Abstract
A genetic screen was performed to find new mutants with an erecta (er) phenotype and to identify genes that may function with ER, a receptor-like kinase. These mutants were named elk (for erecta-like) and were placed into five complementation groups. We positionally cloned ELK4 and determined that it encodes AGB1, a putative heterotrimeric G-protein β subunit. Therefore, elk4 was renamed agb1. agb1-1 plants express similar fruit phenotypes, as seen in er plants, but differ from er in that the stem is only slightly shorter than that in the wild type, the pedicel is slightly longer than that in the wild type, and the leaves are rounder than those in er mutants. Molecular analysis of agb1-1 indicates that it is likely a null allele. AGB1 mRNA is expressed in all tissues tested but is highest in the silique. Analysis of agb1-1 er double mutants suggests that AGB1 may function in an ER developmental pathway regulating silique width but that it functions in parallel pathways affecting silique length as well as leaf and stem development. The finding that AGB1 is involved in the control of organ shape suggests that heterotrimeric G-protein signaling is a developmental regulator in Arabidopsis.
INTRODUCTION
Sequencing of the Arabidopsis genome has provided a number of insights into plant signal transduction and revealed interesting differences with other multicellular organisms. Whereas G-protein-coupled receptors (GPCRs) predominate in Drosophila, Caenorhabditis elegans, and humans, there are relatively few GPCRs in Arabidopsis (Arabidopsis Genome Initiative, 2000; Venter et al., 2001). Furthermore, multiple genes encode each of three heterotrimeric G-protein subunits (α, β, and γ) in animals, whereas Arabidopsis appears to have single genes encoding α and β subunits and two genes encoding γ subunits (Arabidopsis Genome Initiative, 2000; Mason and Botella, 2001; Venter et al., 2001). However, genes encoding Gγ have low sequence similarity, and the presence of additional Gγ genes in the genome cannot be excluded (Mason and Botella, 2000).
GPCRs interact with α, β, and γ heterotrimeric G-protein subunits (Hamm, 1998). During GPCR activation, the GPCR acts as a guanine nucleotide exchange factor, causing the α subunit to exchange guanosine-diphosphate (GDP) for guanosine-triphosphate (GTP). Subsequently, α-GTP separates from the βγ dimer, and disassociation of all three subunits from the receptor occurs. Both α-GTP and βγ transduce the signal of the activated receptor to downstream effectors. The GTP on the α subunit is hydrolyzed to GDP, inactivating the α subunit and allowing its reassociation with βγ to reform the inactive heterotrimer complex.
The definition of heterotrimeric G-protein subunits in plants is largely based on sequence similarities with animal heterotrimeric G-protein subunits. The Arabidopsis α subunit GPA1 has 36% identity and 73% similarity with animal α subunits (Ma et al., 1990), the β subunit has 50% identity with some animal counterparts (Weiss et al., 1994), and Arabidopsis γ subunits show some sequence similarity with human γ subunits (Mason and Botella, 2000, 2001). In addition to sequence homologies, biochemical similarities between animal and plant heterotrimeric G proteins have been demonstrated. The rice α subunit binds and hydrolyzes GTP (Seo et al., 1997). Furthermore, the α and β subunits are membrane localized (Weiss et al., 1997; Obrdlik et al., 2000), and the β and γ subunits have been shown to bind to each other in vitro (Mason and Botella, 2000, 2001). However, it has not been demonstrated that the plant α, β, and γ subunits can form a trimer, nor has any subunit been shown to bind to any particular putative GPCR in plants.
Previously, the functions of heterotrimeric G-protein signaling in plants have been largely inferred from pharmacological studies (Wu and Assmann, 1994; Jones et al., 1998; Ritchie and Gilroy, 2000). These studies have suggested functions for heterotrimeric G-protein signaling in the regulation of ion channels, gibberellin signal transduction, abscisic acid signaling, as well as other possible functions.
Recently, loss-of-function mutants in the heterotrimeric G-protein α subunits of rice and Arabidopsis have been described (Ashikari et al., 1999; Fujisawa et al., 1999; Ueguchi-Tanaka et al., 2000; Ullah et al., 2001; Wang et al., 2001). Although G-protein α-subunit null mutants from both species are completely viable, they show several developmental defects. The rice mutant exhibits shortened internodes, rounded seeds, and partial insensitivity to gibberellin, whereas the Arabidopsis gpa1 mutants have rounded leaves and altered sensitivity to a number of phytohormones, including gibberellin. Furthermore, gpa1 affects either cell division or cell elongation, depending on the organ type. Moreover, abscisic acid–regulated inhibition of stomatal opening requires GPA1 function.
Together with the Gα null alleles, plant Gα overexpression studies have documented the importance of G-protein signaling (Okamoto et al., 2001; Ullah et al., 2001). Ectopic overexpression of GPA1 increased cell division, led to formation of adventitious meristems, and increased developmental sensitivity to low levels of light. In contrast to Gα, neither gain-of-function nor loss-of-function mutants for any plant heterotrimeric G-protein β or γ subunits have been reported. Therefore, the relative physiological importance of these subunits has been unclear.
A genetic screen aimed at identifying genes functioning in the receptor-like kinase ERECTA (ER) signaling pathway was performed. The ER gene is predicted to encode a protein with 20 leucine-rich repeats in its extracellular domain, a single transmembrane domain, and an intracellular serine/threonine protein kinase domain (Torii et al., 1996). er mutants have pleiotropic phenotypes affecting the development of leaves, stems, flowers, and fruits (Rédei, 1962; Bowman, 1994; Torii et al., 1996). One of the mutants we identified in our screen encodes a mutant allele of the Arabidopsis heterotrimeric G-protein β subunit (AGB1). agb1-1 exhibits several defects, including short, blunt fruits, rounded leaves, and shortened floral buds. The phenotypic characterization of agb1-1 demonstrates that heterotrimeric G proteins play a role in plant development and contribute to our understanding of plant cell signaling.
RESULTS
Identification of agb1
We initiated a genetic screen to find mutants representing components of an ER signaling pathway, based on the hypothesis that other loci can be mutated to give an er phenotype. er mutants have rounded leaves, shortened petioles, pedicels, and stems, with flowers clustered together at the inflorescence apex (Rédei, 1962; Bowman, 1994; Torii et al., 1996). In addition, the siliques of er mutants are shorter, wider, and have a blunt tip. To test our hypothesis, we screened ethyl methanesulfonic acid–mutagenized populations of Arabidopsis for the clustered flower phenotype, which we followed with observations of the silique morphology. As summarized in Table 1, the mutants were placed into five complementation groups and named elk1 through elk5 (for erecta-like). Each elk mutant was mapped, as summarized in Table 2. Because of a similar map position and phenotypes, complementation testing was performed between elk1 and transport inhibitor resistant3 (tir3) (Ruegger et al., 1997), which revealed that they are allelic. Therefore, elk1 alleles were renamed tir3-101 to tir3-110. The remainder of the elk mutants do not map near previously described mutants with similar phenotypes. As a first step toward ascertaining which mutants represent genes that may function with ER, we performed silique length measurements of elk mutants (Table 3). All of the elk loci, except elk4, have a shorter silique than does a complete loss-of-function er reference allele. Therefore, tir3, elk2, elk3, and elk5 mutants were not studied further because they likely function in either separate and/or additional developmental pathways, with ER controlling silique shape. However, elk4, represented by a single allele, has a silique length almost identical to that of er. Therefore, we performed more detailed studies with elk4. Upon molecular cloning of the ELK4 locus, elk4 was renamed agb1.
Table 1.
Summary of elk Mutants
| Locus | No. of Independent Alleles |
|---|---|
| elk1 (tir3) | 10 |
| elk2 | 2 |
| elk3 | 1 |
| elk4 (agb1) | 1 |
| elk5 | 1 |
Table 2.
Map Positions of elk Mutants
| Locus | Upper Marker | Lower Marker | Recombinant Inbred Map Position |
No. of Recombinants | Estimated Position |
|---|---|---|---|---|---|
| elk1 (tir3) | ca1 | III: 4.24 | 2/134 | III: ∼6 cMa | |
| nga172 | III: 6.91 | 1/144 | |||
| elk2 | nga128 | I: 83.32 | 5/140 | I: ∼85 cM | |
| nF5I14 | I: 92.08 | 5/140 | |||
| elk3 | nga248 | I: 42.17 | 10/70 | I: ∼60 cM | |
| nF5I14 | I: 92.08 | 18/70 | |||
| elk4 (agb1) | RPS2 | IV: 75.69 | 16/52 | IV: ∼83 cM | |
| DHS1 | IV: 108.54 | 5/52 | |||
| elk5 | nga280 | I: 83.83 | 9/72 | I: ∼112 cM | |
| athatpase | I: 117.86 | 3/72 |
a cM, centimorgans.
Table 3.
Analysis of elk Mutant Silique Lengths
| Genotype | Silique Length (mm) |
|---|---|
| Wild type | 14.46 ± 0.89 |
| er-105 | 11.10 ± 0.84a |
| elk1 (tir3) | 8.48 ± 1.22a |
| elk2 | 8.40 ± 0.60a |
| elk3 | 6.95 ± 1.41a |
| elk4 (agb1) | 11.17 ± 0.82a |
| elk5 | 2.95 ± 0.95a |
a Significantly different from the wild type (P ≤ 0.00001, Student's t test).
agb1 Affects Silique Morphology
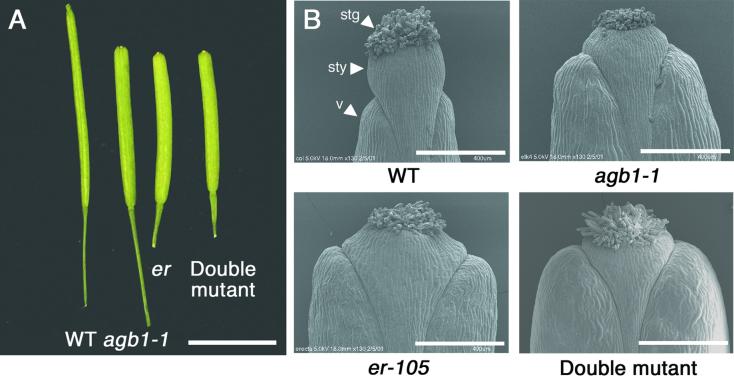
A striking phenotype of agb1 is its silique morphology. Figure 1 shows that the development of the silique is altered in agb1 and is similar in appearance to the silique morphology of er. The tip of the wild-type silique has an acute appearance, because the width of the valves tapers apically and the valves contact the style at its base. Moreover, the style begins near the distal end of the valves and projects well past them. In contrast, the silique tips of agb1 and er plants have a blunt appearance. The valves do not taper apically and contact the style at its midpoint, and styles do not extend as far past the valves as they do in the wild type.
Figure 1.
agb1 and er Cause Similar Changes in Silique Morphology.
(A) Excised siliques from wild-type plants (WT; ecotype Columbia [Col]), agb1-1, er-105, and agb1-1 er-105 double mutant. Bar = 5 mm.
(B) Scanning electron micrographs of silique tips from wild-type plants (WT; Col ecotype), agb1-1, er-105, and agb1-1 er-105 double mutant. stg, stigma; sty, style; v, valve. Bars = 400 μm.
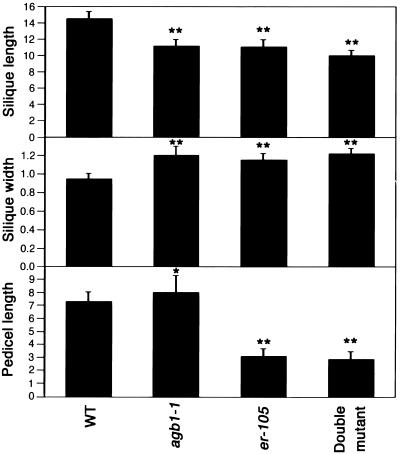
The similarity in the silique morphology between agb1 and er prompted us to analyze the morphology of the double mutant silique. In Figure 2 and Table 4, statistical analysis of quantitative measurements of silique length, silique width, pedicel length, and silique tip angle is presented. In the single agb1 or er mutants, the length of the silique is decreased, whereas the width is increased. Furthermore, the angle of the fruit tip is smaller in agb1 and er than it is in the wild type, reflecting the blunt tip morphology. However, agb1 pedicel length is slightly longer than it is in the wild type, whereas er has a shorter pedicel. Double mutants were constructed to examine the interaction between agb1 and er in the control of silique form. Of the silique and pedicel traits measured, the silique length of the double mutant was significantly different from that of er, whereas the pedicel length, silique width, and angle of the silique tip were not significantly different from those of the er single mutant.
Figure 2.
Morphometric Analysis of agb1 and er Silique Traits.
Comparison of silique and pedicel traits among wild-type plants (WT; Col ecotype), agb1-1, er-105, and agb1-1 er-105 double mutant. The first five fruits from eight to 10 plants were measured for each genotype. Means ±sd are in millimeters and were for 40 to 50 siliques per genotype. *Significantly different from the wild type (P ≤ 0.001). **Significantly different from the wild type (P ≤ 0.00001, Student's t test). er-105 is not significantly different from the double mutant for pedicel length (P ≥ 0.057, Student's t test) or silique width (P ≥ 0.301, Student's t test), but it is significantly different from the double mutant for silique length (P ≤ 0.00001, Student's t test). er-105 is significantly different from agb1-1 for pedicel length (P ≤ 0.00001, Student's t test) and for silique width (P ≥ 0.034), but it is not significantly different from agb1-1 for silique length (P ≥ 0.642, Student's t test). agb1-1 is significantly different from the double mutant for pedicel length (P ≤ 0.00001, Student's t test), silique length (P ≤ 0.00001, Student's t test), and silique width (P ≤ 0.00001, Student's t test).
Table 4.
Measurements of Silique Tip Angles
| Genotype | Angle of Silique Tip (degrees) |
|---|---|
| Wild type | 155.6 ± 5.6 |
| agb1-1 | 144.2 ± 5.9a,c |
| er-105 | 142.0 ± 5.6a,b |
| Double mutant | 143.7 ± 6.6a,b,c |
Significantly different from the wild type (P ≤ 0.00001, Student's t test).
Not significantly different from agb1 (P ≥ 0.06, Student's t test).
Not significantly different from er (P ≥ 0.06, Student's t test).
In addition to the alterations in silique morphology, agb1 causes a modest shortening of floral bud length, as shown in Figure 3. The floral buds at the inflorescence apex appear more tightly clustered together than they are in the wild type, but not as clustered as in er. This is because agb1 does not greatly affect pedicel or stem length, which are both shortened in er mutants (Bowman, 1994; Torii et al., 1996). Stem length is slightly decreased in agb1 relative to the wild type, but it is not as short as in er, as shown in Figure 3B and Table 5. The agb1 er double mutant is shorter than either agb1 or er single mutants.
Figure 3.
agb1 Affects Flower Shape and Inflorescence Length.
(A) Comparison of inflorescence apices among wild-type plants (WT; Col ecotype), agb1-1, er-105, and agb1-1 er-105 double mutants ∼5 days after flowering. Bar = 1 mm.
(B) Comparison of 5-week-old adult plants. Bar = 2 cm.
Table 5.
Analysis of agb1 Inflorescence Length
| Genotype | Length (cm)a |
|---|---|
| Wild type | 31.8 ± 3.9b,c |
| agb1 | 27.6 ± 3.8c,d |
| er | 18.2 ± 1.8b,e |
| Double mutant | 15.1 ± 1.5b,c,e |
a Measurements are from 5-week-old plants (n = 30) and represent mean ±sd.
b Significantly different from agb1 (P ≤ 0.00001, Student's t test).
c Significantly different from er (P ≤ 0.00001, Student's t test).
d Significantly different from the wild type (P ≤ 0.0001, Student's t test).
e Significantly different from the wild type (P ≤ 0.00001, Student's t test).
agb1 plants also have rounded leaves and short petioles, which is shown by a comparison of rosette leaves in Figure 4. From our initial comparisons of leaf characteristics between agb1 and the wild type, the ninth leaf appeared to show the greatest differences between the two genotypes. Therefore, we chose the ninth leaf for performing quantitative measurements. The statistical analyses of petiole length, lamina length, and lamina width are presented in Table 6. agb1 has petioles ∼50% as long as those in the wild type, whereas the lamina in agb1 is ∼125% wider and ∼85% as long as that in the wild type. These traits contribute to giving the agb1 rosette a more compact, rounded appearance than that of wild-type plants (data not shown).
Figure 4.
agb1 Affects Leaf Shape.
Comparison of excised leaves from 4-week-old wild-type plants (WT; Col ecotype), agb1-1, er-105, and agb1-1 er-105 double mutants. The first leaves through the 10th leaves are seen left to right. Bar = 1 cm.
Table 6.
Analysis of agb1 Leaf Traitsa
| Genotype | Petiole Length | Lamina Length | Lamina Width |
|---|---|---|---|
| Wild type | 10.77 ± 1.77b,c | 24.56 ± 3.01b | 9.59 ± 1.26c |
| agb1 | 4.66 ± 1.49c,d | 20.65 ± 3.02c,e | 12.03 ± 1.80c,d |
| er | 8.80 ± 1.66b,f | 25.6 ± 3.68b | 10.33 ± 1.28b |
| Double mutant | 3.59 ± 0.93b,c,d | 18.49 ± 2.26c,d | 11.34 ± 1.41b,c,e |
Measurements are in millimeters and represent mean ±sd for n ≥ 20 of ninth leaves from 4-week-old plants.
Significantly different from agb1 (P ≤ 0.02).
Significantly different from er (P ≤ 0.02).
Significantly different from the wild type (P ≤ 0.00001, Student's t test).
Significantly different from the wild type (P ≤ 0.0001, Student's t test).
Significantly different from the wild type (P ≤ 0.001, Student's t test).
Because agb1 and er affect both leaf shape and petiole length (Figure 4, Table 6), we addressed the interaction between agb1 and er in the control of leaf shape by analyzing the agb1 er double mutant. With one exception, the double mutant has a shorter petiole, a shorter lamina, and a wider lamina than does either single mutant. The exception is that leaf width in agb1 and the double mutant do not show a statistically significant difference.
agb1 Encodes a Mutant Heterotrimeric G-Protein β Subunit
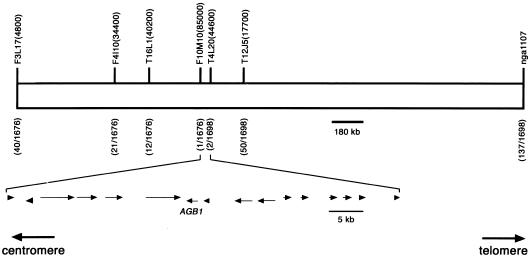
Molecular identification of the agb1 mutant was performed by following a positional cloning strategy, as illustrated in Figure 5. agb1 was found to map between the markers F3L17(4800) and nga1107 on the bottom arm of chromosome four. F2 plants that were recombinant for these flanking markers were assayed with additional markers internal to the first pair to define a smaller pool of recombinants. This process was repeated in an iterative manner until the genomic region encompassing the AGB1 locus was delimited to 61 kb. At this point there was one recombinant on the centromeric side of AGB1 and two recombinants at a marker telomeric to AGB1.
Figure 5.
Mapping of the AGB1 Locus to a 61-kb Interval.
Above the chromosome are the genetic markers used in the mapping studies. Below each marker are the number of recombinants observed for that marker out of the total number of chromosomes analyzed. The 61-kb mapping interval contains 16 genes. The transcriptional orientation and relative size of each gene are shown by arrows.
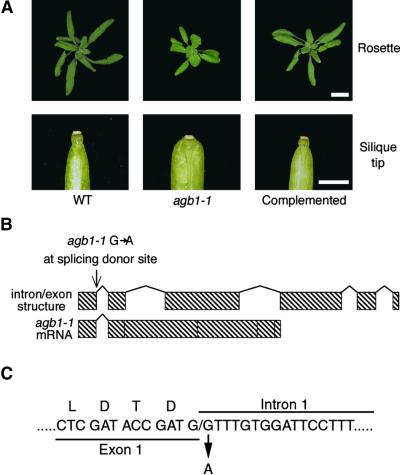
Sixteen annotated transcription units were present in the 61-kb interval. We transformed agb1-1 with each of the 16 genes to test for complementation. The genes were tested singly, or in three cases, two adjacent genes were tested simultaneously. On the basis of the gene annotation, polymerase chain reaction (PCR) products amplified with the wild-type Columbia (Col) ecotype genomic DNA template were cloned into a binary vector for Agrobacterium-mediated transformation. We found that a genomic fragment containing only a gene encoding a heterotrimeric G-protein β subunit was able to complement agb1-1, as documented in Figure 6A. The cDNA for this gene was previously identified and named AGB1, for Arabidopsis Gβ 1 (Weiss et al., 1994). Thirty independent transformants were obtained with the AGB1-containing construct, all of which showed complementation. For example, the rounded leaf phenotype of agb1-1 was restored to its normal shape. Also, the blunt silique tip of agb1-1 was complemented, having the acute tip seen in wild-type fruits. DNA gel blot analysis confirmed the presence of the construct used to complement agb1 in the genome of complemented plants (data not shown).
Figure 6.
Complementation of agb1 and Identification of the Molecular Lesion in agb1-1.
(A) Comparison of phenotypic traits among wild-type plants (WT; Col ecotype), agb1-1, and complemented plants (homozygous agb1-1 transformed with pCAMBIA2300-T4L20.4). At top, rosette phenotypes of 3-week-old plants. Bar = 1 cm. At bottom, silique tip phenotypes from each genotype. Bar = 1 mm.
(B) At top, the AGB1 locus is comprised of six exons and five introns. Sequence analysis of agb1-1 genomic DNA and AGB1 cDNA from agb1-1 showed that the molecular lesion is a transition mutation altering the splicing donor site at the 5′ end of the first intron of AGB1. At bottom is the mature AGB1 mRNA from agb1-1.
(C) A portion of the AGB1 genomic DNA sequence flanking the first exon/intron junction, showing the molecular lesion in agb1-1. Above the exon DNA sequence, the amino acid residues for the last four entire codons before the first intron are shown.
agb1-1 Is a Null Allele
AGB1 genomic sequence in agb1-1 was analyzed, and a transition mutation at the splicing donor site of the first intron was found, as shown in Figure 6B. Sequencing of AGB1 cDNA isolated from agb1-1 revealed that the mutation results in a failure to splice out the first intron. RNA gel blot analysis of steady state levels of AGB1 in agb1-1 showed that the transcript is slightly larger in size than it is in the wild-type controls. The size of the mRNA from agb1-1 plants corresponds to the size of the transcript if the first intron is not removed. Failure to splice out the first intron appears to destabilize the transcript, because AGB1 mRNA is less abundant in agb1-1 (Figure 7). When conceptually translated, the mature mRNA in agb1-1 is predicted to express the first exon, which consists of 35 amino acids, and then undergo a frameshift resulting in the addition of 20 novel amino acids and a premature stop. The predicted AGB1 protein in agb1-1 is truncated before the first of the seven WD-40 repeats that comprise AGB1 (Weiss et al., 1994). Thus, agb1-1 is likely to be a null allele.
Figure 7.
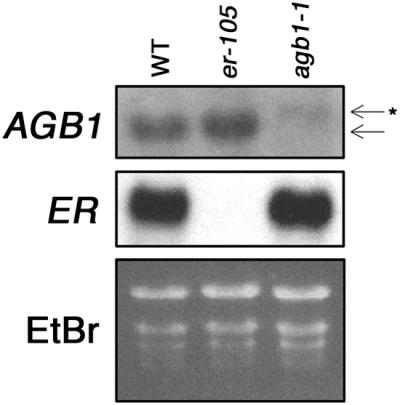
AGB1 mRNA Is Larger in Size and Less Abundant in the agb1-1 Background.
RNA gel blot analysis of steady state levels of mRNA expression in wild-type plants (WT; Col ecotype), er-105, and agb1-1. The blot was sequentially hybridized with AGB1 and ER 32P-labeled probes. At top, AGB1 steady state mRNA levels. The arrow indicates the wild-type AGB1 mRNA; the arrow with the asterisk indicates the aberrantly spliced AGB1 mRNA seen in agb1-1. At center, ER steady state mRNA levels. At bottom, ethidium bromide (EtBr)–staining of RNA to show loading. When normalized for loading, AGB1 transcript in er-105 is 90% of that in the wild type, AGB1 transcript in agb1-1 is 11% of that in the wild type, ER transcript in er-105 is 0% of that in the wild type, and ER transcript in agb1-1 is 74% of that in the wild type.
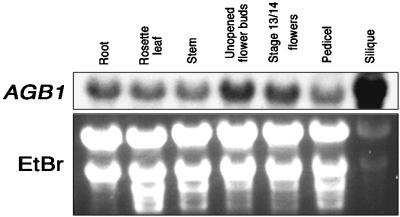
Expression of ELK4 (AGB1) was found in all tissues tested (Figure 8). agb1-1 has the shortened, blunt silique phenotype of er but not the shortened pedicel phenotype. Consistent with the phenotypes observed in agb1-1, expression was high in the silique relative to the pedicel.
Figure 8.
AGB1 Is Expressed in All Tissues Examined and Is Highest in Siliques.
At top, AGB1 mRNA signals from RNA gel blot analysis of wild-type plant tissues with a 32P-labeled AGB1 probe. From left, roots of 3-week-old plants, rosette leaves of 3-week-old plants, stems of 4-week-old plants (2 to 5 cm from the apex), unopened flower buds, open flowers (including pedicels) from 4-week-old plants, pedicels from stage 15/16 fruits (stages according to Smyth et al. [1990]), and stage 15/16 siliques. At bottom, ethidium bromide (EtBr)–staining of RNA to show loading.
DISCUSSION
agb1 affects the shape of leaves, floral buds, and fruits. The silique phenotypes of agb1-1 are reminiscent of those exhibited by the er mutant (Rédei, 1962; Bowman, 1994; Torii et al., 1996), which raises the possibility that AGB1 functions in a common silique developmental pathway with ER. This possibility was addressed by examination of agb1 er double mutants. The two simplest cases are that either AGB1 and ER function in parallel pathways or they function in a common developmental pathway. For example, if AGB1 and ER function in parallel pathways, the double mutant would be expected to show a more severe phenotype, evidenced by shorter, wider siliques. For this test to be meaningful, Arabidopsis fruits must have the capacity of being either shorter or wider than those seen in agb1 or er mutants. This requirement is satisfied because mutants have been reported that make the silique either shorter or wider than either agb1 or er. For example, tir3 mutants have siliques that are <60% as long as those in the wild type (Ruegger et al., 1997), and overexpression of CYP78A9 results in Arabidopsis fruits that are 40% wider than are er fruits (Ito and Meyerowitz, 2000).
Statistical analysis of the agb1 er double mutant shows that the silique is significantly shorter than that in either the agb1 or er single mutant. Similarly, for leaf and stem phenotypes, the double mutants were more severe than were the er or agb1 single mutants. These observations suggest that AGB1 and ER function in parallel pathways that control the development of these traits. If they do function in parallel pathways, it is possible that agb1 simply phenocopies some aspects of er mutants but that each mutant has a different physiological basis for the similar aspects of their morphology.
However, statistical analysis of silique width, as well as tip angle measurements of agb1 er double mutants, showed these traits are not significantly different from those of either agb1 or er single mutant siliques. Futhermore, pedicel length in the double mutant is not significantly different from that in the er single mutant. These observations support the hypothesis that AGB1 functions in a common developmental pathway, with ER controlling these characteristics.
If AGB1 and ER function in a common developmental pathway, they might function in a common signal transduction cascade. Many signal transduction pathways modulate the expression of genes to bring about a cellular response (Wodarz and Nusse, 1998). One possibility we considered was that ER is transcriptionally activated by a GPCR pathway involving AGB1. If this case were true, we would expect to observe lower levels of ER expression in agb1-1. However, steady state levels of ER mRNA are similar in an agb1-1 background. A second possibility is that AGB1 expression requires functional ER. In this case, we would expect to see loss of AGB1 expression in an er background. This scenario is unlikely because steady state levels of AGB1 mRNA are only slightly reduced in er.
Another possibility is that AGB1 and ER may physically interact. To do so, two proteins must be co-localized. Both ER and AGB1 proteins are reported to be localized to the plasma membrane (Torii et al., 1996; Obrdlik et al., 2000). The possibility of interaction is further supported by the presence of a motif in ER that has been proposed to be important for interactions between the Gβ subunit and its effectors, QXXER (Chen et al., 1995). In subdomain XI of the protein kinase domain of ER, a QXXDR sequence is present (Torii et al., 1996), suggesting the potential for interaction. However, in preliminary protein gel blot and glutathione S-transferase pull-down binding studies, we have not been able to detect a direct interaction between these two proteins. Other experimental approaches, which are closer to physiological conditions, may be required to detect an interaction. Alternatively, the genetic results we observed may not reflect a direct physical interaction between AGB1 and ER. Although the genetic results are tantalizing because they suggest a connection between receptor-like kinase and GPCR signaling pathways, the biochemical link, if one exists, remains to be determined.
Weiss et al. (1994) first cloned the AGB1 cDNA. They observed that AGB1 consists of seven WD-40 repeats and that it shows significant similarity to animal Gβ subunits. However, numerous proteins contain the WD-40 repeat but are not heterotrimeric G-protein β subunits (Arabidopsis Genome Initiative, 2000). For example, CONSTITUTIVE PHOTOMORPHOGENIC1 and TRANSPARENT TESTA GLABRA1 contain WD-40 repeats, but they are not Gβ subunits (Deng et al., 1992; Walker et al., 1999). Does AGB1 encode a bona fide heterotrimeric G-protein β subunit? Three lines of evidence from published literature support this hypothesis. First, by sequence homology, AGB1 shows 50% amino acid sequence identity to human Gβ subunits 2 and 3 (Weiss et al., 1994). Second, a biochemical expectation of Gβ subunits in animal and yeast cells is that the Gβ subunit exists as a dimer with the γ subunit (Hamm, 1998). AGB1 can bind to Arabidopsis heterotrimeric G-protein γ subunits in vitro (Mason and Botella, 2000, 2001). Third, it was shown that AGB1 is membrane localized, which is consistent with the expectation of membrane localization (Hamm, 1998; Obrdlik et al., 2000). These observations indicate that AGB1 encodes a heterotrimeric G-protein β subunit.
A question of central importance in interpreting the phenotypes of agb1-1 is whether it represents a loss-of-function allele, and if it does, whether it is a complete loss-of-function allele or only a partial loss-of-function one? When conceptually translated, agb1-1 is predicted to encode the N-terminal 35 amino acids, followed by 20 novel amino acids and a stop codon. This truncation eliminates 342 of the amino acids found in the wild-type protein and would result in loss of the WD-40 repeats that are required for Gβ function (Hamm, 1998). Previous studies have also shown that a truncation mutant consisting of the first 41 amino acids of AGB1 cannot interact with the Arabidopsis Gγ subunit (Mason and Botella, 2000). However, because an N-terminal portion of AGB1 is predicted to be expressed in agb1-1, it is possible that it represents a gain-of-function mutation. We think this is unlikely because gain-of-function mutations are typically dominant, whereas agb1-1 is completely recessive. Recessive mutants generally represent loss-of-function mutants. Because the predicted truncation of AGB1 occurs very close to the N terminus, and the mutant is recessive, it is likely that agb1-1 represents a null allele.
Little is known about GPCR signaling in plants. Mutant alleles of putative GPCRs in plants have not been reported. Although loss-of-function alleles of rice and Arabidopsis α subunits have been described (Ashikari et al., 1999; Fujisawa et al., 1999; Ueguchi-Tanaka et al., 2000), no plant heterotrimeric Gγ subunit mutants have been identified. agb1-1, a null allele of the Arabidopsis Gβ 1 gene, is viable but causes an array of defects, including short, blunt fruits, rounded leaves, and shortened floral buds. This suggests that either GPCR signaling has a restricted role in normal growth and development or there are additional proteins that function as Gβ that are not discernable by sequence homology.
In the mammalian GPCR signaling paradigm, the Gβγ complex associates with Gα-GDP (Hamm, 1998). This interaction terminates the ability of Gβγ to interact with other downstream effectors. If Gα is not functioning, as in the mutant backgrounds gpa1 or the rice Gα mutant dwarf1, the Gβγ dimer is predicted to be constitutively signaling. Therefore, Gα mutant phenotypes could be attributed to either an absence of Gα signaling or an overabundance of Gβγ signaling. On the other hand, if Gβ is not functioning, as in the case of agb1, it is expected that the Gα cannot reassemble with a GPCR. In this scenario, the Gα will be unable to exchange GDP for GTP; as a result, all of the Gα is predicted to be “free” and in the GDP-bound inactive form. Thus, the phenotype of the Gβ mutant agb1 may reflect a loss of both Gα and the Gβγ signaling pathways. The recent report (Ullah et al., 2001) of a null allele for the Arabidopsis Gα, gpa1, and the identification of agb1-1 offers the opportunity to genetically test these models.
Although a direct comparison of gpa1 and agb1-1 under the same growing conditions has not been made, we note some similarities between agb1 and the phenotypes documented for gpa1. Light-grown gpa1 has rounded leaves and shortened petioles (Ullah et al., 2001), similar to those observed in agb1. These leaf traits may reflect a Gα signaling pathway, because both Gα and Gβ mutants appear to share this trait. On the other hand, the silique phenotypes of agb1 were not described in the initial description of gpa1. Therefore, these traits may reflect a Gβγ signaling pathway. A more conclusive determination of which physiological effects can be attributed to either Gα or Gβγ requires a direct side-by-side comparison and construction of the double mutant with gpa1 and agb1. Whatever the case may be, the identification and characterization of agb1 open up new avenues for investigation and contribute to a better understanding of plant cell signaling.
METHODS
Identification of agb1-1 and Other Plant Material Used
agb1-1 was identified from an ethyl methanesulfonic acid–mutagenized Columbia (Col) ecotype M2 population of 100,000 plants from 40,000 M1 seeds (Lehle Seeds, Round Rock, TX). We screened for inflorescence apices bearing clustered flowers, resembling the erecta mutant. In addition to many new er alleles (Lease et al., 2001), five complementation groups were found. agb1-1 was backcrossed to wild-type Col two times before beginning genetic or morphological studies. agb1-1 segregates as a monogenic recessive in F2 crosses with Col (197 wild type: 55 agb1-1; χ2 = 1.35; df = 1; 0.3 > P > 0.2) (Russell, 1996). F1 progeny from crosses between agb1-1 and er-105 exhibit a wild-type phenotype. Wild-type Col and Wassilewskija (WS) ecotype seeds were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). agb1-1 er-105 double mutants were constructed by crossing homozygous agb1-1 and er-105, allowing the F1 to self-fertilize, and generating a segregating F2. The double mutant was identified by choosing several plants with the er phenotype, backcrossing each plant to both er-105 and agb1-1, and finding an individual that complemented neither agb1-1 nor er-105.
Plant Growth and Measurements
Plants were grown under 100 μmol·m−2·sec−1 continuous white light at 23°C and fertilized weekly with Miracle Gro (Scotts, Port Washington, NY). The first five siliques from eight to 10 plants per genotype were used for measurement. The plants were 5 weeks old, and siliques were at stage 15/16 (Smyth et al., 1990), which are fully expanded siliques. The silique and pedicel lengths were measured by excising siliques and pedicels from the primary inflorescence, placing them on acetate sheets, photocopying, and measuring the photocopies using the software SigmaScan (Jandel Scientific, San Rafael, CA). Petiole length, lamina length, and lamina width were measured from leaf traces of the ninth leaf from at least 20 4-week-old plants per genotype by using SigmaScan. Lamina width was measured across the widest portion of the lamina. Inflorescence length of 5-week-old plants (n = 30) was measured with a ruler to the nearest millimeter. Silique width and the silique tip angle were measured with Metamorph (Universal Imaging Corp., Downingtown, PA) from digital images of siliques magnified with a dissecting microscope. Silique widths were measured at the lengthwise midpoint of each silique. The silique tip angle was determined by measuring the angle between a line tangential to the midpoint of the side of the silique and a line tangential to the apical tip of one of the two valves. Silique measurements represent the mean ±sd, with n = 40 to 50 organs measured for each genotype. For statistical analysis of quantitative data, Microsoft Excel was used to perform two-tailed Student's t tests assuming equal variances, with 0 for expected difference and α = 0.05.
Microscopy
Scanning electron microscopy samples were fixed in 2.5% glutaraldehyde buffered with 0.1 M cacodylate, pH 7, postfixed with 1% osmium tetroxide, dehydrated through an ethanol series, critical point-dried, sputter-coated with platinum, mounted on stubs, and viewed with a Hitachi (Tokyo, Japan) S4700 FESEM.
Mapping
For mapping purposes, homozygous mutants were crossed with WS ecotype, the F1 was allowed to self-fertilize, and a segregating F2 population was generated. Recombinant inbred map positions for markers cited in Table 2 are based on the Lister and Dean (1993) map maintained on the Arabidopsis Information Resource website (Huala et al., 2001). Genomic DNA was isolated from 850 F2 plants from the mapping population that expressed the agb1 phenotype. SSLP and CAPS markers were used to map the elk loci (Konieczny and Ausubel, 1993; Bell and Ecker, 1994). Additional markers were generated during the course of mapping based on analysis of bacterial artificial chromosome (BAC) sequences for simple sequence repeats. The markers nga1107 and F3L17(4800), which flank the AGB1 locus, were tested with all 850 individuals. Those F2 individuals determined to be recombinant for the flanking markers were assayed with additional markers internal to the original flanking markers, to define a smaller set of recombinants. This process was repeated iteratively until the interval delimited by the markers encompassed ∼61 kb, spanning portions of the overlapping BAC clones F10M10 and T4L20. Nga1107 was analyzed on 4.5% agarose gels. All other SSLPs were analyzed by 32P-labeling the forward primer, separating the polymerase chain reaction (PCR) products on 5% denaturing polyacrylamide gels, and autoradiography. Marker names are a composite of the BAC sequence from which they were derived, followed by the approximate location in basepairs. The primers for the novel SSLP markers used in mapping are as follows: F3L17(4800): forward, 5′-ACATAACATGTTTGATCTAGCAC-3′; reverse, 5′-CTGCTTTTTGTT-CACACTGAACAT-3′; F4I10(34,400): forward, 5′-AAGGAAGAAGAAGACTGTTGAA-3′; reverse, 5′-CTCGTCCGTACCGTTCTCTTCC-3′; T16L1(40,200): forward, 5′-GGAAACTTAGATTGTAAAGCTTG-3′; reverse, 5′-AGCAGCAATCTCAGAGAAACATA-3′; F10M10(85,000): forward, 5′-CTGAATACAATATCTAATCTTTGA-3′; reverse, 5′-AAG-ACAAATATACAGTTTTCGACC-3′; T4L20(44,600): forward, 5′-CTAGACAAAAGAGAATTCAAAAGG-3′; reverse, 5′-TAAGGCAAAGTT-ACAAGATTACGT-3′; and T12J5(17,700): forward, 5′-ATCTCACTA-AATATTGACTAAGAG-3′; reverse, 5′-ATGTCTAGATTCCAATTG-TTTCA-3′.
Molecular Complementation of agb1
For brevity, only the construct that successfully complemented agb1-1 is described. The following primers, which include SalI and KpnI restriction sites at their 5′ end to facilitate cloning, were used to generate a PCR product used for complementation: T4L20.4 forward, 5′-CTTGTCGACGGGAAAGCATGGATGAAGAAGATGAGCG-3′; and T4L20.4 reverse, 5′-CTTGGTACCGCTGTTCGTAAGGAGAATCAATGGGCT-3′. This construct includes 791 bp upstream of the initiation ATG and 1041 bp downstream of the stop codon. PCR was performed with the wild-type Col genomic DNA template; the PCR products were purified using Concert PCR purification kit (Invitrogen, Carlsbad, CA), digested sequentially with KpnI and SalI, and cloned into KpnI and SalI sites of pCAMBIA2300 (GenBank accession numbers AF234290–AF234316). This construct, pCAMBIA2300-T4L20.4, was transformed into Agrobacterium tumafaciens strain GV3101 via electroporation. Homozygous agb1-1 plants were transformed (Clough and Bent, 1998), the seeds were collected, and transformants were selected on medium containing half-strength Murashige and Skoog Salt Mix (Invitrogen, Carlsbad, CA), 50 μg·mL−1 kanamycin, and 1% agar.
Molecular Analysis of agb1-1
A genomic fragment was amplified from agb1-1 by using the same primers as those used for molecular complementation, and the coding portion of AGB1 was completely sequenced on both strands. The molecular lesion in agb1-1 is at nucleotide 15,169 of BAC T4L20 (GenBank accession number AL023094). Total RNA was isolated using the RNeasy midi kit (Qiagen, Valencia, CA). An AGB1 cDNA was isolated from agb1-1 by using reverse transcription–PCR with agb1-1 total RNA from leaves and flowers, using the following primers: forward, 5′-TCCGGTACCATGTCTGTCTCCGAGCTCAAAGAA-3′; and reverse, 5′-TCCGGTACCTCAAATCAC-TCTCCTGTGTCCTCC-3′. The cDNA was completely sequenced on both strands. For comparison of mRNA expression among genotypes, total RNA was isolated from rosette leaves of 3-week-old plants. To analyze AGB1 mRNA tissue-specific expression, total RNA was isolated from various tissue types of wild-type Col. For RNA gel blot analyses, 20 μg of total RNA was separated on a 1% MOPS-formaldehyde gel, the ethidium bromide–stained RNAs were photographed, and the RNA was transferred to a nylon membrane, cross-linked to the membrane by UV irradiation, hybridized with a 32P-labeled probe, and exposed to film for 2 days. AGB1 and ER probes were generated from a full-length cDNA template that was randomly primed. The relative level of expression was normalized by dividing the intensity of the ER or AGB1 signal by the intensity of the large rRNA from the ethidium bromide–stained gel. The result for each of the three genotypes was divided by the value obtained from the wild type to determine the relative difference in the level of expression in each mutant background compared with that of the wild type.
Acknowledgments
We thank the members of the Walker and Liscum laboratories for support and discussions and the following facilities and individuals for technical assistance: Jessica Wagner and Shawn Bailes (University of Missouri Molecular Cytology Core facility), Cheryl Jensen and Randy Tindall (University of Missouri Electron Microscopy Core facility), Dean Bergstrom, and Barb Sonderman. er-105 seeds were a generous gift of Keiko Torii. tir3-1 seeds were a generous gift of Mark Estelle and Elizabeth Dewey. We also thank Drs. Thomas Guilfoyle and Jane Murfett for critical reading of the manuscript. K.A.L. was supported by a predoctoral fellowship from the University of Missouri Maize Biology Training Program and supplemented by the Molecular Biology Program. This work was supported by U.S. Department of Agriculture–CSREES Grant No. 99-35304-8060 to E.L., and by grants from the University of Missouri Food for the Twenty-First Century Program and National Science Foundation Grant No. MCB 9809884 to J.C.W.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010315.
References
- Arabidopsis Genome Initiative (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Ashikari, M., Wu, J., Yano, M., Sasaki, T., and Yoshimura, A. (1999). Rice gibberellin-insensitive dwarf mutant gene Dwarf1 encodes the α-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. USA 96, 10284–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, C.J., and Ecker, J.C. (1994). Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19, 137–144. [DOI] [PubMed] [Google Scholar]
- Bowman, J., ed (1994). Arabidopsis: An Atlas of Morphology and Development. (New York: Springer-Verlag).
- Chen, J., et al. (1995). A region of adenylyl cyclase 2 critical for regulation by G protein beta gamma subunits. Science 268, 1166–1169. [DOI] [PubMed] [Google Scholar]
- Clough, S.J., and Bent, A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Deng, X.W., Matsui, M., Wei, N., Wagner, D., Chu, A.M., Feldmann, K.A., and Quail, P.H. (1992). COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G beta homologous domain. Cell 71, 791–801. [DOI] [PubMed] [Google Scholar]
- Fujisawa, Y., Kato, T., Ohki, S., Ishikawa, A., Kitano, H., Sasaki, T., Asahi, T., and Iwasaki, Y. (1999). Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice. Proc. Natl. Acad. Sci. USA 96, 7575–7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm, H.E. (1998). The many faces of G protein signaling. J. Biol. Chem. 273, 669–672. [DOI] [PubMed] [Google Scholar]
- Huala, E., et al. (2001). The Arabidopsis Information Resource (TAIR): A comprehensive database and web-based information retrieval, analysis, and visualization system for a model plant. Nucleic Acids Res. 29, 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, T., and Meyerowitz, E.M. (2000). Overexpression of a gene encoding a cytochrome P450, CYP78A9, induces large and seedless fruit in Arabidopsis. Plant Cell 12, 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, H.D., Smith, S.J., Desikan, R., Plakidou-Dymock, S., Lovegrove, A., and Hooley, R. (1998). Heterotrimeric G proteins are implicated in gibberellin induction of α-amylase gene expression in wild oat aleurone. Plant Cell 10, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny, A., and Ausubel, F.M. (1993). A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J. 4, 403–410. [DOI] [PubMed] [Google Scholar]
- Lease, K.A., Lau, N.Y., Schuster, R.A., Torii, K.U., and Walker, J.C. (2001). Receptor serine/threonine protein kinases in signalling: Analysis of the erecta receptor-like kinase of Arabidopsis thaliana. New Phytol. 151, 133–143. [DOI] [PubMed] [Google Scholar]
- Lister, C., and Dean, C. (1993). Recombinant inbred lines for mapping RFLP and phenotypic markers in Arabidopsis thaliana. Plant J. 4, 745–750. [DOI] [PubMed] [Google Scholar]
- Ma, H., Yanofsky, M.F., and Meyerowitz, E.M. (1990). Molecular cloning and characterization of GPA1, a G protein α subunit gene from Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 87, 3821–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, M.G., and Botella, J.R. (2000). Completing the heterotrimer: Isolation and characterization of an Arabidopsis thaliana G protein γ-subunit cDNA. Proc. Natl. Acad. Sci. USA 97, 14784–14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, M.G., and Botella, J.R. (2001). Isolation of a novel G-protein γ-subunit from Arabidopsis thaliana and its interaction with Gβ. Biochem. Biophys. Acta 1520, 147–153. [DOI] [PubMed] [Google Scholar]
- Obrdlik, P., Neuhaus, G., and Merkle, T. (2000). Plant heterotrimeric G protein β subunit is associated with membranes via protein interactions involving coiled-coil formation. FEBS Lett. 476, 208–212. [DOI] [PubMed] [Google Scholar]
- Okamoto, H., Matsui, M., and Deng, X.W. (2001). Overexpression of the heterotrimeric G-protein α-subunit enhances phytochrome-mediated inhibition of hypocotyl elongation in Arabidopsis. Plant Cell 13, 1369–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rédei, G.P. (1962). Single locus heterosis. Z. Vererbungsl. 93, 164–170. [Google Scholar]
- Ritchie, S., and Gilroy, S. (2000). Abscisic acid stimulation of phospholipase D in the barley aleurone is G-protein-mediated and localized to the plasma membrane. Plant Physiol. 124, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegger, M., Dewey, E., Hobbie, L., Brown, D., Bernasconi, P., Turner, J., Muday, G., and Estelle, M. (1997). Reduced naphthylphthalamic acid binding in the tir3 mutant of Arabidopsis is associated with a reduction in polar auxin transport and diverse morphological defects. Plant Cell 9, 745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, P.J. (1996). Genetics. (New York: HarperCollins).
- Seo, H.S., Choi, C.H., Lee, S.Y., Cho, M.J., and Bahk, J.D. (1997). Biochemical properties of a rice (Oryza sativa L., IR36) G-protein alpha-subunit expressed in Escherichia coli. Biochem. J. 324, 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, D.R., Bowman, J.L., and Meyerowitz, E.M.. (1990). Early flower development in Arabidopsis. Plant Cell 2, 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii, K.U., Mitsukawa, N., Oosumi, T., Matsuura, Y., Yokoyama, R., Whittier, R.F., and Komeda, Y. (1996). The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8, 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueguchi-Tanaka, M., Fujisawa, Y., Kobayashi, M., Ashikari, M., Iwasaki, Y., Kitano, H., and Matsuoka, M. (2000). Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA 97, 11638–11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah, H., Chen, J., Young, J., Im, K., Sussman, M., and Jones, A. (2001). Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292, 2066–2069. [DOI] [PubMed] [Google Scholar]
- Venter, J.C., et al. (2001). The sequence of the human genome. Science 291, 1304–1351. [DOI] [PubMed] [Google Scholar]
- Walker, A.R., Davison, P.A., Bolognesi-Winfield, A.C., James, C.M., Srinivasan, N., Blundell, T.L., Esch, J.J., Marks, M.D., and Gray, J.C. (1999). The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. Plant Cell 11, 1337–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Ullah, H., Jones, A., and Assmann, S. (2001). G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells. Science 292, 2070–2072. [DOI] [PubMed] [Google Scholar]
- Weiss, C.A., Garnaat, C.W., Mukai, K., Hu, Y., and Ma, H. (1994). Isolation of cDNAs encoding guanine nucleotide-binding protein beta-subunit homologues from maize (ZGB1) and Arabidopsis (AGB1). Proc. Natl. Acad. Sci. USA 91, 9554–9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, C.A., White, E., Huang, H., and Ma, H. (1997). The G protein alpha subunit (GP alpha1) is associated with the ER and the plasma membrane in meristematic cells of Arabidopsis and cauliflower. FEBS Lett. 407, 361–367. [DOI] [PubMed] [Google Scholar]
- Wodarz, A., and Nusse, R. (1998). Mechanisms of Wnt signaling in development. Annu. Rev. Cell Dev. Biol. 14, 59–88. [DOI] [PubMed] [Google Scholar]
- Wu, W.H., and Assmann, S.M. (1994). A membrane-delimited pathway of G-protein regulation of the guard-cell inward K+ channel. Proc. Natl. Acad. Sci. USA 91, 6310–6314. [DOI] [PMC free article] [PubMed] [Google Scholar]