Abstract
The hormone abscisic acid (ABA) regulates stress responses and developmental processes in plants. Calcium-permeable channels activated by reactive oxygen species (ROS) have been shown recently to function in the ABA signaling network in Arabidopsis guard cells. Here, we report that ABA activation of these ICa Ca2+ channels requires the presence of NAD(P)H in the cytosol. The protein phosphatase 2C (PP2C) mutant abi1-1 disrupted ABA activation of ICa channels. Moreover, in abi1-1, ABA did not induce ROS production. Consistent with these findings, in abi1-1, H2O2 activation of ICa channels and H2O2-induced stomatal closing were not disrupted, suggesting that abi1-1 impairs ABA signaling between ABA reception and ROS production. The abi2-1 mutation, which lies in a distinct PP2C gene, also disrupted ABA activation of ICa. However, in contrast to abi1-1, abi2-1 impaired both H2O2 activation of ICa and H2O2-induced stomatal closing. Furthermore, ABA elicited ROS production in abi2-1. These data suggest a model with the following sequence of events in early ABA signal transduction: ABA, abi1-1, NAD(P)H-dependent ROS production, abi2-1, ICa Ca2+ channel activation followed by stomatal closing.
INTRODUCTION
The plant hormone abscisic acid (ABA) regulates a range of physiological processes, including seed maturation, control of vegetative growth, and promotion of dormancy, as well as tolerance of plants to adverse environmental conditions such as drought, cold, and salinity (Koornneef et al., 1998; Leung and Giraudat, 1998). In response to drought, ABA causes closing of stomatal pores, which are formed by pairs of guard cells in the epidermis of leaves and other aerial tissues. Stomatal closing results in a reduction of plant transpirational water loss. ABA induces an increase in cytosolic Ca2+ in guard cells, which precedes the reduction in stomatal aperture (McAinsh et al., 1990). Cytosolic Ca2+ elevation in turn activates slow (S-type) anion channels and downregulates inward K+ channels in guard cells (Schroeder and Hagiwara, 1989), resulting in net ion release and turgor reduction leading to stomatal closing. ABA increases Ca2+ by inducing both Ca2+ release from intracellular stores and Ca2+ influx from the extracellular space (Schroeder and Hagiwara, 1990; Grabov and Blatt, 1998; Leckie et al., 1998; Staxen et al., 1999; Hamilton et al., 2000; MacRobbie, 2000; Pei et al., 2000). ABA activation of plasma membrane Ca2+ influx also is required in Arabidopsis suspension culture cells, suggesting that ABA activation of Ca2+ influx is a more general component of ABA signaling in plants (Ghelis et al., 2000b). More than one type of plasma membrane Ca2+ channel may exist in guard cells (Schroeder and Hagiwara, 1990; Hamilton et al., 2000; Pei et al., 2000). The second messengers inositol 1,4,5-trisphosphate, cyclic ADP ribose, and calcium have been suggested to cause Ca2+ release via different endomembrane Ca2+ channels in response to ABA in guard cells (Gilroy et al., 1990; Ward and Schroeder, 1994; Parmar and Brearley, 1995; Lee et al., 1996; Leckie et al., 1998; Bewell et al., 1999; Staxen et al., 1999). Furthermore, a recent study also implicates inositol hexakisphosphate in Ca2+-dependent stomatal movements (Lemtiri-Chlieh et al., 2000).
ABA triggers Ca2+ influx via nonselective Ca2+-permeable channels in Vicia guard cells (Schroeder and Hagiwara, 1990). Cellular mechanisms that activate guard cell plasma membrane Ca2+ channels have been identified. Studies show that membrane hyperpolarization causes cytosolic Ca2+ increases in guard cells (Gilroy et al., 1991; Grabov and Blatt, 1998; Allen et al., 2000). Hyperpolarization-activated Ca2+ (ICa) channels were identified in Arabidopsis and Vicia guard cells (Hamilton et al., 2000; Pei et al., 2000). In Arabidopsis the Ica currents were shown to be carried by non-selective cation channels (Pei et al., 2000). In Vicia guard cells, intracellular ABA transiently enhanced the activity of hyperpolarization-activated Ca2+ channels (Hamilton et al., 2000). In Arabidopsis guard cells, H2O2 and ABA stimulated hyperpolarization-activated Ca2+-permeable ICa channels (Pei et al., 2000).
ABA was shown to induce the production of reactive oxygen species (ROS) in Arabidopsis guard cells (Pei et al., 2000). H2O2 activation of ICa channels and H2O2-induced stomatal closing were abolished in the ABA-insensitive mutant gca2 (Himmelbach et al., 1998), providing genetic evidence for roles of ROS and ICa channels in ABA signaling (Pei et al., 2000). Interestingly, in maize embryos and Vicia guard cells, ABA was shown recently to increase H2O2 levels (Guan et al., 2000; Zhang et al., 2001), indicating that ABA-induced ROS production may be of more general importance for ABA signaling. Diphenylene iodonium chloride (DPI), an inhibitor of NAD(P)H oxidases, partially inhibited ABA-induced stomatal closing (Pei et al., 2000). DPI also can inhibit other flavoenzymes (O'Donnell et al., 1993); therefore, further analyses are required to determine whether NAD(P)H contributes to the guard cell ABA response.
The dominant mutations abi1-1 and abi2-1 lie in two distinct type 2C protein phosphatases (PP2Cs) (Koornneef et al., 1984; Leung et al., 1994; Meyer et al., 1994; Grill and Himmelbach, 1998; Leung and Giraudat, 1998). The abi1-1 and abi2-1 mutations reduce ABA-induced cytosolic Ca2+ increases in guard cells (Allen et al., 1999a). Furthermore, experimentally imposing cytosolic Ca2+ ([Ca2+]cyt) elevations bypasses these mutants and restores S-type anion channel activation and stomatal closing (Allen et al., 1999a), demonstrating that abi1-1 and abi2-1 disrupt early ABA signaling at the level of, or upstream of, ABA-induced [Ca2+]cyt increases. However, it remains unknown where these PP2C mutants act in the early signaling cascade and whether they affect ABA activation of ICa channels.
In this article, we investigate the link of ABA signaling to ROS production and ICa activation, and we analyze whether ABA activation of ICa depends on cytosolic NAD(P)H. Furthermore, we analyze whether abi1-1 and abi2-1 affect this newly recognized branch of ABA signaling, and if so, at what points in the signaling pathway. The results show a requirement of NAD(P)H in ABA activation of ICa and demonstrate, via several independent analyses, that abi1-1 and abi2-1 differentially disrupt ABA activation of ICa.
RESULTS
NAD(P)H Requirement for ABA Activation of Ca2+ Channels
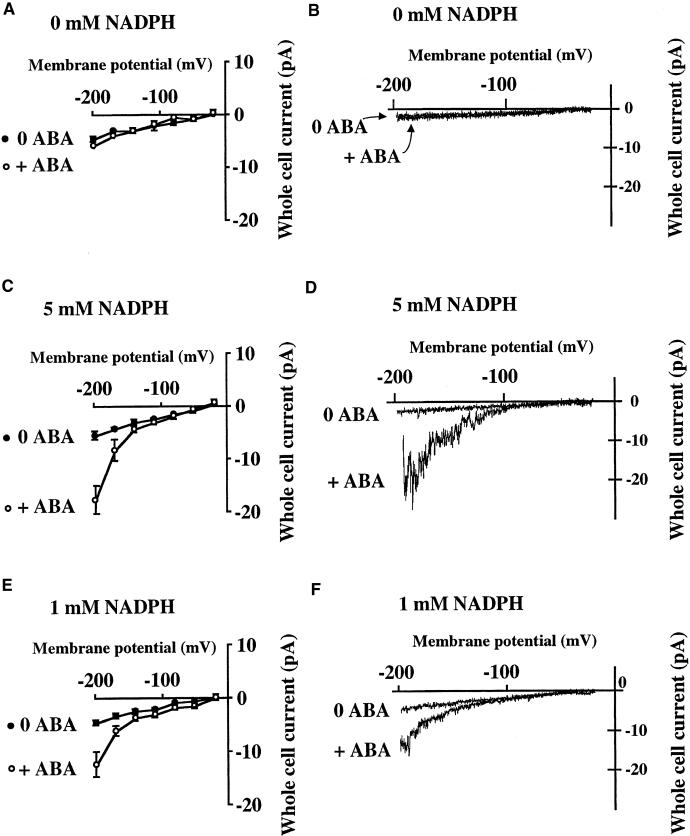
To examine whether NAD(P)H may play a role in the ABA stimulation of hyperpolarization-activated calcium-permeable channels, we analyzed ABA effects on ICa in the presence or absence of cytosolic NADPH. ABA and H2O2 were applied to patch-clamped guard cells, and responses were recorded. The presence of 0.1 mM DTT in the patch clamp pipette and bath solutions inhibited the spontaneous activation of ICa currents, as reported previously (Pei et al., 2000). In the absence of DTT, some guard cells showed constitutive ICa activity (Pei et al., 2000), which supports the findings that oxidative processes activate these Ca2+ channels.
Arabidopsis guard cells (Landsberg erecta ecotype) were patch clamped for 10 min in the whole-cell mode before extracellular ABA application. When NADPH, the cytoplasm of guard cells, was not included in the patch clamp pipette solution which dialyzes, ABA did not activate ICa currents, as shown in Figures 1A and 1B (n = 10). However, when 5 mM NADPH was added to the pipette solution, ABA activated ICa currents (n = 13), as reported previously (Figures 1C and 1D) (Pei et al., 2000). Addition of the NADPH oxidase inhibitor DPI (12.5 μM) to the pipette solution inhibited the ABA activation of ICa (n = 3; data not shown). ABA also activated ICa in the presence of 1 mM NADPH (P < 0.01) (Figures 1E and 1F; n = 8). The average amplitudes of ABA-activated whole-cell ICa currents at −198 mV were statistically similar at 1 μM and 5 mM NADPH (P > 0.11).
Figure 1.
ABA Activation of Ca2+-Permeable ICa Currents in Arabidopsis Guard Cell Protoplasts Requires Cytosolic NADPH.
(A) and (B) ABA (50 μM) did not activate ICa calcium channels when the pipette solution did not include NADPH or NADH. (B) shows two overlapping traces from a guard cell before and after ABA application.
(C) and (D) ABA (50 μM) activated ICa calcium currents when 5 mM NADPH was added to the pipette solution.
(E) and (F) ABA activated ICa when 1 mM NADPH was added to the pipette solution.
(A), (C), and (E) show average responses, and (B), (D), and (F) show responses in individual cells before and 5 min after ABA application. ABA was added ∼10 min after establishing whole-cell recordings, and whole-cell currents were measured before ABA application and in the same cells 5 min after extracellular ABA application in all recordings. The numbers of cells averaged are given in the text. Closed circles, before ABA addition to batch solution; open circles, 5 min after ABA addition to the same cells. Error bars represent sem.
In additional sets of experiments, 5 mM cytosolic NADPH caused an activation of ICa in the absence of added ABA in some cells, whereas with 1 mM cytosolic NADPH, ABA activation of ICa occurred (I.C. Mori, G.J. Allen, and J.I. Schroeder, data not shown). NADPH oxidation has been reported to be similar to NADH oxidation; therefore, not only NADPH but also NADH functions as a substrate of peroxidases to produce ROS in higher plants (Bestwick et al., 1998). When 5 mM NADPH was replaced with 5 mM NADH in the pipette solution, ABA activated ICa to a lesser extent, with an average amplitude of −7.8 ± 1.6 pA at −198 mV (n = 9; data not shown).
abi1-1 and abi2-1 PP2C Mutants Disrupt the ABA Activation of ICa
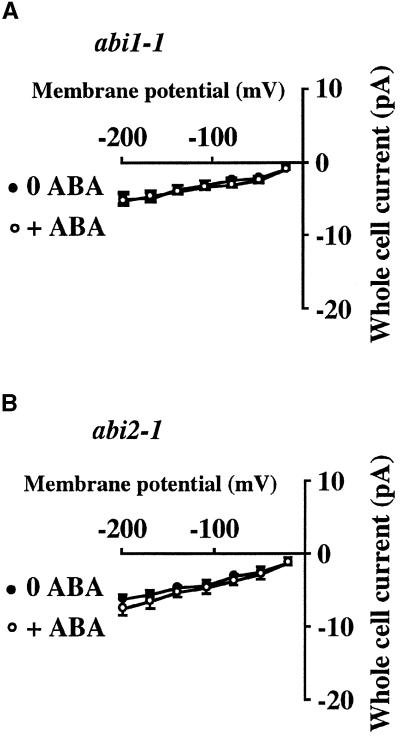
To determine whether the abi1-1 and abi2-1 mutations affect the ABA activation of ICa, guard cell protoplasts of the abi1-1 and abi2-1 mutants were first patch clamped for ∼10 min in the whole-cell mode. Subsequently, the same cells were treated with ABA by bath perfusion. Figure 2 shows the effects of abi1-1 and abi2-1 on the ABA activation of ICa. ABA did not activate ICa in either mutant in the presence of 5 mM cytosolic NADPH (n = 5, P > 0.72 for abi1-1; n = 5, P > 0.35 for abi2-1).
Figure 2.
ABA Failed to Activate Ca2+ Channel Currents in abi1-1 and abi2-1 PP2C Mutant Guard Cells.
(A) ABA (50 μM) did not activate ICa in abi1-1 guard cells with 5 mM NADPH included in the pipette solution (n = 5).
(B) ABA (50 μM) did not activate ICa in abi2-1 guard cells with 5 mM NADPH added to the pipette solution (n = 5).
Experiments were performed as described in Figure 1. Error bars represent sem.
H2O2-Induced Responses Are Impaired in abi2-1 but Not in abi1-1
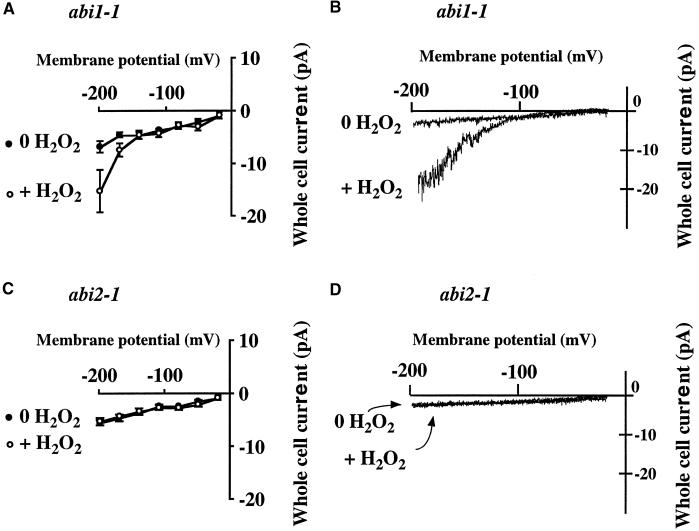
To examine whether the abi1-1 and abi2-1 mutations impair the activation of ICa upstream or downstream of or parallel to ROS production, we first analyzed H2O2 activation of the hyperpolarization-activated Ca2+ channel currents in abi1-1 and abi2-1. H2O2 (100 μM) clearly activated ICa in abi1-1 guard cells, as shown in Figures 3A and 3B (n = 6, P < 0.04). Interestingly, however, H2O2 failed to activate ICa in abi2-1 guard cells (Figures 3C and 3D) (n = 5, P > 0.84).
Figure 3.
H2O2 Activates Ca2+-Permeable Currents in abi1-1 but Not abi2-1 Arabidopsis Guard Cell Protoplasts.
(A) and (B) H2O2 (100 μM) activates ICa in abi1-1 guard cells (n = 6).
(C) and (D) H2O2 (100 μM) did not activate ICa in abi2-1 guard cells (n = 5). (D) shows two overlapping traces.
Experiments were performed as described in Figure 1 except that H2O2 was used instead of ABA. Error bars represent sem.
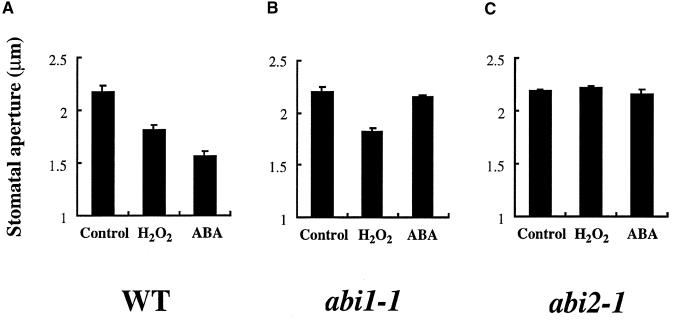
Exposure of stomates to H2O2 induces [Ca2+]cyt increases and partial stomatal closing in Commelina and Arabidopsis (McAinsh et al., 1996; Pei et al., 2000). To further analyze H2O2-mediated signal transduction in abi1-1 and abi2-1, we performed stomatal closing assays in wild-type, abi1-1, and abi2-1 leaves. As reported previously, extracellular Ca2+ is required for H2O2 induction of stomatal closing and [Ca2+]cyt increases (Pei et al., 2000). When 0.1 mM CaCl2 was added to the bath solution, partial stomatal closing occurred (DeSilva et al., 1985; Allen et al., 1999a). However, buffering the total free Ca2+ concentration to ∼0.1 mM in the cell wall space of epidermal strips with a bath solution containing 0.1 mM EGTA and 0.2 mM Ca2+ minimized Ca2+-induced stomatal closing and allowed analysis of H2O2 responses (Pei et al., 2000). H2O2 at 100 μM triggered a reduction in stomatal aperture in the wild type (Landsberg erecta ecotype), as illustrated in Figure 4A (n = 3 experiments, P < 0.01). The partial H2O2 response compared with the ABA response (Figure 4A) is consistent with the proposed model of parallel branches together mediating early ABA signaling (see Figure 5f in the article by Pei et al., 2000). H2O2 also triggered a reduction in stomatal aperture in the abi1-1 mutant (Figure 4B; n = 3, P < 0.02). However, stomatal aperture measurements showed that H2O2-induced stomatal closing was impaired in the abi2-1 mutant (Figure 4C; n = 3, P > 0.46). Stomatal movement results were confirmed in additional control and blind experiments (see Methods).
Figure 4.
Differential Effects of H2O2 on Stomatal Apertures of abi1-1 and abi2-1.
(A) Both ABA (50 μM) and H2O2 (100 μM) induced stomatal closing in the wild type (Landsberg erecta ecotype).
(B) ABA did not cause stomatal closing, but H2O2 elicited partial stomatal closing in abi1-1.
(C) Neither ABA nor H2O2 elicited stomatal closing of abi2-1.
Averages from n = three leaf epidermal experiments are shown (60 stomates per bar). Error bars represent sem.
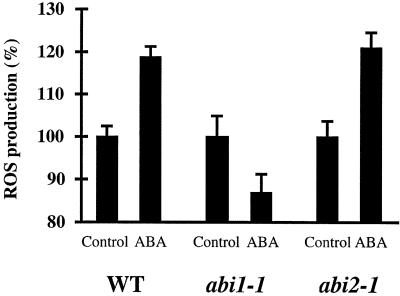
Figure 5.
Differential Production of ROS in abi1-1 and abi2-1 Guard Cells Treated with ABA.
ABA (50 μM) increased ROS production in the wild type (WT) (four experiments, n = 161 cells before ABA treatment, n = 123 cells after ABA treatment) and abi2-1 (nine experiments, n = 221 cells before ABA treatment, n = 207 cells after ABA treatment). ABA did not increase ROS production in abi1-1 (six experiments, n = 110 cells before ABA treatment, n = 150 cells after ABA treatment). Changes in ROS levels were analyzed by measuring H2DCF fluorescence levels in guard cells in response to ABA or solvent (0.1% ethanol) control applications. Error bars represent sem.
ABA Enhances ROS Levels in the Wild Type and abi2-1 but Not in abi1-1
To analyze ABA-dependent ROS production in abi1-1 and abi2-1, ROS levels were analyzed in populations of wild-type, abi1-1, and abi2-1 guard cells using the fluorescent dye 2′,7′-dichlorofluorescin diacetate (H2DCF-DA), which reports changes in the oxidative state of guard cells (Ohba et al., 1994; Lee et al., 1999; Pei et al., 2000). As shown in Figure 5, the relative fluorescence emission increased after treatment of wild-type guard cells with 50 μM ABA (four experiments, P < 0.02). However, ROS measurements showed that 50 μM ABA did not increase the relative fluorescence emission in abi1-1 guard cells (six experiments). A slight ABA-induced decrease in ROS levels was observed in abi1-1 guard cells, which was not significant in all data sets (P = 0.02 to 0.053) (Figure 5). Conversely, ABA at 50 μM increased the relative fluorescence emission in abi2-1 guard cells (nine experiments, P < 0.001) (Figure 5). Impairment of ABA-induced ROS production in abi1-1 was significant compared with that in the wild type (P < 0.001) and abi2-1 (P < 0.001). Differential ABA-induced fluorescence responses in abi1-1 and abi2-1 were confirmed in additional blind experiments (see Methods).
DISCUSSION
ABA induces an increase in [Ca2+]cyt, leading to a reduction in stomatal aperture. Recent studies of Arabidopsis mesophyll suspension culture cells showed that ABA induction of Rab18 gene expression requires ABA activation of plasma membrane Ca2+ influx followed by S-type anion channel activation, suggesting that the analyzed early signaling mechanisms in guard cells may be components of ABA signaling in many plant cell types (Ghelis et al., 2000a, 2000b). ABA stimulates hyperpolarization-activated ICa Ca2+ channels via ROS production, suggesting a new branch in early ABA signaling (Pei et al., 2000). ABA also increases the endogenous level of ROS in maize embryos, suggesting that H2O2 functions in ABA regulation of seed maturation (Guan et al., 2000). Furthermore, recent studies have linked ABA with oxidative responses (Bueno et al., 1998; Gong et al., 1998). These studies suggest that ROS may be of more general importance for ABA signal transduction in plants.
Cytosolic NADPH Is Necessary for the ABA Activation of ICa
ROS is a term for radicals and other reactive species derived from oxygen. ROS have been implicated in numerous signal transduction pathways in both plant and animal cells (Lamb and Dixon, 1997; Rhee et al., 2000). In plants, ROS, including the superoxide radical and H2O2, act as important second messengers in defense responses triggered by pathogens and elicitors (Levine et al., 1994; Lamb and Dixon, 1997). Many enzymes can produce ROS in plant cells. Activation of these enzymes is closely associated with oxidative bursts (Lamb and Dixon, 1997). A plasma membrane oxidase generates superoxide (Keller et al., 1998), a peroxidase produces H2O2 directly, and an oxalate oxidase also generates H2O2 (Baker and Orlandi, 1995). DPI, an inhibitor of neutrophil NADPH oxidases (Cross and Jones, 1986, 1991), partially inhibits ABA-induced stomatal closing (Pei et al., 2000). The elicitors oligogalacturonic acid and chitosan reduce stomatal aperture and induce the production of ROS in tomato and Commelina guard cells (Lee et al., 1999). The ROS activation of ICa channels has been proposed to represent a possible joint branch of multiple stress signaling pathways (Pei et al., 2000; Schroeder et al., 2001).
Previous studies have shown that the ABA activation of Ca2+ channels in Vicia guard cells is transient and attenuated (Schroeder and Hagiwara, 1990; Hamilton et al., 2000). In the present study of Arabidopsis guard cells, we obtained ABA activation of ICa in patch-clamped whole cells by adding NADPH or NADH via the patch pipette to the cytosol of guard cells. However, no ABA response was found in the relatively small Arabidopsis guard cells when no NAD(P)H was added to the patch pipette. The requirement of NADPH or NADH, ABA-induced ROS production, and the inhibitory effects of DPI suggest that NAD(P)H oxidases and/or redox control of sulfhydryl groups contributes to ABA signal transduction. NAD(P)H is formed by the reduction of NAD(P) via light-supplied energy in guard cell chloroplasts (Shimazaki et al., 1989). A previous study showed that ABA-induced stomatal closing requires guard cell metabolism (Weyers et al., 1982). In this respect, the results presented here indicate a possible link between guard cell metabolism and ion channel regulation. ABA signaling requires hydrolyzable ATP in guard cells (Schmidt et al., 1995) and phosphorylation events as positive transducers of stomatal closing, which also would require intact guard cell metabolism.
Plasma Membrane Ca2+ Channel Activity in Guard Cells
Guard cells show spontaneous activity of hyperpolarization-induced Ca2+ increases (Gilroy et al., 1991; Grabov and Blatt, 1998; Allen et al., 1999b) and spontaneous activity of plasma membrane Ca2+ currents (Hamilton et al., 2000; Pei et al., 2000). Whether the spontaneous Ca2+ influx and ICa are mediated by the same Ca2+ channel remains unknown. Interestingly, the spontaneous activity of hyperpolarization-induced Ca2+ currents was inhibited by adding DTT to the patch clamp pipette (cytosolic) solution and bath solutions (Pei et al., 2000; this study).
Fungal elicitors have been shown to induce hyperpolarization-activated Ca2+ channels in tomato suspension culture cells (Gelli et al., 1997). These Ca2+ channels show a similar ICa-like activation by hyperpolarization and a more pronounced time-dependent activation. Interestingly, in some tomato cells, spontaneous activity of hyperpolarization-activated Ca2+ channels was observed, which also was inhibited by 1 mM DTT (A. Gelli and E. Blumwald, personal communication). Pathogenic elicitors cause ROS production in plants, with Ca2+ influx occurring both before and after ROS production (Knight et al., 1991; Price et al., 1994; Lamb and Dixon, 1997; Kawano et al., 1998), suggesting that more than one Ca2+ channel or activation mechanism may contribute to this response. ICa-like Ca2+ channels may contribute to the secondary response that follows ROS production.
Interestingly, hyperpolarization-activated Ca2+ channels also have been identified in Arabidopsis cells from the cortical elongation zone of roots and the epidermis of the growing root tip, but not in mature epidermis or in pericycle cells (Kiegle et al., 2000) or in the apex of Arabidopsis root hair cells (Very and Davies, 2000). These studies suggest that hyperpolarization-activated Ca2+ channels may contribute to various signal transduction and growth processes in plants, and their opposite voltage dependence compared with depolarization-activated Ca2+ channels (Huang et al., 1994; Marshall et al., 1994; Thuleau et al., 1994) suggests activation during different signaling processes.
Differential Disruption by abi1-1 and abi2-1 PP2Cs
Stomata of the abi1-1 and abi2-1 mutants are insensitive to ABA (Finkelstein and Somerville, 1990; Roelfsema and Prins, 1995; Pei et al., 1997). ABA regulation of K+ channels is impaired by abi1-1 expression (Armstrong et al., 1995), and ABA activation of S-type anion channels is impaired in abi1-1 and abi2-1 (Pei et al., 1997). The Arabidopsis abi1-1 and abi2-1 mutations impair ABA-induced cytoplasmic Ca2+ increases in guard cells (Allen et al., 1999a). ABA induces both Ca2+ release from intracellular stores and Ca2+ influx from the extracellular space. However, it remained unknown which Ca2+ increase mechanisms were affected by abi1-1 and abi2-1. The identification of ROS and ICa as early ABA signaling intermediates has allowed a direct analysis of the effects of the abi PP2C mutations on early signal transduction mechanisms.
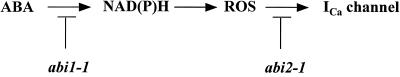
Here, we show that the PP2C mutations abi1-1 and abi2-1 both disrupt the ABA activation of ICa. ABA did not induce the production of ROS in the abi1-1 mutant. H2O2-activated ICa and H2O2-induced stomatal closing were not impaired in abi1-1. These findings suggest that the abi1-1 mutation disrupts ABA signaling upstream of ROS production, as illustrated in Figure 6. In contrast, ABA elicited ROS production in the abi2-1 mutant, but H2O2 did not activate ICa and did not induce stomatal closing in abi2-1. These data suggest that the abi2-1 mutation impairs ABA signaling downstream of ROS production (Figure 6). These data lead to a simple model for the positioning of ICa, ROS production, and the abi1-1 and abi2-1 protein phosphatases in the ABA signal transduction cascade in Arabidopsis guard cells, as illustrated in Figure 6. Note that ABA may function by downregulating ROS-scavenging enzymes such as catalase. The data further suggest that abi1-1 interacts with early signal transduction mechanisms upstream of ROS production. Previous studies have suggested that abi1-1 and abi2-1 have distinct functions, even though they both disrupt ABA signaling in general (Gilmour and Thomashow, 1991; Vartanian et al., 1994; Gosti et al., 1995; Bruxelles et al., 1996; Söderman et al., 1996; Pei et al., 1997; Strizhov et al., 1997). Our data are consistent with these findings and provide a working model for the ABA signal transduction pathway to explain the differential effects of abi1-1 and abi2-1 (Figure 6).
Figure 6.
Model Summarizing the Differential Disruption of ABA Activation of ICa Ca2+ Channels by the Two abi1-1 and abi2-1 PP2C Mutants.
ABA activation of Ca2+ channels required cytosolic NAD(P)H, was inhibited by DPI, and was accompanied by ROS production.
It remains unknown whether abi1-1 and abi2-1 additionally affect Ca2+ release from intracellular stores and a possible parallel Ca2+-independent pathway (Allan et al., 1994; Allen et al., 1999a). Ca2+ release can occur parallel to Ca2+ influx at low ABA concentrations (MacRobbie, 2000), and perhaps also downstream of ICa activation, based on Ca2+ activation of plant phospholipase C isoforms (Staxen et al., 1999) and on the proposed Ca2+-induced Ca2+ release via vacuolar slow vacuolar (SV) channels (Ward and Schroeder, 1994; Bewell et al., 1999). If abi1-1 impairs an ABA receptor, parallel mechanisms could be accounted for by one effect of abi1-1. Note that the abi1-1 and abi2-1 PP2Cs may interact with more than one protein. Future research is needed to analyze the effects of abi1-1 and abi2-1 on Ca2+ release mechanisms.
In summary, the present study provides strong support for the new model that ABA-induced ROS production and ICa Ca2+-permeable channel activation are important components of ABA signal transduction. The cytosolic NAD(P)H requirement for ABA activation of ICa channels, together with DTT and DPI inhibition of ICa, suggests that NAD(P)H oxidases and/or redox control of sulfhydryl groups contributes to early ABA signaling. Furthermore, abi1-1 and abi2-1 interact with important and distinct transducers of this early ABA signaling branch upstream and downstream of ROS production, respectively.
METHODS
Isolation of Arabidopsis Guard Cells
Arabidopsis thaliana wild type (Landsberg erecta ecotype) and the abi1-1 and abi2-1 mutant lines were used in this study. The mutant genotypes and homozygosity were confirmed using a previously described polymerase chain reaction method (Leung et al., 1997). The abscisic acid (ABA) insensitivity was further confirmed in seed germination assays (Pei et al., 1997; Allen et al., 1999a). Note that during the course of this work, a separate set of abi1-1 and abi2-1 seed was obtained from the Arabidopsis stock center (Ohio State University, Columbus). However, seed germination and polymerase chain reaction tests showed that these consisted of a mixed population. The stock center was informed, and these seed were not used further. Plants were grown in soil in plant growth chambers with a 16-hr-light (80 μE light fluence rate) and 8-hr-dark regimen and watered with deionized water every day. Arabidopsis guard cell protoplasts were isolated enzymatically from leaf epidermal strips of 4- to 6-week-old plants. Arabidopsis rosette leaves were blended in a commercial blender in deionized water three times for 5 sec each and collected using a nylon mesh (pore size, 62 μm). The collected epidermal tissue was incubated in 10 mL of medium containing 1% Cellulase R-10, 0.5% Macerozyme R-10 (Yakult, Japan), 0.5% BSA, 0.5 M mannitol, 0.1 mM KCl, 0.1 mM CaCl2, 10 mM ascorbic acid, and 0.1% kanamycin sulfate, pH 5.5 (with KOH), for 15 to 17 hr at 24°C on a shaker. Isolated guard cell protoplasts were collected and washed twice as described previously (Pei et al., 1997).
Patch Clamp and Data Acquisition
Whole-cell patch clamp recordings from Arabidopsis guard cells were made using Axopatch 200 and 200A amplifiers (Axon Instruments, Union City, CA) that were connected to microcomputers via interfaces as described (Pei et al., 1997). Seal resistances were >10 GΩ. Liquid junction potentials were corrected (Ward and Schroeder, 1994). Initially, upon establishment of whole-cell recordings, a current was observed in some guard cells. This current, however, disappeared within 30 sec to 4 min after establishing whole-cell recordings in 90% of the guard cells analyzed. The frequency of occurrence of this initial current varied from cell preparation to cell preparation. After the initial current had vanished and ∼10 min after establishing whole-cell recordings, ABA and H2O2 were applied by bath perfusion to patch-clamped guard cells and responses were recorded. pClamp software (Axon Instruments) was used to acquire and analyze whole-cell currents. The standard voltage protocol ramped from −18 to −198 mV (ramp speed, 180 mV/sec). The interpulse period was 1 min. Whole-cell currents were not leak subtracted. Data were analyzed using Axograph software (Axon Instruments, Inc., Foster City, CA). The bath solution used in patch clamp experiments contained 100 mM BaCl2, 0.1 mM DTT, and 10 mM Mes titrated to pH 5.6 with Tris, and the pipette solution was composed of 10 mM BaCl2, 0.1 mM DTT, 4 mM EGTA, 10 mM Hepes adjusted to pH 7.1, and Tris. To investigate the effects of ABA on a Ca2+-permeable, non-selective cation current (ICa), NADPH or NADH was added to the pipette solution at the indicated concentrations in the text. To analyze the hyperpolarization-activated currents, we used Ba2+ ions, which are permeable to ICa channels (Pei et al., 2000).
Stomatal Aperture Measurements
Stomatal movement analyses were performed as described previously (Pei et al., 2000). Rosette leaves from 4- to 6-week-old plants were exposed to white light (125 μE fluence rate) while floating in a solution containing 10 mM KCl, 0.2 mM CaCl2, 0.1 mM EGTA (free extracellular Ca2+ concentration buffered to ∼0.1 mM), and 10 mM Mes titrated to pH 6.15 with KOH. Subsequently, 100 μM H2O2 and 50 μM ABA were added to the bath solution as indicated in the Figures and text. After treatment for 2 hr in white light (125 μE fluence rate), leaves were blended and stomatal apertures were measured by focusing on the inner lips of stomates (away from the focal plane of guard cells) as described (Ichida et al., 1997). In each epidermal peel experiment, 20 stomatal apertures were measured at each condition. In additional control and blind experiments, the ability of H2O2 to cause stomatal closing in wild-type and abi1-1 leaves, but not abi2-1 leaves, was reproduced (n = 3 experiments per line; wild type ± H2O2, P < 0.03; abi1-1 ± H2O2, P < 0.02; abi2-1 ± H2O2, P > 0.36). Standard errors were determined relative to the square root of the number of epidermal strip experiments, as in previous studies (Ichida et al., 1997; Pei et al., 2000). All statistical analyses were performed using the TTEST program in Excel 5.0 software (Microsoft, Redmond, WA). Values of P < 0.05 were considered to show statistically significant differences.
Reactive Oxygen Species Detection in Guard Cells
Reactive oxygen species (ROS) production in guard cells was analyzed using 2′,7′-dichlorofluorescin diacetate (H2DCF-DA) (Ohba et al., 1994; Lee et al., 1999). This nonfluorescent compound is permeable to the plasma membrane and is converted to dichlorofluorescin (H2DCF), which is impermeable. H2DCF can be oxidized by peroxidases and H2O2. Epidermal tissues were isolated from 6-week-old plants with a commercial blender. The epidermal tissues were incubated in 30 mM KCl and 10 mM Mes-KOH, pH 6.15, in the light at room temperature for 2 hr (Pei et al., 2000). Note that immediately after epidermal tissue isolations, guard cells showed increased ROS levels, likely as a result of mechanical perturbation from epidermis excision. However, after 2-hr incubations of epidermal tissues in white light (125 μE), ROS levels decreased. Fifty micromolar H2DCF-DA was added to the incubation medium and then either 0.1% ethanol (control) or 50 μM ABA was added to the incubation medium after 20 to 30 min of dye loading. The epidermal tissues were collected using a nylon mesh and washed with distilled water twice after 20 to 30 min of ABA or ethanol control treatments. Guard cells were observed under a fluorescence microscope equipped with a cooled charge-coupled device camera. Note that prolonged exposure of H2DCF-loaded guard cells to excitation light led to a transient increase in ROS and subsequent bleaching of the dye. Therefore, to compare fluorescence responses in control and ABA-treated samples, excitation light exposure was reduced using neutral density filters and limited to a 10-sec exposure, and only one image was captured per sample. All experiments reported here (Figure 5) were confirmed in additional blind experiments (abi2-1 ± ABA, five experiments, n = 234 guard cells, P < 0.01). abi1-1 showed a slight but statistically insignificant ABA-induced reduction in ROS (five experiments, n = 152 guard cells, P = 0.053). Images were acquired and the fluorescence emission of guard cells was analyzed using Adobe Photoshop 5.0 (Mountain View, CA).
Acknowledgments
We thank Gethyn Allen for reading the manuscript, Lien Dang for conducting blind stomatal aperture experiments, and Jared Young for help with blind ROS experiments. This research was supported by grants from the National Institutes of Health (R01 GM60396-01) and the Department of Energy (FG03-94-ER20148) to J.S. and by a Japan Society for the Promotion of Sciences fellowship (11000465) to I.C.M.
Article, publication date, and citation information can be found at www.aspb.org/cgi/doi/10.1105/tpc.010210.
References
- Allan, A.C., Fricker, M.D., Ward, J.L., Beale, M.H., and Trewavas, A.J. (1994). Two transduction pathways mediate rapid effects of abscisic acid in Commelina guard cells. Plant Cell 6 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, G.J., Kuchitsu, K., Chu, S.P., Murata, Y., and Schroeder, J.I. (1999. a). Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11 1785–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, G.J., Kwak, J.M., Chu, S.P., Llopis, J., Tsien, R.Y., Harper, J.F., and Schroeder, J.I. (1999. b). Cameleon calcium indicator reports cytoplasmic calcium dynamics in Arabidopsis guard cells. Plant J. 19 735–747. [DOI] [PubMed] [Google Scholar]
- Allen, G.J., Chu, S.P., Schumacher, K., Shimazaki, C.T., Vafeados, D., Kemper, A., Hawke, S.D., Tallman, G., Tsien, R.Y., Harper, J.F., Chory, J., and Schroeder, J.I. (2000). Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289 2338–2342. [DOI] [PubMed] [Google Scholar]
- Armstrong, F., Leung, J., Grabov, A., Brearley, J., Giraudat, J., and Blatt, M.R. (1995). Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc. Natl. Acad. Sci. USA 92 9520–9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, C.J., and Orlandi, E.W. (1995). Active oxygen in plant pathogenesis. Annu. Rev. Phytopathol. 33 299–321. [DOI] [PubMed] [Google Scholar]
- Bestwick, C.S., Brown, I.R., and Mansfield, J.W. (1998). Localized changes in peroxidase activity accompany hydrogen peroxide generation during the development of a nonhost hypersensitive reaction in lettuce. Plant Physiol. 118 1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewell, M.A., Maathuis, F.J.M., Allen, G.J., and Sanders, D. (1999). Calcium-induced calcium release mediated by a voltage-activated cation channel in vacuolar vesicles from red beet. FEBS Lett. 458 41–44. [DOI] [PubMed] [Google Scholar]
- Bruxelles, G.L., Peacock, W.J., Dennis, E.S., and Dolferus, R. (1996). Abscisic acid induces the alcohol dehydrogenase gene in Arabidopsis. Plant Physiol. 111 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno, P., Piqueras, A., Kurepa, J., Savoure, A., Verbruggen, N., Van Montagu, M., and Inze, D. (1998). Expression of antioxidant enzymes in response to abscisic acid and high osmoticum in tobacco BY-2 cell cultures. Plant Sci. 138 27–34. [Google Scholar]
- Cross, A.R., and Jones, O.T. (1986). The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils: Specific labelling of a component polypeptide of the oxidase. Biochem. J. 237 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, A.R., and Jones, O.T. (1991). Enzymic mechanisms of superoxide production. Biochim. Biophys. Acta 1057 281–298. [DOI] [PubMed] [Google Scholar]
- DeSilva, D.L.R., Cox, R.C., Hetherington, A.M., and Mansfield, T.A. (1985). Synergism between calcium ions and abscisic acid in preventing stomatal opening. New Phytol. 101 555–563. [Google Scholar]
- Finkelstein, R.R., and Somerville, C.R. (1990). Three classes of abscisic acid (ABA)–insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol. 94 1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelli, A., Higgins, V.J., and Blumwald, E. (1997). Activation of plant plasma membrane Ca2+-permeable channels by race-specific fungal elicitors. Plant Physiol. 113 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghelis, T., Dellis, O., Jeannette, E., Bardat, F., Cornel, D., Miginiac, E., Rona, J.-P., and Sotta, B. (2000. a). Abscisic acid specific expression of RAB18 involves activation of anion channels in Arabidopsis thaliana suspension cells. FEBS Lett. 474 43–47. [DOI] [PubMed] [Google Scholar]
- Ghelis, T., Dellis, O., Jeannette, E., Bardat, F., Miginiac, E., and Sotta, B. (2000. b). Abscisic acid plasmalemma perception triggers a calcium influx essential for RAB18 gene expression in Arabidopsis thaliana suspension cells. FEBS Lett. 483 67–70. [DOI] [PubMed] [Google Scholar]
- Gilmour, S.J., and Thomashow, M.F. (1991). Cold acclimation and cold-regulated gene expression in ABA mutants of Arabidopsis thaliana. Plant Mol. Biol. 17 1233–1240. [DOI] [PubMed] [Google Scholar]
- Gilroy, S., Read, N.D., and Trewavas, A.J. (1990). Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature 346 769–771. [DOI] [PubMed] [Google Scholar]
- Gilroy, S., Fricker, M.D., Read, N.D., and Trewavas, A.J. (1991). Role of calcium in signal transduction of Commelina guard cells. Plant Cell 3 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong, M., Li, Y.-J., and Chen, S.-Z. (1998). Abscisic acid–induced thermotolerance in maize seedling is mediated by calcium and associated with antioxidant system. J. Plant Physiol. 153 488–496. [Google Scholar]
- Gosti, F., Bertauche, N., Vartanian, N., and Giraudat, J. (1995). Abscisic acid–dependent and –independent regulation of gene expression by progressive drought in Arabidopsis thaliana. Mol. Gen. Genet. 246 10–18. [DOI] [PubMed] [Google Scholar]
- Grabov, A., and Blatt, M.R. (1998). Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc. Natl. Acad. Sci. USA 95 4778–4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill, E., and Himmelbach, A. (1998). ABA signal transduction. Curr. Opin. Plant Biol. 1 412–418. [DOI] [PubMed] [Google Scholar]
- Guan, L.M., Zhao, J., and Scandalios, J.G. (2000). Cis-elements and trans-factors that regulate expression of the maize Cat1 antioxidant gene in response to ABA and osmotic stress: H2O2 is the likely intermediary signaling molecule for the response. Plant J. 22 87–95. [DOI] [PubMed] [Google Scholar]
- Hamilton, D.W.A., Hills, A., Köhler, B., and Blatt, M.R. (2000). Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc. Natl. Acad. Sci. USA 97 4967–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelbach, A., Iten, M., and Grill, E. (1998). Signaling of abscisic acid to regulate plant growth. Philos. Trans. R. Soc. Lond. B 353 1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J.W., Grunes, D.L., and Kochian, L.V. (1994). Voltage-dependent Ca2+ influx into right-side-out plasma membrane vesicles isolated from wheat roots: Characterization of a putative Ca2+ channel. Proc. Natl. Acad. Sci. USA 91 3473–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida, A.M., Pei, Z.M., Baizabal-Aguirre, V.M., Turner, K.J., and Schroeder, J.I. (1997). Expression of a Cs+-resistant guard cell K+ channel confers Cs+-resistant, light-induced stomatal opening in transgenic Arabidopsis. Plant Cell 9 1843–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano, T., Sahashi, N., Takahashi, K., Uozumi, N., and Muto, S. (1998). Salicylic acid induces extracellular superoxide generation followed by an increase in cytosolic calcium ion in tobacco suspension culture: The earliest events in salicylic acid signal transduction. Plant Cell Physiol. 39 721–730. [Google Scholar]
- Keller, T., Damunde, H.G., Werner, D., Doerner, P., Dixon, R.A., and Lamb, C. (1998). A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10 255–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiegle, E., Gilliham, M., Haseloff, J., and Tester, M. (2000). Hyperpolarization-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J. 21 225–229. [DOI] [PubMed] [Google Scholar]
- Knight, M.R., Campbell, A.K., Smith, S.M., and Trewavas, A.J. (1991). Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352 524–526. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., Reuling, G., and Karssen, C.M. (1984). The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 61 377–383. [Google Scholar]
- Koornneef, M., Leon-Kloosterziel, K.M., Schwartz, S.H., and Zeevaart, J.A.D. (1998). The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol. Biochem. 36 83–89. [Google Scholar]
- Lamb, C.J., and Dixon, R.A. (1997). The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 251–275. [DOI] [PubMed] [Google Scholar]
- Leckie, C.P., McAinsh, M.R., Allen, G.J., Sanders, D., and Hetherington, A.M. (1998). Abscisic acid–induced stomatal closure mediated by cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA 95 15837–15842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S., Choi, H., Suh, S., Doo, I.S., Oh, K.Y., Choi, E.J., Taylor, A.T.S., Low, P.S., and Lee, Y. (1999). Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol. 121 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y., Choi, Y.B., Suh, S., Lee, J., and Assmann, S.M. (1996). Abscisic acid–induced phosphoinositide turnover in guard cell protoplasts of Vicia faba. Plant Physiol. 110 987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh, F., MacRobbie, E.A.C., and Brearley, C.A. (2000). Inositol hexakisphosphate is a physiological signal regulating the K+-inward rectifying conductance in guard cells. Proc. Natl. Acad. Sci. USA 97 8687–8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, J., and Giraudat, J. (1998). Abscisic acid signal transduction. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49 199–222. [DOI] [PubMed] [Google Scholar]
- Leung, J., Bouvier-Durand, M., Morris, P.-C., Guerrier, D., Chefdor, F., and Giraudat, J. (1994). Arabidopsis ABA response gene ABI1: Features of a calcium-modulated protein phosphatase. Science 264 1448–1452. [DOI] [PubMed] [Google Scholar]
- Leung, J., Merlot, S., and Giraudat, J. (1997). The Arabidopsis ABSCISIC ACID–INSENSITIVE (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine, A., Tenhaken, R., Dixon, R., and Lamb, C. (1994). H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79 583–593. [DOI] [PubMed] [Google Scholar]
- MacRobbie, E.A.C. (2000). ABA activates multiple Ca2+ fluxes in stomatal guard cells, triggering vacuolar K+ (Rb+) release. Proc. Natl. Acad. Sci. USA 97 12361–12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, J., Corzo, A., Leigh, R.A., and Sanders, D. (1994). Membrane potential-dependent calcium transport in right-side-out plasma membrane vesicles from Zea mays L. roots. Plant J. 5 683–694. [Google Scholar]
- McAinsh, M.R., Brownlee, C., and Hetherington, A.M. (1990). Abscisic acid–induced elevation of guard cell cytoplasmic Ca2+ precedes stomatal closure. Nature 343 186–188. [Google Scholar]
- McAinsh, M.R., Clayton, H., Mansfield, T.A., and Hetherington, A.M. (1996). Changes in stomatal behavior and guard cell cytosolic free calcium in response to oxidative stress. Plant Physiol. 111 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer, K., Leube, M.P., and Grill, E. (1994). A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264 1452–1455. [DOI] [PubMed] [Google Scholar]
- O'Donnell, B.V., Tew, D.G., Jones, O.T., and England, P.J. (1993). Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 290 41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba, M., Shibanuma, M., Kuroki, T., and Nose, K. (1994). Production of hydrogen peroxide by transforming growth factor–β1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J. Cell Biol. 126 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar, P.N., and Brearley, C.A. (1995). Metabolism of 3- and 4-phosphorylated phosphatidylinositols in stomatal guard cells of Commelina communis L. Plant J. 8 425–433. [Google Scholar]
- Pei, Z.-M., Kuchitsu, K., Ward, J.M., Schwarz, M., and Schroeder, J.I. (1997). Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9 409–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei, Z.M., Murata, Y., Benning, G., Thomine, S., Klusener, B., Allen, G.J., Grill, E., and Schroeder, J.I. (2000). Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734. [DOI] [PubMed] [Google Scholar]
- Price, A.H., Taylor, S., Ripley, S.J., Griffiths, A., Trewavas, J., and Knight, M.R. (1994). Oxidative signals in tobacco increase cytosolic calcium. Plant Cell 6 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee, S.G., Bae, Y.S., Lee, S.R., and Kwon, J. (2000). Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Science's STKE (2001), http://stke.sciencemag.org/cgi/content/full/OC_sigtrans;2000/53/pe1. [DOI] [PubMed]
- Roelfsema, M.R.G., and Prins, H.B.A. (1995). Effect of abscisic acid on stomatal opening in isolated epidermal strips of abi mutants of Arabidopsis thaliana. Physiol. Plant. 95 373–378. [Google Scholar]
- Schmidt, C., Schelle, I., Liao, Y.J., and Schroeder, J.I. (1995). Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc. Natl. Acad. Sci. USA 92 9535–9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I., and Hagiwara, S. (1989). Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338 427–430. [Google Scholar]
- Schroeder, J.I., and Hagiwara, S. (1990). Repetitive increases in cytosolic Ca2+ of guard cells by abscisic acid activation of nonselective Ca2+ permeable channels. Proc. Natl. Acad. Sci. USA 87 9305–9309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder, J.I., Kwak, J.M., and Allen, G.J. (2001). Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature 410 327–330. [DOI] [PubMed] [Google Scholar]
- Shimazaki, K., Terada, J., Tanaka, K., and Kondo, N. (1989). Calvin-Benson cycle enzymes in guard-cell protoplasts from Vicia faba L.: Implications for the greater utilization of phosphoglycerate/dihydroxyacetone phosphate shuttle between chloroplasts and the cytosol. Plant Physiol. 90 1057–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderman, E., Mattsson, J., and Engström, P. (1996). The Arabidopsis homeobox gene ATHB-7 is induced by water deficit and by abscisic acid. Plant J. 10 375–381. [DOI] [PubMed] [Google Scholar]
- Staxen, I., Pical, C., Montgomery, L.T., Gray, J.E., Hetherington, A.M., and McAinsh, M.R. (1999). Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc. Natl. Acad. Sci. USA 96 1779–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strizhov, N., Abraham, E., Okresz, L., Blickling, S., Zilberstein, A., Schell, J., Koncz, C., and Szabados, L. (1997). Differential expression of two P5CS genes controlling proline accumulation during salt stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J. 12 557–569. [DOI] [PubMed] [Google Scholar]
- Thuleau, P., Ward, J.M., Ranjeva, R., and Schroeder, J.I. (1994). Voltage-dependent calcium-permeable channels in the plasma membrane of a higher plant cell. EMBO J. 13 2970–2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian, N., Marcotte, L., and Giraudat, J. (1994). Drought rhizogenesis in Arabidopsis thaliana: Differential responses of hormonal mutants. Plant Physiol. 104 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Very, A.A., and Davies, J.M. (2000). Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc. Natl. Acad. Sci. USA 97 9801–9806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, J.M., and Schroeder, J.I. (1994). Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6 669–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyers, J.D.B., Paterson, N.W., Fitzsimons, P.J., and Dudley, J.M. (1982). Metabolic inhibitors block ABA-induced stomatal closure. J. Exp. Bot. 33 1270–1278. [Google Scholar]
- Zhang, X., Zhang, L., Dong, F., Gao, J., Galbraith, D.W., and Song, C.-P. (2001). Hydrogen peroxide is involved in abscisic acid–induced stomatal closure in Vicia faba. Plant Physiol. 126 1438–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]