Abstract
Divergent architecture of shoot models in flowering plants reflects the pattern of production of vegetative and reproductive organs from the apical meristem. The SELF-PRUNING (SP) gene of tomato is a member of a novel CETS family of regulatory genes (CEN, TFL1, and FT) that controls this process. We have identified and describe here several proteins that interact with SP (SIPs) and with its homologs from other species: a NIMA-like kinase (SPAK), a bZIP factor, a novel 10-kD protein, and 14-3-3 isoforms. SPAK, by analogy with Raf1, has two potential binding sites for 14-3-3 proteins, one of which is shared with SP. Surprisingly, overexpression of 14-3-3 proteins partially ameliorates the effect of the sp mutation. Analysis of the binding potential of chosen mutant SP variants, in relation to conformational features known to be conserved in this new family of regulatory proteins, suggests that associations with other proteins are required for the biological function of SP and that ligand binding and protein–protein association domains of SP may be separated. We suggest that CETS genes encode a family of modulator proteins with the potential to interact with a variety of signaling proteins in a manner analogous to that of 14-3-3 proteins.
INTRODUCTION
The shoot systems of flowering plants display great variation in their architecture and growth habit. The majority of this variation can be attributed to modifications of the fundamental branching pattern (Bell, 1992). These modifications result from alternate states of the shoot apical meristem, which can show either determinate or indeterminate growth, and either vegetative or reproductive development.
Two model plant species, Arabidopsis and Antirrhinum, have simple (monopodial) shoot architecture, that is, the apical meristem is indeterminate and active throughout the plant life cycle so that all appendages (leaves, side branches, and floral buds) are clearly lateral. A decision to flower is made only once during the life cycle of these species, after which the main shoot and axillary buds develop reproductive organs. This results in a clear distinction between vegetative and reproductive phases.
In contrast, vegetative and reproductive phases alternate regularly along the compound (sympodial) shoots of tomato. The primary vegetative apex is terminated by an inflorescence after six to 20 leaves have formed, but upward growth then continues from a new vegetative shoot arising from the uppermost axillary bud just below the terminating inflorescence. From then on, the stem is composed of reiterated units, each with three nodal leaves and a terminal inflorescence. Termination of each vegetative apex is thus synonymous with the transition to flowering in tomato but not in Arabidopsis or Antirrhinum. These basic differences in the meristematic environments are reflected in the changes incurred by homologous mutations on the overall architecture of the two plant species (Pnueli et al., 1998).
Mutations in the TERMINAL FLOWER1 (TFL1) gene of Arabidopsis or in the CENTRORADIALIS (CEN) gene in Antirrhinum (Shannon and Meeks-Wagner, 1991; Alvarez et al., 1992; Bradley et al., 1996, 1997) convert the inflorescence apical meristem from a normally indeterminate to a determinate state after a few flowers are formed. This results primarily in a shorter inflorescence shoot bearing a terminal flower. Yet an altered termination pattern in tomato results in an overall dramatic change in the plant architecture. A recessive mutation in the SELF-PRUNING (SP) gene (Yeager, 1927; MacArthur, 1932; Pnueli et al., 1998) confers accelerated termination of stem units until the shoot is eventually terminated by two consecutive inflorescences. Furthermore, in contrast to mutations in the CEN and TFL genes, sp mutation is inconsequential for the architecture of the inflorescence itself, because, unlike that in Arabidopsis, the inflorescence in tomato is inherently determinate (Pnueli et al., 1998). The “determinate” habit of the main shoot of tomato is repeated in side shoots, resulting in a limited growth of each shoot, a bushy compact constitution, and nearly homogeneous fruit setting. Introduction of the recessive sp gene into tomato cultivars revolutionized the tomato industry because the determinate growth habit facilitates mechanical harvesting (Atherton and Harris, 1986). Determinate sp plants also display a range of pleiotropic effects: internodes are shorter, more flowers are formed per inflorescence, control of apical dominance is relaxed, seed germination is accelerated, and plants are more prone to auxin treatment. In wild-type plants, auxin and its synergists induce determinacy and other characteristics of the sp phenotype (Zimmerman and Wilcoxon, 1942; Zimmerman and Hitchcock, 1949; Teubner and Wittwer, 1957; Gardner and Hedger, 1959; Lifschitz, 1965). In addition, the phenotypic expression of sp varies considerably in different genetic backgrounds (Pnueli et al., 1998), suggesting to us that the function of the SP gene is directly, or indirectly, mediated by auxin.
The CEN, TFL, and SP genes have been cloned and shown to be closely related (Bradley et al., 1996, 1997; Pnueli et al., 1998). Thus, although homologous gene mutations have different consequences in different shoot systems, in each case it seems that these genes determine the potential for continuous growth of the shoot apical meristem. This is because contrary to the mutant effect, overexpression of CEN, TFL1, or SP prolongs the vegetative stage. In extreme cases, expression of CEN (Amaya et al., 1999) or SP in tobacco (L. Pnueli and E. Lifschitz, unpublished data) may delay the transition to flowering for years. Interestingly, another gene of Arabidopsis, FLOWERING LOCUS T (FT), also has been shown to be a member of the same gene family (Kardailsky et al., 1999; Kobayashi et al., 1999). The product of this gene is apparently antagonistic to that of TFL1 in that overexpression of FT mimics the loss of TFL1 function, and vice versa (Koornneef et al., 1991; Ratcliffe et al., 1998).
CEN/TFL/SP and FT are members of a small gene family, with approximately six members in tomato (L. Carmel-Goren, personal communication) and six in Arabidopsis (D. Weigel, personal communication), that encodes 23-kD proteins. They share sequence similarity with a group of mammalian polypeptides designated phosphatidylethanolamine binding proteins (PEBPs; Grandy et al., 1990; Schoentgen and Jolles, 1995). The ability of PEBPs to bind phospholipids in vitro promoted the suggestion (Bradley et al., 1997) that they play a role in signaling. The crystal structures of PEBP from human and bovine sources (Banfield et al., 1998; Serre et al., 1998) and that of the CEN protein from Antirrhinum (Banfield and Brady, 2000) have been determined. Structural analysis of CEN suggests that its ligand binding pocket is incapable of accommodating phospholipid and thus is unlikely to function via direct contact with lipid bilayers. It is capable of accommodating phosphoryl groups, however, suggesting that PEBP proteins may mediate signaling via their association with phosphorylated proteins. Indeed, a mouse PEBP, RKIP, was recently shown to interact with and inhibit activity of the kinase Raf1 (Yeung et al., 1999, 2000). Rather than using the inappropriate designation PEBP, we suggest that this gene family be named the CETS genes after the first three plant genes with identified biological functions—CEN, TFL1, and SP.
CETS genes play a critical role in shaping plant architecture that is conserved among species. CETS genes do not, however, encode proteins that belong to families of DNA binding proteins, transcription activators, kinases, or receptors that are known to regulate major developmental programs in plants. CETS genes have no effect on cell survival, cell fate, or organ identity. We believe that in meristems, they decide the timing of potential switching from vegetative to reproductive growth.
To understand the molecular function of this new group of plant regulatory factors, we have initiated the identification and isolation of SP-interacting proteins (SIPs). It is believed that the combination of mutant phenotypes, interacting proteins, and resolved crystal structures will lead eventually to a comprehensive understanding of the mechanisms that facilitate the reproductive, species-specific architecture of flowering plants.
RESULTS
Molecular Identity of the SIPs Suggests a Role for SP in Molecular Signaling
The full-length cDNA of the SP gene was used as bait to screen 106 clones of an activation-domain fusion library prepared from RNA of shoot apices of wild-type tomato (see Methods). The scale is not exhaustive, and thus far five putative SIPs were identified. Their basic characteristics are shown in Table 1.
Table 1.
Characteristics of SIPs
| Number | Type | Two Hybrida | cDNAa | Locusb |
|---|---|---|---|---|
| SIP2 | 14-3-3/2 | 258 | 258 | 11-1, 11-2 |
| SIP74 | 14-3-3/74 | 252 | 252 | 4-3 |
| SIP3 | SPAKc | 339,220 | 609 | 2-1, 2-2 (?) |
| SIP4 | Novel | 98 | 99 | 11-1 |
| SIP8 | SPGBd | 62 | NDe | 2-4, 2-5 |
a Length (amino acids).
b Linkage assignments were obtained using 50 tomato lines containing overlapping insertions of the heterologous Lycopersicon pennellii wild species (Eshed and Zamir, 1995). SIP2, for example, was mapped to a region on chromosome 11 shared by insertions 11-1 and 11-2. The question mark in the last column indicates that the mapping of SIP3 to IL2-2 was not conclusive. Only clones that were identified in the primary screen, using SP as a bait, are included in the table.
c SPAK, NIMA-like kinase.
d SPGB, SIP8.
e ND, not determined.
SIP2 and SIP74 (Table 1) represent isoforms of the 14-3-3 family. One other interacting member of the 14-3-3 family, 14-3-3/5, is not included in Table 1 because it was isolated in a subsequent, limited, two-hybrid screen using the shorter SIP3 clone (Figrure 1A) as bait, and it has not been genetically mapped. SIP2 is a novel isoform of 14-3-3, and SIP74 represents the epsilon isoform. Members of this family are referred to in this article as 14-3-3/2, 14-3-3/74, and 14-3-3/5, respectively. 14-3-3s are a class of adapter proteins involved in signaling, transcription, and compartmentalization via their ability to stabilize, dimerize, or bridge their substrates, for example, Raf1, Cdc25, and BAD (Aitken, 1996; Muslin et al., 1996; Zha et al., 1996; Peng et al., 1997; Roberts et al., 1997; Brunet et al., 1999; Lopez-Girona et al., 1999; Pan et al., 1999; Yang et al., 1999).
SIP3 is a serine/threonine kinase, designated SPAK (for SP-associated kinase). SPAK shares ∼60 to 65% similarity, in its catalytic domain, with the NIMA-like kinases (for never in mitosis A). Two independent clones extending for different lengths to the N terminus were isolated (Table 1). A full-length cDNA, encoding 609 amino acids, was isolated subsequently from our regular cDNA library. Six related sequences were identified in the Arabidopsis sequence database. The NIMA kinase regulates early and late progression of G2 stages in Aspergillus and mammalian cells and is required for entry into mitosis and for the nuclear localization of the cyclin B/cdc2 complex (Osmani et al., 1988, 1991; Fry and Nigg, 1995; Lu et al., 1993; Lu and Hunter, 1995; Ye et al., 1995; Wu et al., 1998). It has been suggested (Cortez and Elledge, 2000) that the NIMA kinase may serve as the target for Chfr, a newly identified mitotic checkpoint protein regulating chromosome condensation (Scolnick and Halazonetis, 2000). SIP4 is a short, 10-kD (99–amino acid) novel protein. It is related to two anonymous genomic sequences from the Arabidopsis genome project. SIP8, named SPGB, is a putative bZIP transcription factor, a subclass of G-box (CCACGTGG) binding proteins (Giuliano et al., 1988; Menkens et al., 1995). It has been shown that G-box factors are phosphorylated before occupying their position in the transcription complex (Harter et al., 1994) and that 14-3-3 isoforms are components of transcription complexes containing G-box binding factors (Lu et al., 1992). On the basis of the 62 C-terminal amino acids sequence currently available, the family member most similar to SPGB is GBF4 (Menkens and Cashmore, 1994).
Tomato SIPs Bind to CETS Proteins from Arabidopsis and Antirrhinum
To explore the relevancy of the SP/SIPs associations, we have investigated whether their interactions are conserved in other species (Figure 1A). The CEN gene of Antirrhinum and TFL1 of Arabidopsis are orthologs of SP (Pnueli et al., 1998) and maintain, in their respective species, the indeterminate state of the inflorescence meristem (Amaya et al., 1999). The determinate phenotype in tomato is complemented by CEN (Pnueli et al., 1998), and SP, like CEN, delays flowering in tobacco plants; it also induces a leafy-like phenotype in tfl1 mutant plants of Arabidopsis (results not shown). FT is a functionally divergent CETS protein from Arabidopsis that apparently plays a role antagonistic to that of TFL1 (Kardailsky et al., 1999; Kobayashi et al., 1999). In addition to these three homologs, we have tested three overlapping peptides of SP spanning residues 1-42, 38-95, and 43-175. They are collectively denoted, in the second column of Figure 1A, by a ΔSP. None of the five SIPs bound to any of the truncated SP proteins. In contrast, both CEN and TFL1 bind SPAK, 14-3-3/74, and the SPGB proteins but do not react significantly with SIP4. FT, the functional antagonist of TFL1, displays the same binding pattern as TFL1. Thus, some of the SIP interactions in tomato are apparently conserved in distantly related plants. The interactions of FT with SIPs, however, do not provide a molecular basis for its antagonistic role in flowering.
Figure 1.
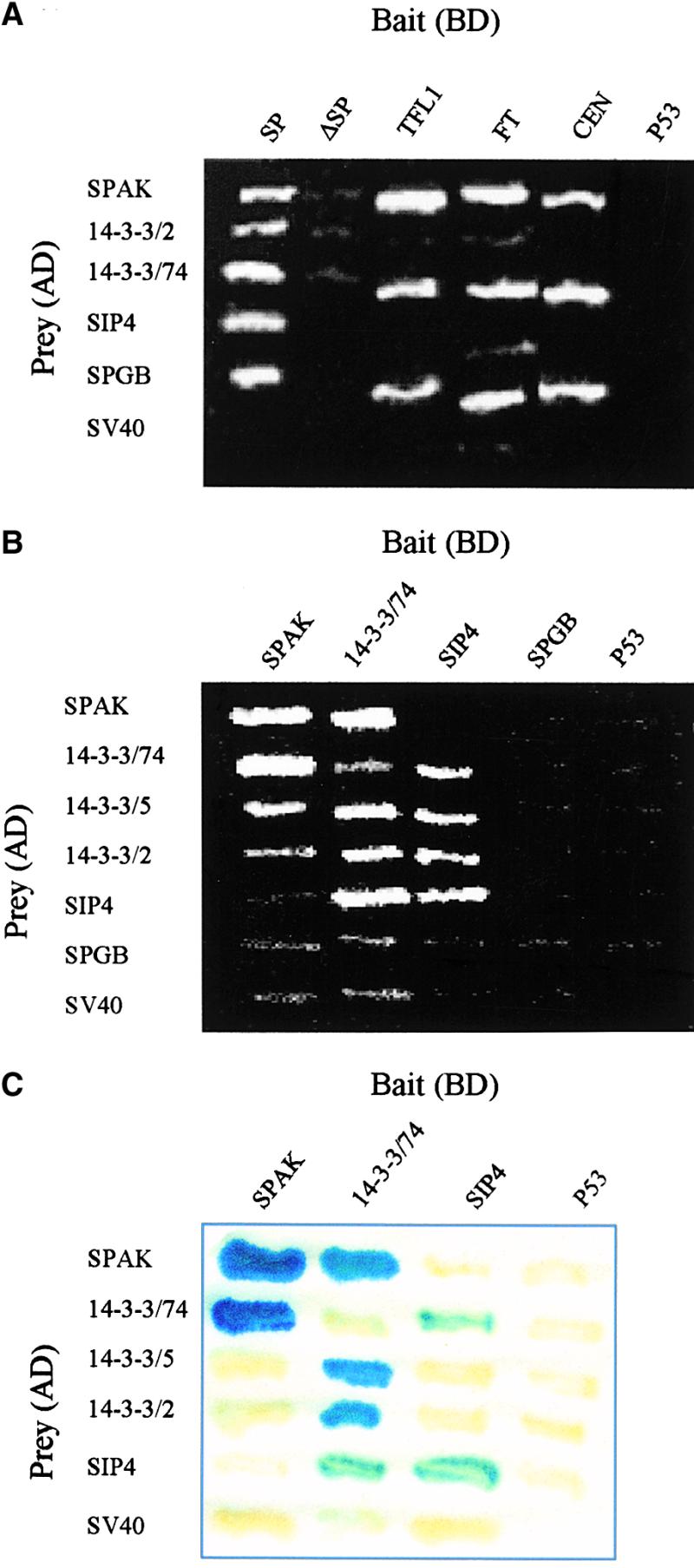
Identification and Characterization of the SIPs in the Yeast Two-Hybrid System.
(A) Conserved interactions between SIPs and SP homologs from Arabidopsis and Antirrhinum. Results of the two-hybrid tests between CETS and SIPs are illustrated. The FT column was inserted from a separate plate because BD-FT displays a residual growth (meaning activation potential) on the −His selective medium, thus requiring tests in plates supplemented with 4 mM triamino triazole (3AT). ΔSP refers collectively to three overlapping peptides of SP (see text), all of which gave the same negative result.
In (B) and (C), 14-3-3 proteins interact with all other SIP proteins.
(B) Bipartite associations between SIP proteins in a survival, two-hybrid test, on the −His selective medium, are shown.
(C) Colony lift, β-galactosidase association assays between SIPs are shown.
The strength of the interactions ranges from 11 Miller units for the SPAK:14-3-3/74 pair to 0.4 units for SPAK:14-3-3/2. Note that for the presence of positive interactions, a positive control between p53 and SV40 is not included here; P53 is used as a negative control.
SIPs Form a Network of Bipartite Interactions
The molecular identity of the SIPs supports their involvement in signaling processes. To test the possibility that they are involved in a SP-dependent signaling network, we examined them for their ability to form associations among themselves. Three 14-3-3s were included in some of the pairwise tests to probe possible differential affinities with the other SIPs or among themselves. Results of the two-hybrid tests are shown in Figures 1B and 1C (and supporting in vitro assays are discussed in relation to experiments documented in Figure 3).
Figure 3.
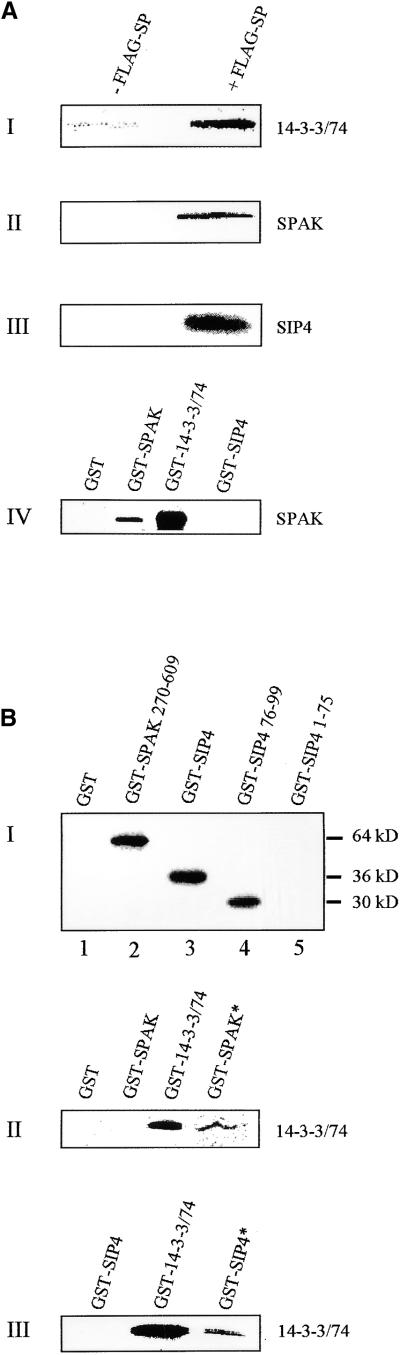
SP and SIPs Interact in Vitro.
(A) In I to III, in vitro association of labeled 14-3-3/74, SPAK, and SIP4, respectively, to FLAG-SP protein is shown. The FLAG-SP gene was expressed in Sf9 cells. Proteins from expressing cells and control cells were immobilized on anti-FLAG agarose beads, incubated with 35S-Met–labeled SIPs, resolved on SDS-PAGE, and visualized by autoradiography. In IV, SPAK polypeptides form homodimers and interact in vitro with 14-3-3/74 but not with SIP4. Note that SPAK in IV, as well as SPAK and SIP4 in II and III, respectively, are phosphorylated.
(B) Phosphorylation-dependent associations between SIP proteins. In I, SPAK auto-phosphorylates and also phosphorylates the C-terminal region of SIP4. A GST fusion with the complete SPAK protein was incubated in a phosphorylation reaction assay with a GST control (lane 1); a GST-SPAK 270-609 protein lacking the kinase domain (60 kD; see Figure 6) (lane 2); a GST-SIP4 fusion protein (36 kD; amino acids 1 to 99) (lane 3); a GST-SIP4 76-99 (30 kD) (lane 4); and a GST-SIP4 1-75 N-terminal domain (lane 5). Proteins were separated by SDS-PAGE and autoradiographed. In II, 14-3-3/74 dimerizes and interacts with phosphorylated (*), but not with nonphosphorylated, GST-SPAK fusion protein. In III, 14-3-3/74 protein binds to GST-SIP4 only if the latter is phosphorylated. In both II and III, in vitro–translated 35S-Met–labeled SIPs (indicated to the right of each gel) were incubated with immobilized GST-SIP fusion proteins (indicated above each gel). The asterisks indicate a GST-SIP previously phosphorylated by SPAK. Samples of bound radioactive proteins were eluted, resolved on SDS-PAGE, and visualized by autoradiography.
Results of survival assays carried on the histidine (−His) selective medium (see Methods) suggest that SPAK, SIP4, and 14-3-3/74 may form homodimers in yeast cells (Figure 1B). SP, by contrast, does not form homodimers (data not shown), which is consistent with the conclusion derived from the structural analysis of CEN (Banfield and Brady, 2000). Evidently, 14-3-3 isoforms, initially recovered as interacting with SP, also interact with SPAK and SIP4. Because G-box binding proteins are known to interact with 14-3-3s, the lack of interactions between 14-3-3s and the SPGB is attributed to the short fragment of the gene available to us. Presumably, SP and 14-3-3s are associated with separate domains of the GB protein.
Corroborating β-galactosidase assays are documented in Figure 1C. Interactions involving SPGB were omitted from this test because they were negative in the much more sensitive assay shown in Figure 1B. Strong interactions occur between SPAK and 14-3-3/74, and between SIP4 and 14-3-3/74. SPAK and SIP4 show weaker, albeit differential, interactions with 14-3-3/5 and 14-3-3/2. Interestingly, 14-3-3/74, which weakly dimerizes, forms a very strong association with 14-3-3/2 and only slightly less so with 14-3-3/5; these, in turn, are the weakest interactors in other combinations. Such differential affinities may form a basis for subtle modifications of the SP system by 14-3-3 factors.
Expression Domains of SPAK, SPGB, and SIP4 Overlap with That of SP
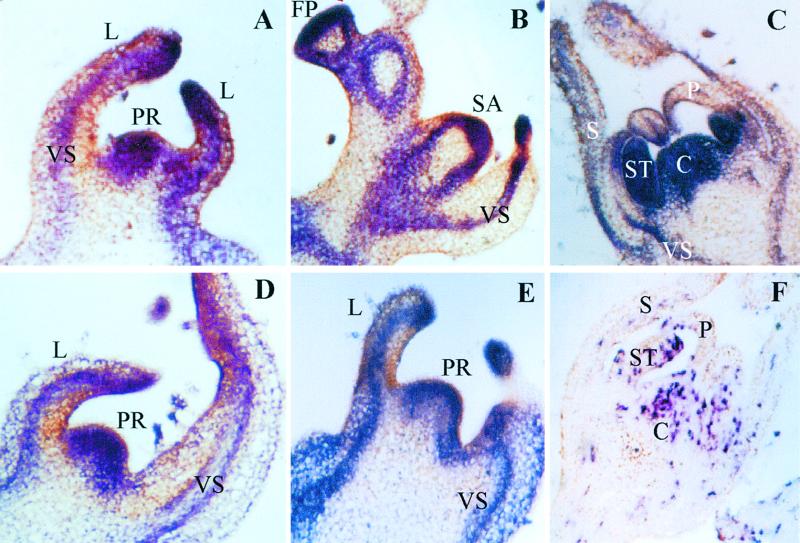
Expression domains of three SIP genes were compared, by in situ hybridization, with the expression pattern of SP. SPAK is expressed from the beginning in the apical meristem and in the leaf and stem vasculature of the primary apex of a young, two-leaf seedling (Figure 2A). A similar pattern is observed in the floral primordia of the primary inflorescence (Figure 2B) and in the vegetative meristem of the first sympodial segment (Figure 2B). In the developing floral bud, SPAK expression is particularly prominent in the stamens and carpels (Figure 2C). This expression pattern of SPAK overlaps spatially and temporarily with the patterns observed for SP and for the tomato LEAFY gene (Pnueli et al., 1998).
Figure 2.
Expression Domains of SPAK, SIP4, and SPGB Overlap with That of SP.
(A) to (C) Localization of SPAK mRNA in the early (two-leaf stage) vegetative apex (A), the first sympodial apex and an early floral primordium (B), and a floral bud (C).
(D) and (E) Localization of SIP4 (D) and SPGB (E) mRNA in early vegetative apices.
(F) Control section displaying contrasting expression pattern of the gene for the small subunit of the tomato ribonucleotide-reductase small subunit gene (RNR2). RNR2 displays a discontinuous pattern, reflecting the distribution of cells in S phase.
Digoxygenin-labeled antisense RNA probes for in situ hybridizations were prepared from inserts cloned in the BS vector using the T7 polymerase. C, carpel; FP, floral primordium; L, leaf; P, petal; PR, primary apex; S, sepal; SA, sympodial apex; ST, stamen; VS, vascular strand.
The in situ localization of transcripts of the SPGB and SIP4 genes is shown only for the apical meristems of young seedling (Figures 2D and 2E) because they too overlap throughout with that of SPAK and SP. In addition, the same pattern was seen for 14-3-3/2 (data not shown). Because all three probes shown in Figures 2A to 2E, along with SP and the T-Leafy probes, mark the same meristematic domains, we have used the tomato ribonucleotide-reductase small subunit gene (RNR2; E. Lifschitz and M. Egea-Cortines, unpublished data) as a positive control probe. This gene is expected to be expressed at high levels in meristematic tissues because it is upregulated specifically during S-phase; thus, it displays a noncontinuous, salt-and-pepper pattern (Figure 2F).
SP Interacts in Vitro with SIPs
In vitro assays were performed to examine the ability of the SIPs to associate with SP and among themselves, independent of potential yeast partners. A FLAG-SP fusion protein, expressed in Sf9 insect cells, was tested in pairwise combinations with radiolabeled 14-3-3/74, SPAK, and SIP4 prepared in the in vitro transcription/translation system and, as shown in Figure 3A, sections I to III, interacts readily with all three tested proteins. Identical results were obtained with FLAG-SP expressed in yeast cells (results not shown). In vitro binding assays shown in Figure 3A, section IV, verified also the in vitro association between SPAK and 14-3-3/74, the lack of association between SPAK and SIP4, and the potential of SPAK to dimerize. The results of the in vitro studies thus are consistent with the results of the two-hybrid experiments illustrated in Figure 1.
SPAK and 14-3-3/74 Interact in a Phosphorylation-Dependent Manner
For the in vitro reciprocal binding assays between pairs of SIPs, we used GST fusion proteins expressed in bacteria and 35S-methionine–labeled proteins translated in vitro. In preliminary experiments, some pairwise reciprocal assays did not give consistent results. It was subsequently determined that SPAK and SIP4 (used, for example, in the experiments described in Figure 3A), but not 14-3-3/74, are actually phosphorylated by kinases present in the in vitro translation system.
Therefore, for the initial evaluation of the role of phosphorylation in interactions between SIP proteins, the possibility that SPAK is autophosphorylated, and that it phosphorylates other SIP proteins, was investigated. For autophosphorylation, we tested the ability of full-length SPAK to phosphorylate a truncated protein deleted for its catalytic domain (amino acids 1 to 270; see Figures 4A to 4C). As shown in Figure 3B, secton I, lanes 1 and 2, the deleted SPAK protein is indeed phosphorylated by full-length SPAK expressed in bacteria. Of the other SIPs, only SIP4 was phosphorylated, in vitro, by SPAK (Figure 3B, section I, lane 3), thus establishing a biochemical function for SPAK. Deletion analysis of SIP4 suggested that the phosphorylation site was present in the C-terminal 20 amino acids (Figure 3B, section I, lanes 4 and 5), where the only candidate is a serine 98, embedded in a potential phosphorylation domain (R/KXXS/T).
Figure 4.
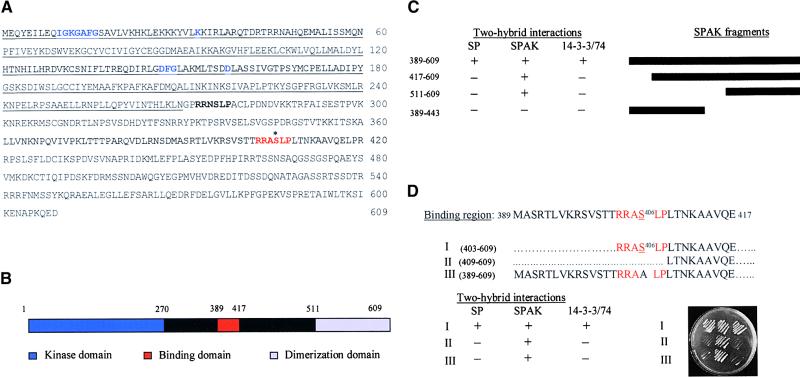
SP and 14-3-3 Share the Same Binding Site in SPAK.
(A) Amino acid sequence of the SPAK protein. Some conserved amino acids characteristic of the catalytic domain of kinases are marked in blue. The two putative 14-3-3 binding sites are shown in boldface and red, respectively. Serine 406 of the C-terminal site shared by 14-3-3 and SP is indicated by an asterisk.
(B) Schematic representation of the major functional domains in the SPAK protein. The scheme is based on data derived from sequence comparisons and deletion analysis in the two-hybrid system.
(C) Identification of the binding region for Sp and 14-3-3. Fragments of the regulatory domain of SPAK were tested in yeast for their interaction with binding domain fusions of SP, the SPAK regulatory domain (amino acids 270 to 609), and 14-3-3/74. The black bars at right show deletions of SPAK used to map the dimerization domain and the SP/14-3-3 binding region. The three columns at center show the results of the yeast two-hybrid assays. The ability (+) or the failure (−) to survive on His selective medium is indicated. Note that binding of SP and 14-3-3 to SPAK is effective only in the presence of the dimerization domain (amino acids 511 to 609).
(D) Fine-scale mapping of the 14-3-3 and SP binding site within SPAK. The amino acid sequence of the putative wild-type SP/14-3-3 binding region (389 to 417) of SPAK is shown. The consensus sequence for the binding of 14-3-3 (Yaffe et al., 1997a) and the conservative serine 406 are highlighted in red. Delineated below are the sequences of the three mutant versions of SPAK 389-417 that were used for the detailed mapping of the common binding site. The first retained the consensus six–amino acid sequence at its N terminus (I), the second polypeptide had this sequence deleted (II), and the third included the 14-3-3 consensus sequence but with serine 406 replaced by alanine (III). Results of two-hybrid tests of interactions between these modified SPAK proteins with SP, SPAK, and 14-3-3/74 are shown at bottom. Serine 406 is required for the binding of both SP and 14-3-3 under these conditions (plate at the bottom right).
Because in many cases 14-3-3 binds phosphorylated proteins (Yaffe et al., 1997b; Finnie et al., 1999), the requirement for SPAK and SIP to be phosphorylated to associate with 14-3-3/74 was examined. Pull-down assays shown in Figure 3B, sections II and III, revealed that in vitro–translated 14-3-3/74, which by itself is not phosphorylated, binds only phosphorylated SPAK (Figure 3B, section II); however, to form homodimers, 17-3-3/74 needs not to be phosphorylated. Likewise, it was found that only a phosphorylated SIP4 binds 14-3-3/74 (Figure 3B, section III).
SP and 14-3-3/74 Share a Six–Amino Acid Binding Domain in the SPAK Protein
The results described thus far show that SPAK binds to both SP and 14-3-3 proteins. Another serine/threonine kinase, Raf1, provides a precedent because it is associated with 14-3-3 (Rommel et al., 1996; Muslin et al., 1996; Yaffe et al., 1997a; Rittinger et al., 1999) and with a mammalian homolog of SP, RKIP (Yeung et al., 1999, 2000). The interactions of 14-3-3 and SP with SPAK were therefore studied in more detail. The amino acid sequence of SPAK is shown in Figure 4A. The deduced catalytic and dimerization domains, and the binding sites for SP and 14-3-3/74, are shown schematically in Figure 4B. Deletion analysis in the yeast two-hybrid system suggests that the C-terminal domain of SPAK, amino acids 511 to 609, is required for homodimerization (Figure 4C). A binding site for SP must also lie within the larger 389 to 609 region because it alone was present in the smaller of the two original cDNA products that bound SP (Table 1). A deletion analysis of SPAK also reveals that a 29–amino acids long peptide, spanning positions 389 to 417, inclusive, is required for the binding of both SP and 14-3-3/74, but binding is effective only in conjunction with a C-terminal dimerization domain (Figure 4C).
The sequence of the putative, 29–amino acid long, binding domain for SP and 14-3-3/74 contains a classical consensus sequence for the binding of 14-3-3 proteins (Yaffe et al., 1997a; Figures 4A and 4D), raising the unexpected possibility that SP and 14-3-3 share the same binding site within SPAK.
To address this possibility, we further dissected the 389-to-417 peptide of SPAK 389-609 into three subregions (Figure 4D) and tested the mutated SPAK proteins against SP and 14-3-3/74 in the yeast two-hybrid system. The results narrowed the essential SPAK site to the six–amino acids sequence (shown in red) that forms the consensus for the binding of 14-3-3 proteins. Included in this peptide is a conservative serine residue in position 406 that is likely to be crucial for effective binding with 14-3-3/74 (Yaffe et al., 1997b; Rittinger et al., 1999). Indeed, replacement of serine 406 by alanine (Figure 4D, lines III) abolished the binding of SPAK 389-609 to 14-3-3/74 and to SP but not the formation of SPAK dimmers, thus providing further evidence that SP and 14-3-3/74 may be interchangeable at, or even compete for, this SPAK site under certain physiological conditions.
In Raf1, two 14-3-3 molecules form a bivalent intramolecular bridge connecting binding sites in its N-terminal, RSTS259TP and C-terminal, RSAS621EP domains (Li et al., 1995; Rommel et al., 1996; Muslin et al., 1996). Likewise there is, in addition to the serine 406 site, another potential binding site for 14-3-3, RRNS274LP, in the N-terminal domain of SPAK. To examine the binding potential of this second site to 14-3-3/74, a full-length SPAK that includes the S274 site but with S406 replaced by alanine was examined. The full-length SPAK S406A retained its binding to 14-3-3, but no binding to SP was detected (results not shown), which perhaps implies that SPAK has only one potential site for SP but, like Raf1, at least two potential binding sites for 14-3-3.
Overexpression of 14-3-3 Genes Compensates for the Loss of Function of SP
To explore the functional role of the SIP genes in the context of plant architecture, we have initiated their study in transgenic plants. At first, the 14-3-3 and SPAK genes were used to transform plants of the determinate double mutant sp an (anantha; Paddock and Alexander, 1952) genotype because anantha plants are more sensitive to the growth-promoting effects of the SP gene in two ways (Pnueli et al., 1998). First, the determinate habit of the sp;an shoots (Figure 5B; cf. Figure 5A) is converted by the overexpression of SP to a highly irregular, indeterminate pattern, with three to six internodes between inflorescences (Figure 5C). Second, the primordia of the sp;an inflorescence, which are normally arrested at a pre-floral, cauliflower-like state (Figure 5E), now develop leaflets and leaves (Figures 5C and 5F). Thus, transgenes affecting the SP system are expected foremost to alter the determinate habit of sp;an shoots and/or the stage in which the primordia of the anantha inflorescence are arrested.
Figure 5.
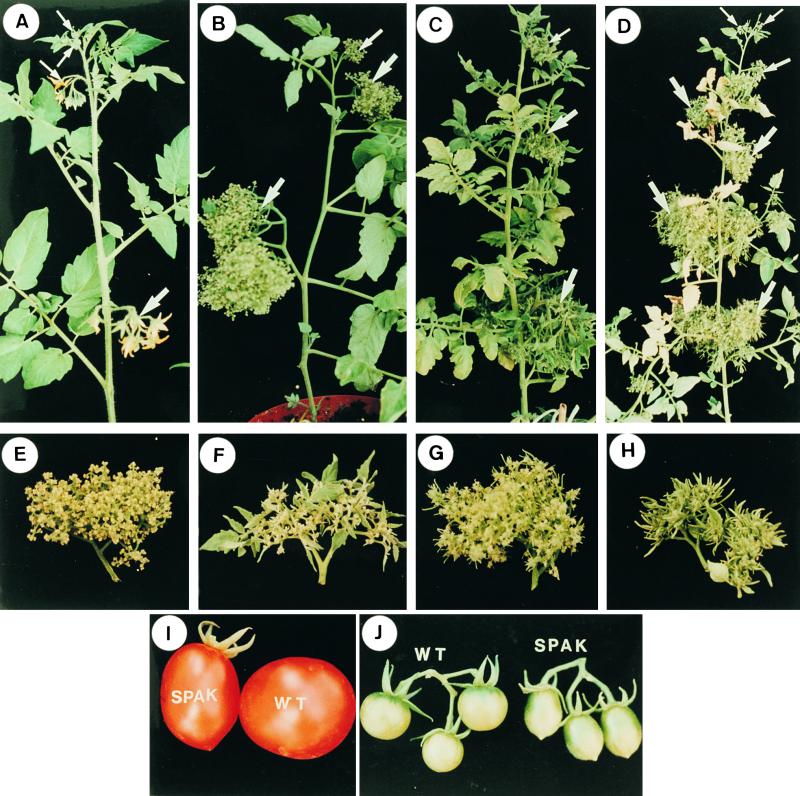
Overexpression of 14-3-3 genes in Determinate anantha Mutant Plants Mimics the Effect of Upregulation of SP.
(A) An indeterminate wild-type shoot. Four successive inflorescences spaced by three leaves each are indicated by arrows.
(B) A shoot from a progenitor determinate double mutant sp an plant. The shoot is terminated by two consecutive inflorescences (upper two arrows), which is the consequence of the sp mutation. The meristems in each inflorescence (arrows) proliferate, and all are arrested in a cauliflower-like stage (the effect of an).
(C) Overexpression of the SP gene in sp an mutant background. Note the increased number of leaves between inflorescences and the generation of leaves by the sp;an inflorescence meristems (arrows).
(D) Overexpression of the 14-3-3/2 gene results in the partial rescue of the mutant determinate habit of sp;an plants. Note the indeterminate pattern of the shoot (seven inflorescences are marked by arrows), and the two to three nodal leaves separating the inflorescence shoots.
(E) A single inflorescence of a progenitor, double mutant sp;an mutant. Meristems are arrested at the cauliflower-like stage, with no leafy primordia formed.
(F) Vegetative growth, that is, production of leaves in the sp;an mutant inflorescence of a plant overexpressing SP.
(G) Promotion of vegetative growth, that is, production of leaf primordia in the inflorescence of a sp;an mutant plant overexpressing 14-3-3/2.
(H) Leafy phenotype of the inflorescence of a sp;an mutant plant overexpressing 14-3-3/74.
(I ) and (J) Suppression of SPAK induces fruit elongation. (I) A regular size fruit of the VF36 line (right) and an elongated, pear shape fruit from plants expressing p35S::SPAK antisense RNA. (J) Round cherry tomato fruits (left) and elongated cherry fruits (right) from plants expressing a dominant-negative form of SPAK.
No developmental changes were observed in sp or sp;an plants expressing antisense RNA of the 14-3-3/2 or 14-3-3/74 genes. However, a clear manifestation of increased vegetative characteristics is evident in the shoots and inflorescence of sp;an plants overexpressing either of the two genes (Figures 5D, 5G, and 5H). A significant attenuation of the determinate phenotype was observed in sp;an plants overexpressing 14-3-3/2 (Figure 5D), although this was not as complete as observed when SP is overexpressed in the same background (Figure 5C). The now indeterminate main and side shoots of sp;an plants expressing 14-3-3/2 feature a variable number of one to three internodes between inflorescences, with spacings of one or two leaves predominating. In addition, the inflorescence is now mildly leafy (Figure 5G). Overexpression of 14-3-3/74 in sp;an plants resulted in only mild, albeit consistent, delay of determinacy, so that many branches generated five to seven inflorescence shoots before termination, as compared with three to four inflorescence shoots in the progenitor line. However, the second growth-promoting parameter, that is, the leafiness of the inflorescence, is much more pronounced (Figure 5H). Thus, increased levels of 14-3-3 proteins seem to compensate for the loss of function of the SP gene, at least in the anantha mutant background, suggesting perhaps some overlapping biochemical functions. It should also be noted that the partial suppression of the determinate sp mutant phenotype that is a semi-determinate habit is also characteristic of several genetic backgrounds such as that of the VF36 line. Modifiers may include variants of SP-interacting genes such as 14-3-3.
It will be interesting to see if overexpression, in the same plants, of 14-3-3/2 and 14-3-3/74, which form strong heterodimers (Figure 1), will result in a more complete complementation of the determinate phenotype.
Antisense expression of SPAK has no obvious effect on growth habit. However, all sp plants expressing antisense RNA form pear-shaped fruits instead of the normal round fruits of the progenitor plants. Pear-shaped, ovate fruits are formed in transgenic plants bearing regular-size fruits as well as in plants bearing cherry fruits (Figures 5I and 5J). Exactly the same results were obtained when a dominant-negative form of SPAK (i.e., amino acids 403 to 609) was expressed in tomato plants. Outcrossing of antisense-expressing plants with different determinate and indeterminate lines showed that formation of ovate fruits is independent of allelic variation in the SP locus itself.
Mutations in Conserved Structural Domain Annul the SP/SIPs Interactions
To substantiate a functional need for SP to interact with SIPs, we took advantage of the recently determined crystal structures of three CETS (PEBP) proteins, from mammals and plants (Banfield et al., 1998; Serre et al., 1998; Banfield and Brady, 2000), and of single amino acid alterations known to inactivate plant CETS genes (Bradley et al., 1996, 1997; Pnueli et al., 1998; Kardailsky et al., 1999; Kobayashi et al., 1999). In principle, it should be possible, under these circumstances, to relate binding potential of mutant SP variants to conformational features, known to be conserved, in this new family of regulatory factors.
For a rational choice of mutant sites to be tested, we have built a structural model of SP, using homology-based modeling (Peitsch, 1996). The resulting three-dimensional model (see Figure 7A) is very similar to the structures reported for PEBP proteins. We have subsequently analyzed the binding properties of SP proteins carrying three known mutations in particularly critical and conservative domains of all CETS/PEBP proteins.
Figure 7.
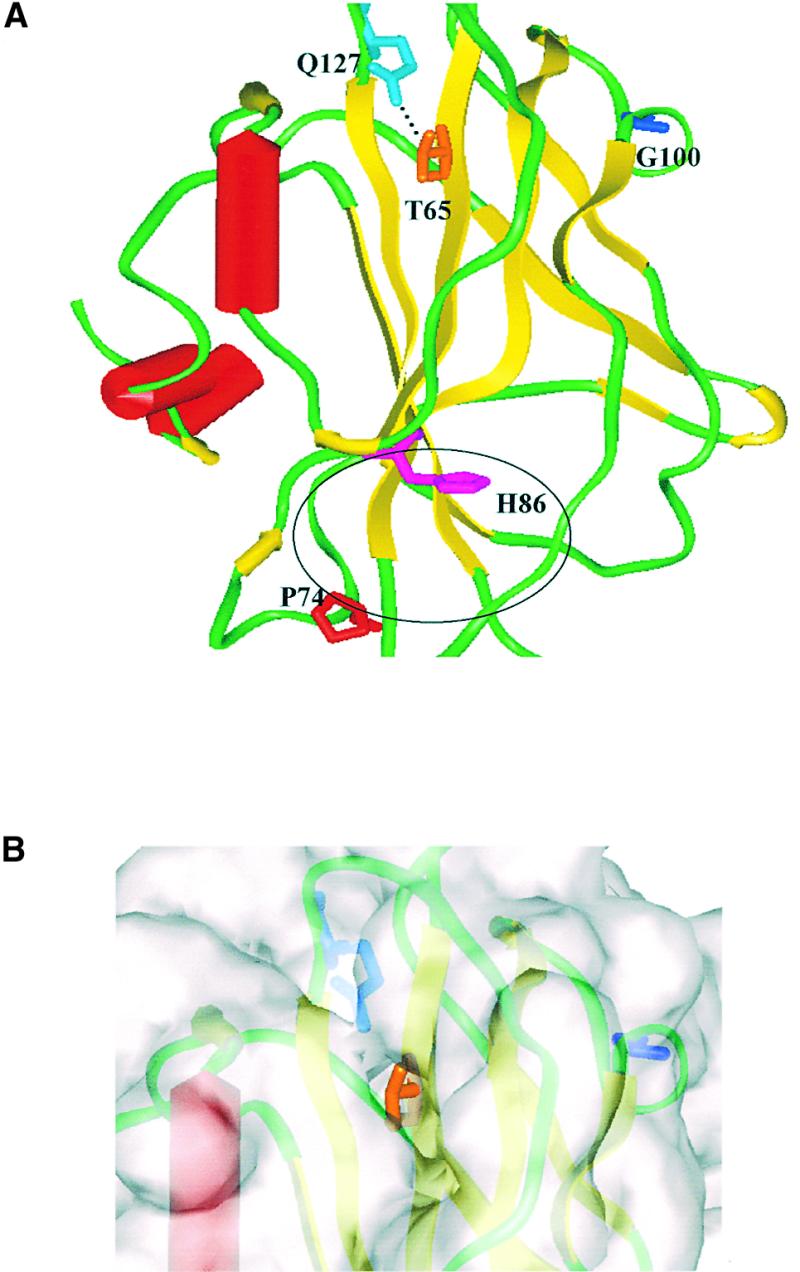
Molecular Model of the SP Protein.
(A) This molecular model of the SP protein was built using the SwissProt automated comparative protein modeling server, based on its sequence homology to three members of the PEBP protein family whose structures have been determined by x-ray crystallographic methods: human PEBP, bovine brain PEBP, and Antirrhinum CEN (Protein databank accession numbers 1BEH, 1A44, and 1QOU, respectively). The overall protein model contains extensive stretches of secondary structure, with a large β-sheet (yellow ribbons) in the center flanked on one side by a smaller β-sheet and on the other side by three short α-helices (red tubes). The putative ligand binding sites, comprised of a number of amino acid residues, which are not a contiguous sequence, have been delineated in all three existing crystal structures as a pocket formed between the helices and the bottom of the central β-sheet. The putative active site is indicated by the position of H86 (purple), surrounded by a black oval. Residues that affected protein–protein interactions when mutated are T65 (orange) and G100 (blue). Mutation of P74 (red) did not affect protein–protein interactions. Q127 (cyan) may form a hydrogen bond to the T65 hydroxyl group.
(B) The molecular surface (transparent gray) superimposed on a close-up of the SP model (the colors are the same as in [A]) in the vicinity of T65 is shown. This residue lies at the bottom of a depression that may be important for protein–protein interactions because mutation of the threonine to isoleucine hinders SP–SIP interactions.
In the recessive sp allele, proline in position 76 (Pnueli et al., 1998), designated here as P74 to conform with the alignment of all the genes in the family (Banfield and Brady, 2000), is replaced by leucine. Proline 74 is within an invariant motif, DPDXP74D, near the putative active site (indicated in Figure 7A by an oval frame and the position of H86), which is conserved in all members of the family (Serre et al., 1998; Banfield and Brady, 2000). Because no other mutant alleles of SP are known, we have exploited two other conserved mutant sites of its homolog, the TFL1 gene of Arabidopsis (Bradley et al., 1997). One disfunctional allele of TFL1 (i.e., a terminal flower and early flowering) carries a threonine-to-isoleucine alteration in position 65 and the other a glycine-to-aspartic acid in position 100 (Bradley et al., 1997). These two sites are more distant from the ligand binding site, and it appears that neither mutation potentially alters SP ligand binding.
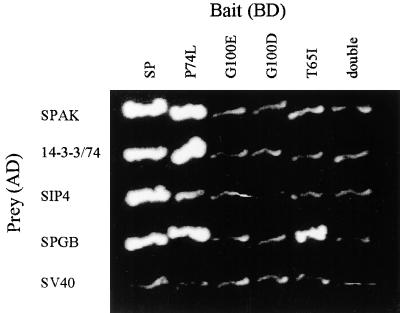
To investigate the effect of these mutations on the binding properties of SP, we introduced identical mutations into the SP gene. A change in one base was sufficient for the T65I alteration, but two steps were required (G to E and E to D) to introduce the G100D change in the SP. The three mutant forms of SP were subsequently tested in yeast for their interaction with the SIP proteins. As shown in Figure 6, the four SIPs interact readily with the SP-P74L mutant protein. In contrast, with the exception of the successful binding between SP T65I and the short peptide representing the SPGB protein, the SP-T65I, SP-G100E, and the SP-G100D mutants, as well as the double mutant SP-T65I G100E, abolished the SP–SIPs interactions in the two-hybrid test.
Figure 6.
Binding Properties of T65I and G100D Mark Putative Conservative Protein–Protein Association Domains of SP.
SP and its mutant derivatives used in this experiment were tagged with FLAG (to verify expression in yeast) and fused to the Gal4 binding domain. The SP bait (double, in the far right column) denotes the double mutant SP T65I:G100E.
DISCUSSION
SPAK, SPGB, and 14-3-3s belong to protein families known to be involved in signaling pathways; the novel SIP4 may be phosphorylated by SPAK; and SP and the other SIPs all interact with 14-3-3s. The observed network of specific interactions between SIPs provides support for the assertion that the SIPs are legitimate components of a putative SP-dependent signaling system. Upstream effectors and downstream targets for the SP system are yet to be discovered, and the full range of the biochemical diversification of CETS proteins has not yet been explored. Even so, it is already possible at this point to discuss the possible biochemical function of SP in conjunction with mutations that interfere with its binding properties and in relation to its interactions with the 14-3-3 and SPAK proteins.
Molecular Interactions between SP, SPAK, and 14-3-3s Suggest Analogy with the Raf1 Mechanism
The SP system shares notable similarities, with possible far-reaching implications, with the Raf1 complex. Because none of the known SP partners seems likely to anchor the system to the membrane, the SP signaling system, like that of Raf1, is more likely to be a transient component of membrane-associated effector complexes. Although Raf1 and SPAK belong to two different families of kinases, they each bind to members of the same protein family: SP, TFL1, and CEN bind to SPAK, and RKIP, an SP homolog protein from mammals, binds to Raf1 (Yeung et al., 1999, 2000). Both SP and RKIP bind only phosphorylated kinases, the two kinases bind 14-3-3s in a phosphorylation-dependent manner, and each has two 14-3-3 binding sites. In fact, we found that the NIMA kinase itself also contains two potential binding sites for 14-3-3 proteins. In this context, it is noteworthy that if Raf1, like SPAK, also uses the 14-3-3 binding site to attract the mammalian SP homolog RKIP, the analogy is extended and an unexpected additional regulatory point for Raf1 is envisaged.
Here, we report that binding of SP to SPAK depends on a serine residue at position 406. The phosphorylation status of this particular serine has not been directly established, but because the very same site is also required for the binding of 14-3-3/74, and because the requirement for a phosphorylated serine in the 14-3-3 class of binding sites has already been established (Muslin et al., 1996), it is likely that serine 406 of SPAK must be phosphorylated for SPAK to bind SP as well. It is not implied, however, that the phosphoryl-serine 406 is actually accommodated by the ligand binding pocket of SP.
In Raf1, two 14-3-3 molecules form bivalent intramolecular bridging sites in the N- and C-terminal domains before being displaced from the N-terminal site by an activated Ras (Li et al., 1995; Rommel et al., 1996; Muslin et al., 1996). Likewise there are two binding sites for 14-3-3 in SPAK, but it seems as if only one is shared by SP. Exclusive SP bridges in SPAK are currently excluded also because SP does not dimerize. However, potentially SP could participate in an intramolecular bridge in cooperation with a 14-3-3 if transient modifications of SPAK by other accessory proteins occur. Pin1, a peptidyl-prolyl isomerase, is one possible agent for such a structural modification of SPAK. Pin1 was initially identified as interacting with, and inhibiting, the mammalian NIMA kinase, thereby blocking entry to mitosis by interacting with phosphorylated regulators such as Cdc25 and Wee1 (Lu et al., 1996; Yaffe et al., 1997b; Shen et al., 1998).
Biological Function of the SP/14-3-3/SPAK Interactions
Suppression of SPAK, by either antisense or dominant-negative expression, does not alter plant architecture. This is not surprising for several reasons. Tomato plants have been cultivated and extensively bred for more then 500 years; however, despite the readily scored phenotype, only one locus for determinate phenotype was found. Suppression of gene activity by the antisense or dominant negative approaches is frequently inefficient, and there are at least five SPAK-like genes with possible redundant function. And because the determinate phenotype is very sensitive to modifiers, it is also possible that the differential response of meristems and fruits to the inactivation of SPAK is mediated by as yet unknown organ-specific modifiers.
It is nevertheless satisfying that suppression of SPAK activity induces elongated fruits, presumably as a result of effect on cell division. In Aspergillus and in mammalian cells, the NIMA kinase is required for entry into mitosis and for the nuclear localization of the cyclin B/cdc2 complex. It may thus participate, redundantly, in regulating the transition to flowering that is always associated with an altered distribution of cell divisions in the apical meristem (Bernier, 1988).
The effects of overexpressing 14-3-3s genes, in complementing the loss of the sp function, are more amenable to interpretation through direct interactions of 14-3-3 with SP and SPAK, even though indirect effects cannot be excluded. Although antisense expression had no effect, overexpression of each of the two 14-3-3 genes ameliorated the effects of sp mutation by increasing the indeterminacy of the shoot apical meristem, and by increasing the vegetative properties of the inflorescence in sp an double mutants. These effects can be accounted for in at least two reasonable ways. It may be that a given overexpressed 14-3-3 replaces the endogenous legitimate 14-3-3 partner of the mutant sp/14-3-3/ SPAK or that the abundance of 14-3-3 proteins now allows the replacement of a defective sp protein with a competitive, partially functional, 14-3-3/14-3-3/SPAK association. Alternatively, if an antagonist of SP, such as FT, functions via a similar SPAK/14-3-3–based system that involves other NIMA-like kinases and 14-3-3 homologs, then overexpression of illegitimate 14-3-3 might reduce the efficiency of this antagonistic system. A dominant-negative effect, via their involvement in assembling signaling complexes, was also suggested for 14-3-3s involved redundantly in the Ras1-Raf1 signaling in Drosophila (Chang and Rubin, 1997). Presumably, interacting with different 14-3-3 isoforms enables SP and SPAK, as well as other CETS proteins, to perform functions uniquely suited to specialized physiological responses.
T65 and G100 May Identify the Protein Association Domain Required for SP Function
The actual mode of interactions of SP and its mutant variants with other proteins can be resolved only by structural studies of each particular case. However, because a high degree of similarity exists between members of the protein family, we can try to relate structure to function by examining the relative positions of altered amino acids in the three-dimensional protein structures.
In general, default binding could be the result of alterations in the actual protein–protein association domains. Alternatively, it may be secondary to alterations in ligand binding or to major conformational changes.
The results presented here show that the T65I and G100E (or D) mutations, but not P74L, alter the pattern of interactions between SP and other proteins. The P74L mutation does not affect the protein–protein interactions reported in this study, suggesting that not every mutation that renders the SP gene inactive also interferes with the physical associations with other proteins. It also implies that the active site, that is, ligand binding and protein association functions, may be separated in SP. This is not surprising because P74L is within the DPDXPD motif and thus expected to disrupt the ligand binding site and not necessarily to interfere with protein associations. A spatial and functional separation of the protein–protein interaction domain and the catalytic site was recently demonstrated, for example, for the Ras-SOS complex (Hall et al., 2001).
The mutations that hinder the binding of SP to SIPs, T65I and G100E, are more distant from the ligand binding site (see the legend to Figure 7A). They may therefore alter directly and thus identify the protein–protein association domains of SP.
The most reasonable structural inference for such a role is provided by the G100E (or D) mutation. G100 is situated at the base of a loop structure that protrudes from the side of the central β-sheet. It is likely that exchange of glycine with the bulky, and potentially charged, glutamate or aspartate side chains will perturb the vicinity of G100, which in turn could directly affect protein–protein interactions.
Interpretation of the T65I mutation is more complicated because it is situated in the top middle section of the main β-sheet, whose integrity is probably very important for obtaining the overall protein structure. Loss of a hydrogen bond between the threonine 65 hydroxyl group and glutamine 127, the result of the substitution by isoleucine (Figure 7A, dotted line), could primarily destabilize the central β-sheet, deforming the surrounding secondary structure, and thus lead to changes in protein–protein interactions.
Alternatively, a more direct role for the T65 site in the protein associations of SP may be envisaged. In the SP model, the threonine side chain lies at the bottom of a surface-accessible hydrophobic depression (Figure 7B) whose outer rim is lined with polar residues, suggesting that this site may be directly involved in protein–protein interactions (Lo Conte et al., 1999). It is very similar to the hydrophobic pocket that is so critical for the formation of the Ras-SOS complex mentioned above. By contrast, in both the human and bovine PEBP structures, the depression is occluded by loops from Gln-127 to Leu-131 and the top of the adjacent helix (Lys-157/Tyr-158). In the CEN structure, the loop between residues 130 and 142 is disordered, and it was not included in the structure (Banfield and Brady, 2000); the plant proteins have significant sequence differences in the disordered area, when compared with the mammalian PEBPs. This may be an indication of association preferences and functional differences between plant and mammalian PEBPs.
Conclusions
Our studies support the view that to regulate shoot architecture, CETS proteins were recruited to function as a hub of signaling systems that have the inherent flexibility and potential to integrate a wide variety of developmental cues. Flexibility and diversity are facilitated by the potential of CETS proteins to bind diverse classes of regulatory proteins. In mammals, RKIP, a PEBP, was shown to interact with Raf1 (Yeung et al., 1999, 2000). More recently, Yeung et al. (2001) also showed that RKIP interacts physically with two other members of the MAPKKK family, NIK and TAK1, to modulate the response of NfkB pathway to TNF-α and other signals. Here, we have expanded the range of interacting proteins to include a newly identified plant kinase of the NIMA class, adapter factors of the 14-3-3 family, a transcription factor of the G-box binding family, and a novel protein, SIP4. Thus, the diversity of phenotypes regulated by CETS genes is likely the result of their temporal and spatial association with these and most probably other factors. We conclude, therefore, that the CETS genes encode a new family of modulator/adapter proteins analogous to those of the 14-3-3 family. They seem likely to participate in many signaling pathways, but their developmental role may be revealed only in systems and organs in which their function is responsible for a rate-limiting process.
METHODS
Plant Material
Lycopersicon esculentum lines, VFNT-Cherry SP (LA2756), VFNT-Cherry/sp2 (LA2705), and anantha seed were provided by R. Chetelate and C.M. Rick (University of California, Davis). VF36 sp seed were provided by S. McCormick. Tomato Lycopersicon pennellii lines for mapping (Eshed and Zamir, 1995) were kindly provided by D. Zamir.
Two-Hybrid Screen
A cDNA library, representing 106 independent EcoRI-XhoI cDNA clones, was prepared in the Hybrizap vector (Stratagene) from the mRNA of wild-type apices, ∼0.5 cm long, containing the second and third shoot segments. The HF7c yeast line was used as the host because its growth on the histidine (−His) selective medium is completely suppressed (Feilotter et al., 1994). Procedures and controls were as recommended by the manufacturer (Stratagene). The bait was a full-length SP protein. Full-length SP and sp P74L clones (Pnueli et al., 1998) were inserted as BamHI blunt/XhoI fragments into EcoRI blunt/SalI site of the GAL4 binding domain in the pBD-GAL4 phagmid vector. SP 1-42 and 43-175 were recovered as EcoRI and EcoRI/XhoI fragments, respectively, and SP 38-95 was a polymerase chain reaction (PCR) product. All were ligated into a blunt EcoRI site of the pBD-GAL4 vector.
Full-length EcoRI-XhoI inserts of 14-3-3/2, 14-3-3/74, SIP4, SPAK 270-609, SPAK 389-609, and the partial SPGB were ligated into the EcoRI-SalI site of the GAL4 BD vector. CEN (a gift of Enrico Coen) and TFL1 and FT (gifts of Detlef Weigel) were cloned into a blunt-ended EcoRI site of the bait plasmid.
SPAK 389-609, 270-609, 417-609, and 511-609 were recovered from the AD clones as above. SPAK 389-443 was an Nde blunt/XhoI derivative of SPAK 389-609.
SPAK 403-609, 409-609, and 389-609 S406A, described in Figure 4D, were amplified by PCR using their own and the T7 primers and inserted in the EcoRI blunt site of the bait vector.
Transgenic Plants
SPAK, 14-3-3/74, and 14-3-3/2 cDNAs were cloned, in both sense and antisense orientations, in pCGN1548 and expressed under the control of the Cauliflower mosaic virus 35S promoter (Benfey and Chua, 1990). RK9/8 (Pnueli et al., 1994; Hareven et al., 1996) and sp;an plants for transformation were maintained in culture vessels by using cuttings. Leaf disc transformation was conducted essentially according to McCormick (1991).
Constructs for Transgenic Plants
The full-length 14-3-3/2 and 14-3-3/74, and the full SPAK clone and the partial SPAK clone 403-609 (Figure 4), were recovered as EcoRI-XhoI fragments, cloned into a blunt SalI-SmaI site of a pUC18 between the cauliflower mosaic virus 35S promoter and the nopaline synthase terminator and subsequently inserted into the pCGN1548 vector as XbaI fragments.
Site-Specific Mutagenesis
The T65I and G100E alleles of SP were obtained by a two-step PCR protocol (Higuchi et al., 1988). The G100D allele of SP was derived by the same procedure from the G100E clone. Entire sequences were verified on both strands.
Cloning for in Vitro Association Assays
For in vitro transcription/translation, full-length EcoRI-XhoI inserts of 14-3-3/74, SIP4, and the full-length SPAK cDNA clone were cloned into pBS SK. To generate GST fusions, full-length SPAK, partial SPAK 270-609 14-3-3/74, SIP4, and the SIP4 fragments 1-75 (EcoRI-ClaI) and 76-99 (ClaI-XhoI) were cloned as overhang or blunt-end fragments in the EcoRI-XhoI site of pGEX 4T-1 (Pharmacia). For expression in insect Sf9 cells, SP was tagged with the FLAG epitope by using regular PCR, and the product was cloned into pFastBac HTb vector in the NcoI/SalI site.
Cytological Procedures
Digoxygenin-labeled (Boehringer Mannheim) RNA probes and hybridization procedures were as referred to in Pnueli et al. (1994) and Hareven et al. (1996). The tomato RNR2 clone (E. Lifschitz and M. Egea-Cortines, unpublished data) was subcloned in the same vector, and the RNA probe was prepared by the same procedure.
In Vitro Binding Assays
35S-methionine–labeled proteins were prepared using the TNT-coupled system (Promega). Binding assays between GST fusion proteins and 35S-methionine–labeled proteins were performed as in Ausubel et al. (1988). The SP protein was expressed using the Bac-to-Bac expression system (Gibco BRL) and immobilized on anti-FLAG agarose beads (Sigma). 35S-labeled protein was added that was supplemented with 10% BSA. The proteins were incubated for 2 hr at room temperature. Binding was performed in 50 mM K4P2O7, pH 7.5, 150 mM KCl, and 1 mM MgCl2. The samples were resolved by SDS-PAGE and visualized using a PhosphorImager (FUJIX BAS 1000, Tokyo, Japan).
Kinase Assays
Full-length GST:SPAK was expressed at 30°C and immobilized, alone or with a target protein, on glutathione beads. Beads were washed with phosphorylation buffer (50 mM Tris-HCl, pH 7.5, and 10 mM MgCl2) and incubated in the presence of 2 mM DTT, 2.5 μM ATP, and 2 pM 32P-ATP (3000 Ci/mmol) for 30 min at room temperature. After three washes in PBS buffer, samples were analyzed on SDS-PAGE or used for binding assays, in which case radioactive ATP was omitted.
Accession Numbers
The GenBank accession numbers for proteins in this article are as follows: SPAK, AF079103; SIP4 (I4), AF175963; 14-3-3/2, AF079104; 14-3-3/74, AF079450; and 14-3-3/5, AF079451.
Acknowledgments
The relentless and diligent efforts of David Smyth in preparing the manuscript are highly appreciated. Thanks are also due to Leo Brady, Ry Wagner, and Stan Alvarez for their helpful suggestions. We thank Detlef Weigel for the unconditional gifts of the FT and TFL1 clones and Enrico Coen for the generous gift of the CEN clone. We thank Dani Zamir for permission to use the tomato insertion lines and Yona Kasir for her continuous advice on the yeast system. The plant architecture research program was supported by a grant to E.L. from the United States–Israel Binational Agricultural Research and Development Fund (BARD), and by additional support from the Israel Academy of Science, the Israel/USA Bi-national Science Foundation (BSF) and by Grant No. QLK5-CT-2000-00357 from the European Commission. Dr. Lilac Pnueli received the Levi Eshkol award from the Israel Ministry of Science.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.010293.
References
- Aitken, A. (1996). 14-3-3 and its possible role in coordinating multiple signalling pathways. Trends Cell Biol. 6, 341–347. [DOI] [PubMed] [Google Scholar]
- Alvarez, J., Guli, C.L., Yu, X.-H., and Smyth, D.R. (1992). Terminal Flower: A gene affecting inflorescence development in Arabidopsis thaliana. Plant J. 2, 103–116. [Google Scholar]
- Amaya, I., Ratcliffe, O.J., and Bradley, D.J. (1999). Expression of CENTRORADIALIS (CEN) and CEN-like genes in tobacco reveals a conserved mechanism controlling phase change in diverse species. Plant Cell 11, 1405–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton, J.G., and Harris, G.P. (1986). Flowering. In The Tomato Crop, J.G. Atherton and J. Rudich, eds (New York/London: Chapman and Hall) , pp. 167–200.
- Ausubel, F.M., Brent, R., Kingston, R.E., Moor, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. (1988). Current Protocols in Molecular Biology. (New York: Wiley).
- Banfield, M.J., and Brady, R.L. (2000). The structure of Antirrhinum CENTRORADIALIS protein (CEN) suggest a role as a kinase regulator. J. Mol. Biol. 297, 1159–1170. [DOI] [PubMed] [Google Scholar]
- Banfield, M.J., Barker, J.J., Perry, A.C., and Brady, R.L. (1998). Function from structure? The crystal structure of human phosphatidylethanolamine-binding protein suggests a role in membrane signal transduction. Structure 6, 1245–1254. [DOI] [PubMed] [Google Scholar]
- Bell, R.P. (1992). Green Plants. Their Origin and Diversity. (Cambridge: Cambridge University Press).
- Benfey, P.N., and Chua, N.-H. (1990). The cauliflower mosaic virus 35S promoter: Combinatorial regulation of transcription in plants. Science 250, 959–966. [DOI] [PubMed] [Google Scholar]
- Bradley, D., Carpenter, R., Copsey, L., Vincent, C., Rothstein, S., and Coen, E.S. (1996). Control of inflorescence architecture in Antirrhinum. Nature 379, 791–797. [DOI] [PubMed] [Google Scholar]
- Bradley, D., Ratcliffe, O.J., Vincent, C., Carpenter, R., and Coen, E.S. (1997). Inflorescence commitment and architecture in Arabidopsis. Science 275, 80–83. [DOI] [PubMed] [Google Scholar]
- Brunet, A., Bonni, A., Zigmond, M.J., Lin, M.Z., Juo, P., Hu, L.S., Anderson, M.J., Arden, K.C., Blenis, J., and Greenberg, M.E.. (1999). Akt promotes cell survival by phosphorylation and inhibiting a Forkhead transcription factor. Cell 96, 857–868. [DOI] [PubMed] [Google Scholar]
- Chang, C.H., and Rubin, G.M. (1997). 14-3-3 positively regulates Ras-mediated signaling in Drosophila. Genes Dev. 11, 1132–1139. [DOI] [PubMed] [Google Scholar]
- Cortez, D., and Elledge, S.J. (2000). Conducting the mitotic symphony. Nature 406, 354–356. [DOI] [PubMed] [Google Scholar]
- Eshed, Y., and Zamir, D. (1995). Introgression line population of Lycopersicon pennellii in the cultivagted tomato enables the identification and fine mapping of yield associated QTL. Genetics 141, 1147–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feilotter, H.E., Hannon, G.J., Ruddell, C.J., and Beach, D. (1994). Construction of an improved host strain for two hybrid screening. Nucleic Acid Res. 22, 1502–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie, C., Borch, J., and Collinge, D.B. (1999). 14-3-3 proteins: Eukariotic regulatory proteins with many functions. Plant Mol. Biol. 40, 545–554. [DOI] [PubMed] [Google Scholar]
- Fry, A.M., and Nigg, E.A. (1995). The NIMA kinase joins forces with Cdc2. Curr. Biol. 5, 1122–1125. [DOI] [PubMed] [Google Scholar]
- Gardner, H.B., and Hedger, G. (1959). Determinateness in the tomato in relation to variety and to application of N-m-tolylphthalamic acid of high concentration. Proc. Am. Soc. Hort. Sci. 73, 323–328. [Google Scholar]
- Giuliano, G., Pichersky, E., Malik, V.S., Timko, M.P., Scolnik, P.A., and Cashmore, A.R. (1988). An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc. Natl. Acad. Sci. USA 85, 7089–7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy, D.K., Hanneman, E., Bunzow, J., Shih, M., Machida, C.A., Bidlack, J.M., and Civelli, O. (1990). Purification, cloning, and tissue distribution of a 23-kDa rat protein isolated by morphine affinity chromatography. Mol. Endocrinol. 4, 1370–1376. [DOI] [PubMed] [Google Scholar]
- Hall, B.E., Yang, S.E., Boriack-Sjodin, P.A., Kurian, J., and Bar-Sagi, D.J. (2001). Structure-based mutagenesis reveals distinct functions for Ras switch 1 and switch 2 in SOS-catalyzed guanin nucleotide exchange. J. Biol. Chem. 276, 27629–27637. [DOI] [PubMed] [Google Scholar]
- Hareven, D., Gutfinger, T., Parnis, A., Eshed, Y., and Lifschitz, E. (1996). The making of a compound leaf: Genetic manipulation of leaf architecture in tomato. Cell 84, 735–744. [DOI] [PubMed] [Google Scholar]
- Harter, K., Kircher, S., Frohnmeyer, H., Krenz, M., Nagy, F., and Schafer, E. (1994). Light-regulated modification and nuclear translocation of cytosolic G-box binding factor in parsley. Plant Cell 6, 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi, R., Krummel, B., and Saiki, P.K. (1988). A general method of in vitro preparation and specific mutagenesis of DNA fragments: Study of protein and DNA interactions. Nucleic Acid Res. 11, 7351–7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky, I., Shukla, V., Ahn, J.H., Dagenais, N., Christensen, S.K., Nguyen, J.T., Chory, J., Harrison, M.J., and Weigel, D. (1999). Activation tagging of the floral inducer FT. Science 286, 1962–1965. [DOI] [PubMed] [Google Scholar]
- Kobayashi, Y., Kaya, H., Goto, K., Iwabuchi, M., and Araki, T. (1999). A pair of related genes with antagonistic roles in mediating flowering signals. Science 286, 1960–1962. [DOI] [PubMed] [Google Scholar]
- Koornneef, M., Hanhart, C.J., and van der Veen, J.H. (1991). A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol. Gen. Genet. 229, 57–66. [DOI] [PubMed] [Google Scholar]
- Li, S., Janosch, P., Tanji, M., Rosenfeld, G.C., Waymire, J.C., Mischak, H., Kolch, W., and Sedivy, J.M. (1995). Regulation of Raf-1 kinase activity by the 14-3-3 family of proteins. EMBO J. 14, 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz, E. (1965). Physiological Differences between Determinate and Indeterminate Tomato Lines. M.Sc. Thesis (Hebrew University, Jerusalem, Israel).
- Lo Conte, L., Chothia, C., and Janin, J. (1999). The atomic structure of protein–protein recognition sites. J. Mol. Biol. 285, 2177–2198. [DOI] [PubMed] [Google Scholar]
- Lopez-Girona, A., Fumari, B., Mondesert, O., and Russel, P. (1999). Nuclear localization of cdc25 is regulated by DNA damage and a 14-3-3 protein. Nature 397, 172–175. [DOI] [PubMed] [Google Scholar]
- Lu, G., Delisle, A.J., de Vetten, N.C., and Ferl, R.J. (1992). Brain proteins in plants: An Arabidopsis homolog to neurotransmitter pathway activators is part of a DNA binding complex. Proc. Natl. Acad. Sci. USA 89, 11490–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, K.P., and Hunter, T. (1995). Evidence for a NIMA-like mitotic pathway in vertebrate cells. Cell 81, 413–424. [DOI] [PubMed] [Google Scholar]
- Lu, K.P., Osmani, S.A., and Means, A.R. (1993). Properties and regulation of the cell cycle–specific NIMA protein kinase of Aspergillus nidulans. J. Biol. Chem. 268, 8769–8776. [PubMed] [Google Scholar]
- Lu, K.P., Hanes, S.D., and Hunter, T. (1996). A human peptidyl-prolyl isomerase essential for regulation of mitosis. Nature 380, 544–547. [DOI] [PubMed] [Google Scholar]
- MacArthur, J.W. (1932). Inherited characters in tomato. I The self pruning habit. J. Hered. 23, 394–395. [Google Scholar]
- McCormick, S. (1991). Transformation of tomato with Agrobacterium tumefaciens. In Tissue Culture Manual B6 (Dordrecht, The Netherlands: Kluwer Academic Publishers), pp. 1–9.
- Menkens, A.E., and Cashmore, A.R. (1994). Isolation and characterization of a fourth Arabidopsis thaliana G-box-binding factor, which has similarities to Fos oncoprotein. Proc. Natl. Acad. Sci. USA 91, 2522–2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkens, A.E., Schindler, U., and Cashmore, A.R. (1995). The G box: A ubiquitous regulatory DNA element in plant bound by the GBF family of bZIP proteins. Trends Biochem. Sci. 20, 506–510. [DOI] [PubMed] [Google Scholar]
- Muslin, A.J., Tanner, J.W., Allen, P.M., and Shaw, A.S. (1996). Interaction of 14-3-3 with signaling proteins is mediated by the recognition of phosphoserine. Cell 84, 889–897. [DOI] [PubMed] [Google Scholar]
- Osmani, A., Pu, R.T., and Morris, N. (1988). Mitotic induction and maintenance by overexpression of a G2-specific gene that encodes a potential protein kinase. Cell 53, 237–244. [DOI] [PubMed] [Google Scholar]
- Osmani, A., O'Donnell, K., Pu, R.T., and Osmani, S.A. (1991). Activation of the nimA protein kinase plays a unique role during mitosis that cannot be bypassed by absence of the bimE checkpoint. EMBO J. 10, 2669–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock, E.F., and Alexander, L.J. (1952). Cauliflower, a new recessive mutation in tomato. Ohio J. Sci. 52, 327–334. [Google Scholar]
- Pan, S., Sehnke, P., Ferl, R.J., and Gurley, W.B. (1999). Specific interactions with TBP and TFIIB in vitro suggest that 14-3-3 proteins may participate in the regulation of transcription when part of a DNA binding complex. Plant Cell 11, 1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitsch, M.C. (1996). ProMod and Swiss-Model: Internet-based tools for automated comparative protein modelling. Biochem. Soc. Trans. 24, 274–279. [DOI] [PubMed] [Google Scholar]
- Peng, C.Y., Graves, P.R., Thoma, R.S., Wu, Z., Shaw, A.S., and Piwnica-Worms, H. (1997). Mitotic and G2 checkpoint control: Regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science 277, 1501–1505. [DOI] [PubMed] [Google Scholar]
- Pnueli, L., Hareven, D., Broday, C., Hurwitz, C., and Lifschitz, E. (1994). The TM5 MADS box gene mediates organ differentiation in the three inner whorls of tomato flowers. Plant Cell 6, 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli, L., Carmel-Goren, L., Hareven, D., Gutfinger, T., Alvarez, J., Ganal, M., Zamir, D., and Lifschitz, E. (1998). The SELF-PRUNING gene of tomato regulates vegetative to reproductive switching of sympodial meristems and is the ortholog of CEN and TFL1. Development 125, 1979–1989. [DOI] [PubMed] [Google Scholar]
- Ratcliffe, O., Amaya, I., Vincent, C., Rothstein, S., Carpenter, E., Coen, E., and Bradley, D. (1998). A common mechanism controls the life cycle and architecture of plants. Development 125, 1609–1615. [DOI] [PubMed] [Google Scholar]
- Rittinger, K., Budman, J., Xu, J., Volinia, S., Cantley, L.C., Smerdon, S.J., Gamblin, S.J., and Yaffe, M.B. (1999). Structural analysis of 14-3-3 phosphopeptide complexes identifies a dual role for the nuclear export signal of 14-3-3 in ligand binding. Mol. Cell 4, 153–166. [DOI] [PubMed] [Google Scholar]
- Roberts, R.L., Mosch, H.-U., and Fink, G.R.. (1997). 14-3-3 proteins are essential for RAS/MAPK cascade signalling during pseudohyphal development in S. cerevisiae. Cell 89, 1055–1065. [DOI] [PubMed] [Google Scholar]
- Rommel, C., Radziwilli, G., Lovric, J., Noeldeke, J., Heinicke, T., Jones, D., Aitken, A., and Moelling, K. (1996). Activated Ras displaces 14-3-3 protein from the amino terminus of c-Raf-1. Oncogene 12, 609–619. [PubMed] [Google Scholar]
- Schoentgen, F., and Jolles, P. (1995). From structure to function: Possible biological roles of a new widespread protein family binding hydrophobic ligands and displaying a nucleotide binding site. FEBS Lett. 369, 22–26. [DOI] [PubMed] [Google Scholar]
- Scolnick, D.M., and Halazonetis, T.D. (2000). Chfr defines a mitotic checkpoint that delays entry into metaphase. Nature 406, 430–435. [DOI] [PubMed] [Google Scholar]
- Serre, L., Vallee, B., Bureaud, N., Schoentgen, F., and Zelwer, C. (1998). Crystal sturucture of the phosphatidylethanolamine-binding protein from bovine: A novel structural class of phospholipid-binding proteins. Structure 6, 1255–1265. [DOI] [PubMed] [Google Scholar]
- Shannon, S., and Meeks-Wagner, D.R. (1991). A mutation in the Arabidopsis TFLI gene affects inflorescence meristem development. Plant Cell 3, 877–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, M., Stukenberg, P.T., Kirschner, M.W., and Lu, K.P. (1998). The essential mitotic peptydyl-prolyl isomerase Pin1 binds and regulates mitosis specific phosphoproteins. Genes Dev. 12, 706–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teubner, F.G., and Wittwer, S.H. (1957). Effect of N-arylphthalamic acid on tomato flower formation. Proc. Am. Soc. Hort. Sci. 69, 343–351. [Google Scholar]
- Wu, L., Osmani, S.A., and Mirabito, P.M. (1998). A role for NIMA in the nuclear localization of cyclin B in Aspergillus nidulans. J. Cell Biol. 141, 1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe, M.B., Rittinger, K., Volinia, S., Caron, P.R., Aitken, A., Leffers, A., Gamblin, H., Smerdon, S.J., and Cantley, L.C. (1997. a). The structural basis for 14-3-3: Phosphopeptide binding specificity. Cell 91, 961–971. [DOI] [PubMed] [Google Scholar]
- Yaffe, M.B., Schutkowski, M., Shen, M., Zhou, X.Z., Stukenberg, P.T., Rahfeld, J., Xu, J., Kuang, J., Kirschner, M.W., Fischer, G., Cantley, L.C., and Lu, K.P. (1997. b). Sequence-specific and phosphorylation-dependent proline isomerization. A potential mitotic regulatory mechanism. Science 278, 1957–1960. [DOI] [PubMed] [Google Scholar]
- Yang, J., Winkler, K., Yoshida, M., and Kornbluth, S. (1999). Maintenance of G2 arrest in the Xenopus ocoyte: A role for 14-3-3 mediated inhibition of Cdc25 nuclear import. EMBO J. 18, 2174–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye, X.S., Xu, G., Fincher, R.R., McGuire, S.L., Osmani, A.H., and Osmani, S.A. (1995). The NIMA protein kinase is hyperphosphorylated and activated downstream of p34cdc2/cyclin B: Coordination of two mitosis promoting kinases. EMBO J. 14, 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager, A.F. (1927). Determinate growth in the tomato. J. Hered. 18, 263–265. [Google Scholar]
- Yeung, K., Seitz, T., Li, S., Janosch, P., McFerran, B., Kaiser, C., Fee, F., Katsanakis, K.D., Rose, D.W., Mischak, H., Sedivy, J.M., and Kolch, W. (1999). Supression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401, 173–177. [DOI] [PubMed] [Google Scholar]
- Yeung, K., Janosch, P., McFerran, B., Rose, D.W., Mischak, H., Sedivy, J.M., and Kolch, W. (2000). Mechanism of suppression of the Raf/MEK/extracellular signal-regulated kinase pathway by the raf kinase inhibitor protein. Mol. Cell Biol. 20, 3079–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung, K., Rose, D.W., Dhillon, A.S., Yaros, D., Gustafsson, M., Chatterjee, D., McFerran, B., Kolch, W., and Sedivy, J.M. (2001). Raf kinase inhibitor protein interacts with NF-κB-inducing kinase and inhibits NF-κB activation. Mol. Cell Biol. 21, 7207–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha, J., Harada, H., Yang, E., Jockel, J., and Korsmeyer, S.J.. (1996). Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-XL. Cell 87, 619–628. [DOI] [PubMed] [Google Scholar]
- Zimmerman, P.W., and Hitchcock, A.E. (1949). TIBA influence flower formation of tomato. Contrib. Boyce Thompson Inst. 15, 359–361. [Google Scholar]
- Zimmerman, P.W., and Wilcoxon, F. (1942). Flowering habit and correlation of organs as modified by TIBA. Contrib. Boyce Thompson Inst. 12, 321–343. [Google Scholar]