport of wild-type Phytepsin, PhytepsinΔPSI was not affected by the overproduction of Sec12p. The cotransfected secretory marker was influenced in a similar manner in both experiments, confirming that inhibition of COPII transport was of comparable efficiency in both cases. These results are consistent with the hypothesis that secretion, and thus ER export, of PhytepsinΔPSI occurs independently of COPII-mediated transport. This suggests either that the PSI contains two sorting signals, one for ER export and one for vacuolar sorting, or that the vacuolar sorting motif itself is recognized at the ER export site.
Figure 7.
PhytepsinΔPSI Is Secreted in a COPII-Independent Manner.
Secretion/retention experiment using transient expression to investigate the cellular localization of wild-type Phytepsin (WT) and PhytepsinΔPSI (ΔPSI) and the effect of increasing levels of Sec12p (Sec12). The secretion index was determined for the internal secretory marker α-amylase (Amy) by calculating the ratio between the extracellular levels and the intracellular levels of the enzyme (Phillipson et al., 2001) and is used to quantify the inhibition of COPII-mediated ER export. Values for the secretion indices are from single experiments corresponding to the same protoplast suspension analyzed in the protein gel blots at bottom. Secretion index values for the experiment with PhytepsinΔPSI are illustrated with white bars, and values for the wild type are illustrated with gray bars. Annotations are as in Figures 1 and 2, and the amounts of cotransfected plasmid encoding Sec12p are given in micrograms. Note that in contrast to the internal secretory marker α-amylase and wild-type Phytepsin (bottom), PhytepsinΔPSI secretion is not notably affected by Sec12p overexpression.
DISCUSSION
The PSI Contains Vacuolar Sorting Information
Our results clearly show that the deletion of the PSI caused secretion of the truncated Phytepsin into the culture medium, where it accumulated to high levels. The possibility that secretion was attributable to malfolding was unlikely, because it would be expected to cause ER retention rather than secretion (Vitale and Denecke, 1999). Maintenance of the general three-dimensional structure was supported by demonstrating that PhytepsinΔPSI is enzymatically active. The possibility that secretion was caused by a higher synthesis rate and subsequent saturation of the vacuolar transport pathway was excluded by pulse-labeling experiments. Therefore, we propose that the vacuolar targeting information of Phytepsin resides in the PSI portion of the protein. Like the linker sequence in proricin, which joins the A and B chains of the ricin precursor and is likely to be responsible for vacuolar sorting (Frigerio et al., 1998b) of the toxin, the PSI represents an internal vacuolar sorting motif that is processed in the vacuole. Although the proricin linker is very short, PSI represents a large protein domain that may contain more than just vacuolar sorting information.
We were not able to demonstrate that the PSI is sufficient for vacuolar sorting, although the negative results obtained with GFP fusion proteins or production of soluble PSI in the ER lumen do not exclude this possibility. We did not attempt to insert the PSI into the center of a secreted protein, because in addition to the uncertainty of PSI folding in a different context, it is not clear that the secreted protein will benefit from the addition of an alien polypeptide of such dimensions. We decided that additional experiments on specific residues within the 104–amino acid saposin-like insert would be more valuable, but these studies are beyond the scope of the current investigation.
Relevance of the Homology between the PSI and Mammalian Saposins
What could be the mechanism for the PSI-mediated targeting of Phytepsin? Of the mature saposins (A, B, C, and D), saposin C shares the greatest homology with the PSI, although all saposins share the same general features (Figure 8). Saposin C is required by glucosylceramidase for the hydrolysis of glucosylceramide to ceramide and glucose. It has been reported to interact with phosphatidylserine and to induce pH-dependent destabilization and fusion of phosphatidylserine-containing vesicles (Vaccaro et al., 1995). Considering the close similarity of PSI to saposin C, a similar mechanism may be involved in mediating the vesicle transport of Phytepsin in plants. Both saposins and PSI have potential amphipathic α-helical regions as well as six cysteine residues at conserved positions that are thought to be crucial in maintaining the three-dimensional structure and the general property of a “physiological detergent” (reviewed by Munford et al., 1995). Thus, the PSI could interact directly with the lipid bilayer. Further work on the PSI will be required to test this hypothesis. It will be interesting to determine if an engineered saposin C molecule is capable of replacing the PSI function in barley Phytepsin, or vice versa, and whether PSI facilitates direct interactions of the precursor with ER or Golgi membranes.
Figure 8.
Scheme of Phytepsin Compared with Mammalian Cathepsin D and Its Associated Partner Saposin C.
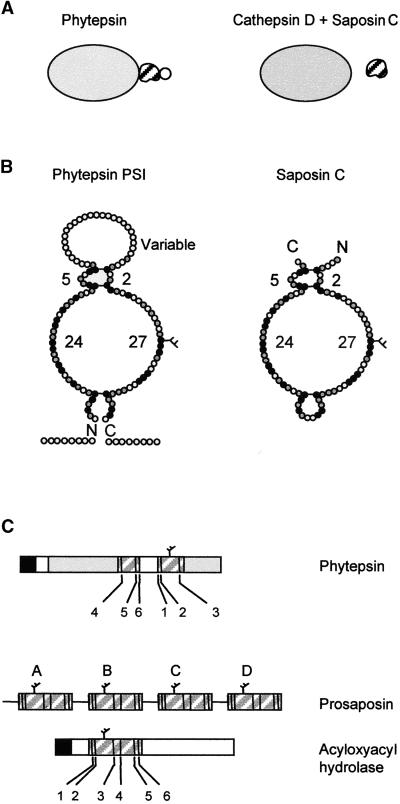
(A) Overall representation showing that the PSI is an integral part of Phytepsin, whereas different genes encode cathepsin D and saposin C. The variable domain of the PSI is indicated by a circle, which is not present in saposin C.
(B) Detailed diagram comparing PSI and saposin C showing the predicted structural similarity. Conserved amino acids are indicated in black and similar amino acids are shown in gray. The conserved disulfide bridges are indicated by horizontal bars. Numbers refer to the number of residues in each loop, which are conserved between the PSI of Phytepsin and saposin C. The C and N termini are indicated (C and N), and the conserved N-linked glycan is indicated by a branch.
(C) Comparison of the domain structure and order of appearance of the conserved cysteine residues (numbered according to saposin nomenclature) of the saposin-like motifs present in barley Phytepsin, the saposin precursor (prosaposin), and human acyloxyacyl hydrolase. Note that in Phytepsin, cysteines 1, 2, and 3 are displaced toward the C terminus of cysteines 4, 5, and 6 and are separated by the variable domain, which is unique to the plant sequence.
In mammalian cells, both procathepsin D and prosaposin have been reported to be associated with the membrane during their biosynthesis (Rijnboutt et al., 1991). It also has been demonstrated that a portion of the synthesized prosaposin is associated with newly synthesized procathepsin D in the ER (Zhu and Conner, 1994). The prosaposin–procathepsin D complex does not bind to the membrane until delivery to the Golgi apparatus, in which the complex becomes associated with the membrane in both a M6P-dependent and a M6P-independent manner. Upon arrival in the lysosomes, the complex dissociates and is released from the membrane. The associated saposin could explain the proposed M6P-independent lysosomal targeting of cathepsin D (Glickman and Kornfeld, 1993). Thus, the targeting of Phytepsin to the plant vacuole may resemble events in the mammalian system, with the exception that the saposin-like PSI and Phytepsin are encoded as a single precursor (Figure 8).
The homology between the PSI and saposin C is not restricted to a few residues but also involves the overall structure as well as the N-linked glycan and three disulfide bridges at conserved positions. Figure 8 shows that all of the loops except for the variable loop of PSI have exactly the same number of residues in the plant and mammalian sequences. However, the variable domain does not have a counterpart in saposins. The second difference between the plant structure and the mammalian equivalent is the difference in the positions of the C and N termini (Ponting and Russell, 1995). The termini of saposin C are replaced by the variable loop in PSI, and the small loop of saposin C is opened up in the PSI, with the two resulting ends being extended by the N- and C-terminal portions of mature Phytepsin. Finally, Phytepsin and the PSI form one precursor molecule, whereas different genes encode cathepsin D and saposin C in mammalian cells. The latter may be a selective advantage because saposin C has additional functions in mammalian cells (Munford et al., 1995).
The localization of a saposin-like sequence in Phytepsin is not a unique phenomenon. A similar saposin-like domain has been reported as part of the small subunit of human acyloxyacyl hydrolase (Staab et al., 1994). Deletion of this saposin-like domain prevented the enzyme from reaching the lysosome. However, the saposin-like domain of acyloxyacyl hydrolase is not swapped as in Phytepsin but has the same order of domains as found in saposin C (Figure 8).
The PSI Specifies COPII-Mediated ER Export
An unexpected result was the observation that deletion of the PSI domain changes the way in which Phytepsin leaves the ER. Whereas the wild-type molecule was clearly transported in a COPII-dependent manner, as shown by the inhibition of ER export via Sec12p overproduction, PhytepsinΔPSI was unaffected by Sec12p. Similar results have been obtained with a GTPase-deficient mutant of Sar1p (Phillipson et al., 2001), the coexpression of which affected the transport of the secretory protein α-amylase and wild-type Phytepsin but not the truncated form lacking the PSI domain (data not shown).
These results could be explained by the hypothesis that PhytepsinΔPSI is exported from the ER in a COPII-independent manner. Because the presence of the PSI domain mediates COPII dependence, the ER export of Phytepsin could involve an active sorting process. This could be controlled either by the vacuolar sorting motif itself or by a second domain present within this 104–amino acid insertion. However, the fact that nonspecific bulk flow of neutral passenger molecules or ER residents occurs in COPII vesicles and is relatively efficient (Phillipson et al., 2001) seems to contradict this. Therefore, an alternative explanation for the observed insensitivity to COPII transport inhibition could be that PhytepsinΔPSI contains a signal that is dominant over bulk flow and diverts the protein from the COPII route. The very efficient secretion of PhytepsinΔPSI, exceeding that of the natural secreted protein α-amylase, seems to support this possibility. The fact that this signal should be exposed only after deletion of the PSI domain could be explained if the latter masks the signal, perhaps via steric hindrance. Clearly, additional research will have to establish which of the two models is correct, but current findings on the route taken by bulk flow markers (Phillipson et al., 2001) support the second model.
Regardless of the model, the results strongly suggest that in addition to mediating vacuolar sorting, the PSI domain has a strong influence on the ER export step. So far, active sorting signals on soluble proteins without specific secondary modifications have not been identified (Klumperman, 2000), and the saposin-like domain could be the first tool to study active ER export mechanisms.
In conclusion, we propose that the PSI represents a novel vacuolar sorting signal that lacks any resemblance to other known vacuolar sorting signals. In addition, we present evidence that the PSI also influences the route Phytepsin takes to exit the ER. This finding opens up the exciting prospect of identifying cargo concentration mechanisms that may operate in addition to bulk flow (Hadlington and Denecke, 2000; Phillipson et al., 2001). Our results also provide direct evidence for a COPII-independent ER export mechanism. This demonstrates that vacuolar sorting may not be restricted to the Golgi apparatus but in fact can commence as early as the ER. Indeed, a unique class of ER-derived vesicles has been identified in plants, and some routes to plant vacuolar compartments may in fact bypass the Golgi apparatus entirely (Gomez and Chrispeels, 1993; Shimada et al., 1997; Hara-Nishimura et al., 1998; Chrispeels and Herman, 2000; Toyooka et al., 2000; Frigerio et al., 2001). Given the fact that even mRNA is sorted on the ER surface before translation (Choi et al., 2000), the early secretory pathway and the process of ER export certainly will remain a subject of intense investigation in the future (Vitale and Galili, 2001).
METHODS
Plant Material
For transient expression experiments, axenic tobacco plant cultures (Nicotiana tabacum cv Petit Havana SR1) (Maliga et al., 1973) were maintained on Murashige and Skoog (1962) medium under a 16-hr-day/8-hr-night regimen (Denecke et al., 1992). Stable tobacco transformants were made from the same SR1 line as the starting material and grown in identical conditions.
Plasmid Constructs
All of the plasmid constructs used are in pUC-based vectors under the 35S promoter of cauliflower mosaic virus. The constructs used in this work were as follows: pKMT46 (35S-Phytepsin; wild-type Phytepsin cDNA), pKMT48 (35S-PhytepsinΔPSI; wild-type Phytepsin cDNA from which the plant-specific insert [PSI] has been deleted), pJLH18 (35S-sp-PSI; soluble PSI), pJLH17 (35S-sp-PSI-HDEL; HDEL-tagged soluble PSI), pFB1 (35S-sp-GFP-HDEL; HDEL-tagged green fluorescent protein [GFP]), and pJLH24 (35S-sp-GFP-PSI; GFP fusion protein containing PSI at the C terminus). Plasmids encoding Sec12 (pSec12) or barley α-amylase were described previously (Crofts et al., 1999; Phillipson et al., 2001).
For Agrobacterium tumefaciens–mediated transformation of tobacco, constructs pKMT46 and pKMT48 were subcloned in the Agrobacterium binary vector pDE1001 (Denecke et al., 1992). Transformation of the recipient Agrobacterium strain C58C1RifR (pGV2260) was performed as described (Denecke et al., 1992).
Stable Plant Transformation
Leaf discs from axenically grown SR1 tobacco plants were transformed with the Agrobacterium strains described by Denecke et al. (1990), except that the selection of transformants was made on kanamycin-containing medium only. The most productive transformants were chosen on the basis of protein gel blotting experiments using leaf material for protein extracts.
Transient Expression Experiments
Protoplast preparation, electroporation, and cultivation of the electroporated protoplasts were as described by Denecke and Vitale (1995) and Hadlington and Denecke (2001). Protoplasts were cultivated for up to 24 hr after electroporation, except in the experiments shown in Figures 4 and 6, in which suspensions were incubated for 48 hr to allow detection of proteins on protein gel blots. Cells and media then were harvested as described previously (Hadlington and Denecke, 2001; Phillipson et al., 2001). The assessment of the distribution of recombinant proteins over the cells and the culture medium was done volumetrically as described by Crofts et al. (1999) and Phillipson et al. (2001). Different amounts of pKMT46 DNA were used for the transfections to monitor the saturation of vacuolar transport. In the experiment illustrated in Figure 1A, 3 μg of pKMT46 plasmid was used to avoid saturation. Modest saturation was found using 10 μg of pKMT46 plasmid (Figure 1B). In the experiment illustrated in Figure 7, 30 μg of either pKMT46 or pKMT50 was used to allow clear detection of secreted wild-type Phytepsin through saturation of the vacuolar transport route. pKMT48 was transfected using 3 μg (Figure 2) and 30 μg (Figure 7) to allow comparison with wild-type Phytepsin. α-Amylase was cotransfected using 1 μg in Figure 7, and α-amylase activity was always measured 24 hr after transfection. To allow measurement of α-amylase and the Phytepsin derivatives in the same protoplast suspension, the suspension was split into two equal portions. pSec12 was transfected at either 10 μg of plasmid (Figures 1B and 5B) or at the amounts indicated in Figure 7.
Organelle Fractionation
Vacuoles were isolated from protoplasts either immediately after enzymatic treatment (stable tobacco transformants) or 16 hr after electroporation (transient expression experiments). In the latter case, protoplasts were incubated in the presence of a specific cellulose synthesis inhibitor, 2,6-dichlorobenzonitrile (5 μM). A total of 107 protoplasts was used as starting material for the vacuole isolations (Gomez and Chrispeels, 1993), except that 10 μg/mL neutral red was used in the lysis medium to visualize the vacuoles in the gradient. For α-mannosidase assays, the protoplasts and vacuoles were resuspended in 0.25 M Na acetate buffer, pH 4.6, sonicated, and centrifuged at 4°C for 10 min at 10,000g, and the supernatant was kept on ice until used for the enzymatic assay.
Enzymatic Assays
α-Mannosidase assays were used for the quantification of vacuole recovery and were performed as follows. Seventy microliters of 250 mM Na acetate buffer, pH 4.6, was added to 20-μL vacuole extracts or cell extracts. The reaction was started by adding 10 μL of the substrate solution (6 mM p-nitrophenyl α-d-mannopyrannoside in extraction buffer), and the mixture was incubated for 30 min at 30°C. The reaction was terminated by adding 160 μL of 1 M Na2CO3. Then, 200 μL was transferred into a microtiter plate and the absorbance was measured in a plate reader at 405 nm. Equal α-mannosidase activities were loaded on SDS-PAGE for cells and vacuoles to monitor vacuolar localization. α-Amylase assays and calculation of the secretion index was performed as described previously (Crofts et al., 1999; Phillipson et al., 2001).
The gel assay for proteinase activity was performed according to the protocol of Bethke et al. (1996) with minor modifications. Crude plant extracts were made in Phytepsin extraction buffer without pepstatin, and 20 μg of protein was loaded per well in the gels. The pH of the lower gel was 8.8 instead of 9.1. After electrophoresis, the control gel was incubated with 5 μM pepstatin instead of 20 μM pepstatin.
Protein Sample Preparation and Protein Gel Blotting
The harvested protoplasts were lysed with Phytepsin extraction buffer (25 mM Tris-HCl, pH 7.5, 250 mM NaCl, 5 mM EDTA, 1% [v/v] Triton X-100, 10 μM leupeptin, 4 mM phenylmethylsulfonyl fluoride, and 0.1 μM pepstatin), sonicated, and centrifuged at maximum speed in a microcentrifuge for 15 min at 4°C. An equal amount of sample buffer (Laemmli, 1970) was added to the supernatant, and the samples were boiled for 4 min. Culture medium of the protoplasts was collected and concentrated by precipitating with 60% ammonium sulfate as described by Hadlington and Denecke (2001).
SDS-PAGE (12% gels) was performed according to Laemmli (1970). Proteins were electrotransferred to a nitrocellulose filter in 0.3% (w/v) Tris, 1.44% (w/v) glycine, and 10% (v/v) methanol, and the protein gel blotting was performed according to the manufacturer's protocol for the enhanced chemiluminescence detection method (Amersham).
In Vivo Pulse Labeling and Immunoprecipitation
Protoplasts were labeled for 1 hr as described (Denecke and Vitale, 1995). All immunoprecipitations were performed as described previously (Denecke and Vitale, 1995; Crofts et al., 1998), except that protease inhibitors present during extraction (10 μM leupeptin and 0.1 μM pepstatin) also were included during the immunoprecipitation and washing steps. Labeled proteins were visualized with a PhosphorImager (Fuji, Tokyo, Japan).
Immunocytochemistry
Small segments of root and leaf tissue were fixed, dehydrated, and embedded according to the protocol described by Sikora et al. (1998), with the following minor alterations: the first 30 min of the primary aldehyde fixation was performed in a vacuum, and the duration of the osmium tetroxide fixation at −20°C was extended to 24 hr. On sections, immunogold labeling was performed by standard procedures (Hohl et al., 1996) using Phytepsin antibodies at a primary dilution of 1:50. Gold-coupled (10 nm) secondary antibodies were presented at a dilution of 1:30. Uranyl acetate/lead citrate poststained sections were examined in a Philips CM10 electron microscope (Philips, Eindhoven, The Netherlands) operating at 80 kV.
Confocal Microscopy
Protoplasts expressing GFP fusion proteins were harvested 24 hr after DNA transfer and embedded in 1% low gelling agarose in TEX medium (Denecke and Vitale, 1995) on a cover slip. The protoplasts were analyzed with a Zeiss LSM 410 confocal laser microscope (Zeiss, Jena, Germany) using a 488-nm argon ion laser in combination with a narrow bandpass filter set (510 to 515 nm). Analysis of fluorescence intensity was performed with the line measure software package provided with the microscope.
Acknowledgments
The authors are indebted to L. Frigerio for helpful comments and S. Vidal for critically reading the manuscript. J. Rogers is thanked for communicating unpublished results and for scientific discussion. A. Dufresne is thanked for inspiration and moral support. K.T. and J.L.H. are indebted to the Finnish Academy and the Biotechnology and Biological Sciences Research Council for studentships. This work was supported by grants from the Biotechnology and Biological Sciences Research Council and the European Union, which we gratefully acknowledge.
References
- Barlowe, C., Orci, L., Yeung, T., Hosobuchi, M., Hamamoto, S., Salama, N., Rexach, M.F., Ravazzola, M., Amherdt, M., and Schekman, R. (1994). COPII: A membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77 895–907. [DOI] [PubMed] [Google Scholar]
- Bethke, P.C., Hillmer, S., and Jones, R.L. (1996). Isolation of intact protein storage vacuoles from barley aleurone: Identification of aspartic and cysteine proteases. Plant Physiol. 110 521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, S.B., Wang, C.L., Muench, D.G., Ozawa, K., Franceschi, V.R., Wu, Y.J., and Okita, T.W. (2000). Messenger RNA targeting of rice seed storage proteins to specific ER subdomains. Nature 407 765–767. [DOI] [PubMed] [Google Scholar]
- Chrispeels, M.J., and Herman, E.M. (2000). Endoplasmic reticulum-derived compartments function in storage and as mediators of vacuolar remodeling via a new type of organelle, precursor protease vesicles. Plant Physiol. 123 1227–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, J., Ashford, D.A., Nimtz, M., Bento, I., Frazão, C., Esteves, C.L., Faro, C.J., Kervinen, J., Pires, E., Veríssimo, P., Wlodawer, A., and Carrondo, M.A. (1997). The glycosylation of the aspartic proteinases from barley (Hordeum vulgare L.) and cardoon (Cynara cardunculus L.). Eur. J. Biochem. 243 695–700. [DOI] [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Pesca, M., Vitale, A., and Denecke, J. (1998). BiP and calreticulin form an abundant complex that is independent of endoplasmic reticulum stress. Plant Cell 10 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts, A.J., Leborgne-Castel, N., Hillmer, S., Robinson, D.G., Phillipson, B., Carlsson, L.E., Ashford, D.A., and Denecke, J. (1999). Saturation of the endoplasmic reticulum retention machinery reveals anterograde bulk flow. Plant Cell 11 2233–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., and Vitale, A. (1995). The use of protoplasts to study protein synthesis and transport by the plant endomembrane system. Methods Cell. Biol. 50 335–348. [DOI] [PubMed] [Google Scholar]
- Denecke, J., Botterman, J., and Deblaere, R. (1990). Protein secretion in plant cells can occur via a default pathway. Plant Cell 2 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecke, J., De Rycke, R., and Botterman, J. (1992). Plant and mammalian sorting signals for protein retention in the endoplasmic reticulum contain a conserved epitope. EMBO J. 11 2345–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sansebastiano, G.P., Paris, N., Marc-Martin, S., and Neuhaus, J.-M. (1998). Specific accumulation of GFP in a non-acidic vacuolar compartment via a C-terminal propeptide-mediated sorting pathway. Plant J. 15 449–457. [DOI] [PubMed] [Google Scholar]
- Frigerio, L., de Virgilio, M., Prada, A., Faoro, F., and Vitale, A. (1998. a). Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., Vitale, A., Lord, J.M., Ceriotti, A., and Roberts, L.M. (1998. b). Free ricin A chain, proricin, and native toxin have different cellular fates when expressed in tobacco protoplasts. J. Biol. Chem. 273 14194–14199. [DOI] [PubMed] [Google Scholar]
- Frigerio, L., Pastres, A., Prada, A., and Vitale, A. (2001). Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: Selective use of an alternative route to vacuoles. Plant Cell 13 1109–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glathe, S., Kervinen, J., Nimtz, M., Li, G.H., Tobin, G.J., Copeland, T.D., Ashford, D.A., Wlodawer, A., and Costa, J. (1998). Transport and activation of the vacuolar aspartic proteinase Phytepsin in barley (Hordeum vulgare L.). J. Biol. Chem. 273 31230–31236. [DOI] [PubMed] [Google Scholar]
- Glickman, J.N., and Kornfeld, S. (1993). Mannose 6-phosphate independent targeting of lysosomal-enzymes in I-cell disease B-lymphoblasts. J. Cell Biol. 123 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, L., and Chrispeels, M.J. (1993). Tonoplast and soluble vacuolar proteins are targeted by different mechanisms. Plant Cell 5 1113–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guruprasad, K., Törmäkangas, K., Kervinen, J., and Blundell, T.L. (1994). Comparative modelling of barley-grain aspartic proteinase: A structural rationale for observed hydrolytic specificity. FEBS Lett. 352 131–136. [DOI] [PubMed] [Google Scholar]
- Hadlington, J.L., and Denecke, J. (2000). Sorting of soluble proteins in the secretory pathway of plants. Curr. Opin. Plant Biol. 10 461–468. [DOI] [PubMed] [Google Scholar]
- Hadlington, J.L., and Denecke, J. (2001). Transient expression, a tool to address questions in plant cell biology. In Plant Cell Biology: A Practical Approach, 2nd ed., C. Hawes and B. Jeunemaitre, eds (Oxford, UK: Oxford University Press), pp. 107–125.
- Hara-Nishimura, I., Shimada, T., Hatano, K., Takeuchi, Y., and Nishimura, M. (1998). Transport of storage proteins to protein storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick, K.G., Boothroyd, J.C., Rudner, A.D., and Pelham, H.R.B. (1992). Genes that allow yeast cells to grow in the absence of the HDEL receptor. EMBO J. 11 4187–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz, G., Hillmer, S., Baumer, M., and Hohl, I. (1999). Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the Golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell 11 1509–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh, B., Hinz, G., Jeong, B.K., and Robinson, D.G. (1995). Protein storage vacuoles form de-novo during pea cotyledon development. J. Cell Sci. 108 299–310. [DOI] [PubMed] [Google Scholar]
- Hohl, I., Robinson, D.G., Chrispeels, M.J., and Hinz, G. (1996). Transport of storage proteins to the vacuole is mediated by vesicles without a clathrin coat. J. Cell Sci. 109 2539–2550. [DOI] [PubMed] [Google Scholar]
- Jauh, G.Y., Fisher, A.M., Grimes, H.D., Ryan, C.A., Jr., and Rogers, J.C. (1998). δ-Tonoplast intrinsic protein defines unique vacuole functions. Proc. Natl. Acad. Sci. USA 95 12995–12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kervinen, J., Tobin, G., Costa, J., Waugh, D.S., Wlodawa, A., and Zdanov, A. (1999). Crystal structure of plant aspartic proteinase proPhytepsin: Inactivation and vacuolar targeting. EMBO J. 18 3947–3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, T., Paris, N., Butler, J.M., Beevers, L., and Rogers, J.C. (1994). Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc. Natl. Acad. Sci. USA 91 3403–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto, Y., Hiraiwa, M., and O'Brien, J.S. (1992). Saposins: Structure, function, distribution, and molecular genetics. J. Lipid Res. 33 1255–1267. [PubMed] [Google Scholar]
- Klumperman, J. (2000). Transport between ER and Golgi. Curr. Opin. Cell Biol. 12 445–449. [DOI] [PubMed] [Google Scholar]
- Laemmli, U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685. [DOI] [PubMed] [Google Scholar]
- Levanony, H., Rubin, R., Altshuler, Y., and Galili, G. (1992). Evidence for a novel route of wheat storage proteins to vacuoles. J. Cell Biol. 119 1117–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X.X., Franceschi, V.R., and Okita, T.W. (1993). Segregation of storage protein messenger-RNAs on the rough endoplasmic-reticulum membranes of rice endosperm cells. Cell 72 869–879. [DOI] [PubMed] [Google Scholar]
- Maliga, P., Breznovits, A., and Marton, L. (1973). Streptomycin-resistant plants from callus culture of haploid tobacco. Nature 244 29–30. [DOI] [PubMed] [Google Scholar]
- Marty, F. (1999). Plant vacuoles. Plant Cell 11 587–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka, K., and Neuhaus, J.-M. (1999). Cis-elements of protein transport to the plant vacuoles. J. Exp. Bot. 50 165–174. [Google Scholar]
- Matsuoka, K., Bassham, D.C., Raikhel, N.V., and Nakamura, K. (1995). Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 130 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford, R.S., Sheppard, P.O., and O'Hara, P.J. (1995). Saposin-like proteins (SAPLIP) carry out diverse functions on a common backbone structure. J. Lipid Res. 36 1653–1663. [PubMed] [Google Scholar]
- Murashige, T., and Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 15 473–497. [Google Scholar]
- Nishikawa, S., Hirata, A., and Nakano, A. (1994). Inhibition of endoplasmic reticulum (ER)-to-Golgi transport induces relocalization of binding protein (BiP) within the ER to form the BiP bodies. Mol. Biol. Cell 5 1129–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien, J.S., and Kishimoto, Y. (1991). Saposin proteins: Structure, function, and role in human lysosomal storage disorders. FASEB J. 5 301–308. [DOI] [PubMed] [Google Scholar]
- Paris, N., Stanley, C.M., Jones, R.L., and Rogers, J.C. (1996). Plant cells contain two functionally distinct vacuolar compartments. Cell 85 563–572. [DOI] [PubMed] [Google Scholar]
- Phillipson, B., Pimpl, P., Crofts, A.J., Movafeghi, A., Taylor, J.P., Robinson, D.G., and Denecke, J. (2001). Secretory bulk flow of soluble proteins is efficient and COPII dependent. Plant Cell 13 2005–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting, C.R., and Russell, R.B. (1995). Swaposins: Circular permutations within genes encoding saposin homologues. Trends Biochem. Sci. 20 179–180. [DOI] [PubMed] [Google Scholar]
- Rijnboutt, S., Aerts, H.M.F.G., Geuze, H.J., Tager, J.M., and Strous, G.J. (1991). Mannose-6-phosphate-independent membrane-association of cathepsin D, glucocerebrosidase, and sphingolipid-activating protein in HepG2 cells. J. Biol. Chem. 266 4862–4868. [PubMed] [Google Scholar]
- Runeberg-Roos, P., Törmäkangas, K., and Östman, A. (1991). Primary structure of a barley-grain aspartic proteinase: A plant aspartic proteinase resembling mammalian cathepsin D. Eur. J. Biochem. 202 1021–1027. [DOI] [PubMed] [Google Scholar]
- Sarkkinen, P., Kalkkinen, N., Tilgmann, C., Siuro, J., and Kervinen, J. (1992). Aspartic proteinase from barley grains is related to mammalian lysosomal cathepsin D. Planta 186 317–323. [DOI] [PubMed] [Google Scholar]
- Shimada, T., Kuroyanagi, M., Nishimura, M., and Hara-Nishimura, I. (1997). A pumpkin 72-kDa membrane protein of precursor-accumulating vesicles has characteristics of a vacuolar sorting receptor. Plant Cell Physiol. 38 1414–1420. [DOI] [PubMed] [Google Scholar]
- Sikora, A., Hillmer, S., and Robinson, D.G (1998). Sucrose starvation causes a loss of immunologically detectable pyrophosphatase and V-ATPase in the tonoplast of suspension-cultured tobacco cells. J. Plant Physiol. 152 207–212. [Google Scholar]
- Staab, J.F., Ginkel, D.L., Rosenberg, G.B., and Munford, R.S. (1994). A saposin-like domain influences the intracellular localization, stability, and catalytical activity of human acyloxyacyl hydrolase. J. Biol. Chem. 269 23736–23742. [PubMed] [Google Scholar]
- Toyooka, K., Okamoto, T., and Minamikawa, T. (2000). Mass transport of preform of a KDEL-tailed cysteine proteinase (SH-EP) to protein storage vacuoles by endoplasmic reticulum-derived vesicle is involved in protein mobilization in germinating seeds. J. Cell Biol. 148 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccaro, A.M., Ciaffoni, F., Tatti, M., Salvioli, R., Barca, A., Tognozzi, D., and Scerch, C. (1995). pH-dependent conformational properties of saposins and their interactions with phospholipid membranes. J. Biol. Chem. 270 30576–30580. [DOI] [PubMed] [Google Scholar]
- Vitale, A., and Denecke, J. (1999). The endoplasmic reticulum: Gateway of the secretory pathway. Plant Cell 11 615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., and Galili, G. (2001). The endomembrane system and the problem of protein sorting. Plant Physiol. 125 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, Y., and Conner, G.E. (1994). Intermolecular association of lysosomal protein precursors during biosynthesis. J. Biol. Chem. 269 3846–3851. [PubMed] [Google Scholar]