Abstract
The Tol-Pal system of gram-negative bacteria is composed of five proteins. TolA, TolQ, and TolR are inner membrane proteins, TolB is a periplasmic protein, and Pal, the peptidoglycan-associated lipoprotein, is anchored to the outer membrane. In this study, the roles of Pal and major lipoprotein Lpp were compared in Escherichia coli. lpp and tol-pal mutations have previously been found to perturb the outer membrane permeability barrier and to cause the release of periplasmic proteins and the formation of outer membrane vesicles. In this study, we showed that the overproduction of Pal is able to restore the outer membrane integrity of an lpp strain but that overproduced Lpp has no effect in a pal strain. Together with the previously reported observation that overproduced TolA complements an lpp but not a pal strain, these results indicate that the cell envelope integrity is efficiently stabilized by an epistatic Tol-Pal system linking inner and outer membranes. The density of Pal was measured and found to be lower than that of Lpp. However, Pal was present in larger amounts compared to TolA and TolR proteins. The oligomeric state of Pal was determined and a new interaction between Pal and Lpp was demonstrated.
The cell envelope of gram-negative bacteria is composed of the inner and outer membranes, and the murein layer within the periplasmic space. The envelope functions as a selective barrier: the lipopolysaccharide (LPS) leaflet prevents the diffusion of toxic compounds through the outer membrane (35) while porins and other specific receptors allow passive or active transport of nutrients. The cell protection is also mediated by efflux systems that pump out toxic compounds to the extracellular medium (32).
The murein layer is the shape-determining component of the cell envelope. It is responsible for the bacterial resistance to osmotic and mechanical stresses. The maintenance of the envelope integrity requires a structural link between the murein layer and other cell wall components. Many inner and outer membrane proteins are bound to the murein. The major lipoprotein Lpp (6, 7) is one of the most abundant outer membrane proteins (32). Its trimerization has been reported (10), and its crystallographic structure has been recently solved (39). About one-third of the major lipoprotein is covalently bound to murein. In the absence of Lpp, or with lpp mutations that affect the covalent attachment to the murein layer, the outer membrane forms outer membrane blebs and cells become hypersensitive to various toxic compounds and release periplasmic proteins to the extracellular medium (42, 48). Other proteins, harboring a peptidoglycan binding sequence (12, 21), appear to interact noncovalently with the murein layer (25). These correspond to some inner membrane proteins, such as MotB, a component of the flagellar motor (3), and various outer membrane proteins, such as OmpA or Pal (12).
Pal belongs to the Tol-Pal system with respect to its interaction with TolB (4) and TolA (8) and its genetic localization (23). The serine residue at position +2 is a sorting signal for the localization of this protein to the outer membrane (16–18). The transport and localization of Pal to the outer membrane require the LolA-B-C-D-E system (28, 29, 44, 47, 49). Pal is anchored to the outer membrane by its amino-terminal N-diacyl glyceride moiety (30) and strongly interacts with the peptidoglycan layer by its carboxy-terminal region (23). The Tol-Pal system consists of five proteins: TolA-Q-R interact with each other via their transmembrane helix in the inner membrane (13, 19, 20, 24), while TolB and Pal form another complex associated with the outer membrane (4, 5, 36). The two complexes are connected by the interaction between Pal and TolA, which requires the proton motive force and TolQ-R proteins (8, 9), and between TolB and TolA (A. Walburger, C. Lazdunski, and Y. Corda, unpublished data). A cytoplasmic protein, YbgC, and a periplasmic protein, YbgF, corresponding, respectively, to the first and last genes of the tol-pal cluster, are also related to the Tol-Pal system (46).
The Tol-Pal system is highly conserved among gram-negative bacteria (41), and mutations that affect each of the tol-pal genes result in outer membrane alterations similar to lpp mutations (2). These effects, the interactions of TolA and TolB with outer membrane porins (14, 37), and the function of TolA in the surface expression of O antigen (15) suggest that the Tol-Pal system is implicated in the maintenance of cell envelope integrity and in the transport of newly synthesized components through the periplasm (27). Thus, the Tol-Pal system could contribute to the outer membrane biogenesis. TolB was also found to interact with Lpp or OmpA in a Pal-dependent manner, indicating that the TolB-Pal complex belongs to the structural network linking murein layer and outer membrane (11).
In this study, we show that Pal lipoprotein plays a critical role in maintaining the cell wall organization. In order to understand the stabilizing effects of Pal and Lpp, the quantification of Pal was determined. In vivo and in vitro cross-linking analyses were performed to define the oligomeric state of Pal and its binding partners.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Strains and plasmids used in this work are listed in Table 1. The pLpp plasmid was constructed by insertion in pT7 of an XbaI-XbaI fragment from a PCR amplification of chromosomal lpp by using 5′-GCGGATCCAGCGTTCGATGCTTC and 5′-AATCTAGAGCGGTAAACGGCAGAC as primers (the XbaI site is underlined and the second XbaI site is present upstream of the Shine-Dalgarno sequence within the PCR DNA fragment). The pPal plasmid was constructed by subcloning a PCR product obtained by the chromosomal amplification of pal and ybgF with 5′-CTGATGGACAGGTCAAATTCC and 5′-CTCAACTGCATATGATTCGCAC as primers in pCR2.1 (Invitrogen). A fragment containing the pal and ybgF genes was obtained by XbaI−BamHI double digestion and thus was cloned in pT7 digested by XbaI−BglII. The resulting plasmid, called pPalYf, was digested by SfiI and BssHII before T4 DNA polymerase action and ligation in order to obtain pPal. All the plasmid constructs were checked by DNA sequencing.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| C600* | thr leuB6 thi fhuA21 supE44 ΔlacU169 | Lab collection |
| C600* tolA | C600* tolA7782 (stop after codon 40) | This study |
| C600* tolB | C600* tolB864 (stop after codon 22) | This study |
| C600* ybgF | C600* ybgF::ΩKm | This study |
| C600* ybgC- tolQRA | C600* ΔybgC-tolQRA::ΩCm | This study |
| C600* lpp | C600* lpp-5508 | This study |
| 1292 | glnV hsdS met gal lacY fhuA | A. Wood |
| JC8056 | 1292 ΔlacU169 | 11 |
| JC8931 | 1292 ompA::TnlacZ | 11 |
| JC8963 | JE5506 lpp-5508 ompA::TnlacZ | 11 |
| KS272 | ΔlacX74 galE galK rpsL | 40 |
| KS303 | KS272 lpp-5508 | 40 |
| JC892 | JC8056 pal-892 (stop after codon 41) | 11 |
| Plasmids | ||
| pT7 | pT7-7 vector, Ampr | 43 |
| pPal | pT7-7 vector, pal Ampr | This study |
| pPalYf | pT7-7 vector, pal-ybgF Ampr | This study |
| pLpp | pT7-7 vector, lpp Ampr | This study |
| pTacompA | pJF118 vector, ompA Ampr | 33 |
Growth conditions.
Routinely, strains were grown aerobically in Luria-Bertani (LB), on minimal-M9 medium, or on agar LB plates. When required, ampicillin (100 μg/ml), chloramphenicol (30 μg/ml), kanamycin (50 μg/ml), or streptomycin (50 μg/ml) was added. For the lpp-ompA strain (JC8963), the medium was supplemented with 10 mM Mg2+.
Cells grown in M9 medium (supplemented with 0.4% glycerol and an amino acids mixture lacking both methionine and cysteine) at 30°C to an A600 of 0.6 were labeled for 10 min with 10 μCi of [35S]methionine/ml and further chased for 10 min with 0.1% cold methionine.
Pal purification.
Native Pal lipoprotein was purified from the lpp-ompA strain carrying pPal under the conditions previously described for the purification of the peptidoglycan-associated lipoprotein from Haemophilus influenzae (50). Pal was extracted at 55°C for 1 h in a 50 mM phosphate buffer-1% Na-deoxycholate, pH 8.0. The detergent was exchanged with 0.08% Triton X-100 by using a PD-10 column (Pharmacia).
Outer membrane permeability assays.
Cells were streaked on LB plates containing 1.5% Torula Yeast RNA (Sigma). The periplasmic RNase I leakage was estimated after overnight incubation by addition of 10% trichloroacetic acid. β-Lactamase activity was monitored in the total soluble cell extract fractions and in the supernatant fractions of cells grown to an A600 of 0.6. The equivalent of a 0.1 A600 sample unit was mixed with 1 ml of chromogenic cephalosporin substrate solution at 25 μg/ml (CENTA; Calbiochem), and the absorbance variation was measured at 410 nm. Electron microscopy analyses and the tolerance for sodium dodecyl sulfate (SDS) and vancomycin were checked as previously described (2, 8).
Membrane preparations.
Labeled cells were suspended to an A600 of 2.5 in 10 mM Tris-HCl (pH 6.8)-30% sucrose-200 μg of lysozyme/ml. After 5 min at room temperature, the same volume of 10 mM Tris-HCl (pH 6.8)-1 mM EDTA was added for 10 min on ice. After five cycles of freeze and thaw and addition of DNase (100 μg/ml) and 10 mM MgCl2, the suspension was centrifuged at 20,000 × g for 30 min and the pellet of membranes was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE).
In vitro and in vivo cross-linking.
In vitro cross-linking experiments were performed with formaldehyde (FA; Merck), ethylene glycol bis(succidimidyl succinate) (EGS; Pierce), or dithiobis(succidimidyl propionate) (DSP; Pierce). Proteins were mixed in a 10 mM sodium phosphate buffer (NaPi), pH 6.3 (for FA and EGS) or 20 mM NaPi (pH 7.0)-150 mM NaCl (for DSP) at a concentration of 10 μM in a total volume of 10 μl, and the mixture was incubated for 15 min at room temperature (FA and EGS) or 37°C (DSP). Formaldehyde (1%), EGS (1 mM in dimethyl sulfoxide), or DSP (1 mM in dimethyl sulfoxide) was added, and the mixture was further incubated for 20 min at room temperature (FA and EGS) or 37°C (DSP). To stop the reaction, Tris-HCl at 10 mM and pH 6.8 (for FA), at 50 mM and pH 7.2 (EGS), or at 20 mM and pH 7.0 (DSP) was added. After 10 min at room temperature, the samples were treated in Laemmli loading buffer without reducing agent, heated at 96°C for 10 min (EGS, DSP, or FA96), or kept at room temperature (FA) and further analyzed by SDS-PAGE and Western blotting.
For serial dilution experiments, the same quantity of protein was mixed in a total volume of 10, 100, or 1,000 μl (corresponding concentrations of 10, 1, or 0.1 μM, respectively). After cross-linking, the volume was adjusted at 1 ml and the samples were precipitated with 10% trichloroacetic acid.
In vivo cross-linking experiments were performed on exponentially growing cells with FA, EGS, or disuccidimidyl tartrate (DST; Pierce). Cells were washed and suspended at an A600 of 0.5 in 10 mM NaPi (pH 6.3) (FA and EGS) or 20 mM NaPi (pH 7.5)-150 mM NaCl (DST) and further incubated for 20 min (FA) or 30 min (EGS and DST) with the addition of FA (1%), EGS (2.5 mM), or DST (5 mM). Cells were treated in loading sample buffer for 15 min at 37°C with the addition of Benzonase (Merck) (FA) or 5 min at 96°C (EGS and DST) and were further analyzed by Western blotting.
Cell surface area measurements.
For the various strains showing visual differences in cell size, measurements were done in order to obtain more precise data of the cell surface area. Fixed cells, stained with AzurII (Merck), were examined using a Zeiss PhoMi III microscope. Images were captured with a video camera (ColorCoolView; Photonic Science) and treated with ImageProPlus software (Media Cybernetics) using the Count and Measure program (size function: length and width). The results were analyzed with Microsoft Excel in order to determine the cell surface area, assuming the shape of the cell as a cylinder with two half-spherical poles.
Miscellaneous.
Anti-Pal (5) and anti-TolB-Pal (4) polyclonal antibodies have been previously described. Anti-OmpA and anti-OmpF antibodies were obtained using standard procedure with purified OmpA and OmpF proteins (generous gifts of F. Pattus and J. Rosenbush, respectively). For OmpF, the protein was heat denatured before rabbit immunization. Standard methods were used for DNA manipulations (38), SDS-PAGE, and electrotransfer onto nitrocellulose (45). After transfer, nitrocellulose membranes were treated with antisera. Secondary antibodies coupled to alkaline phosphatase were revealed using 5-bromo-4-chloro-3-indolylphosphate (BCIP) and nitroblue tetrazolium.
RESULTS AND DISCUSSION
Overexpressed Pal restores outer membrane integrity of lpp strains.
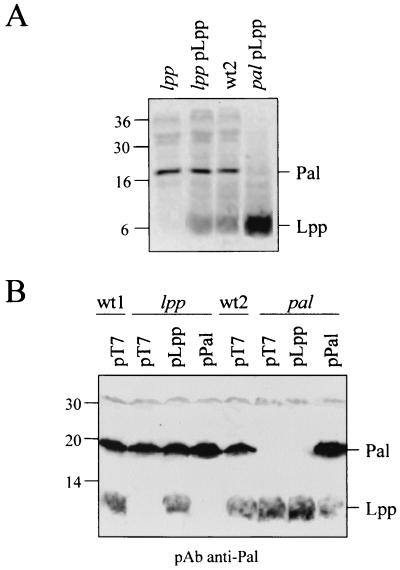
To compare the outer membrane alterations of lpp and pal cells, we investigated several distinct criteria: hypersensitivity to antibiotics and detergents, periplasmic leakage, and outer membrane vesicle formation. lpp cells (KS303) and pal cells (JC892) were analyzed for the periplasmic release of RNase I and β-lactamase, the formation of microvesicles, and SDS and vancomycin susceptibility (Table 2). Both mutants showed similar cell envelope defects as far as the release of β-lactamase, RNase I, and SDS sensitivity are concerned. As previously observed, the pal strain produced fewer vesicles than the lpp mutant (2) and showed a greater sensitivity to vancomycin (8). Plasmids overproducing either Lpp or Pal were constructed. These plasmids, pLpp and pPal, respectively, contain the lpp or pal coding sequence (preceded by their own Shine-Dalgarno sequence) transcribed from the bla promoter of pT7. The localization and the level of expression of these lipoproteins were checked either by immunoprecipitations of methionine-labeled membrane proteins (Fig. 1A) or by immunoblot analyses of total-cell extracts (Fig. 1B) using an anti-Pal antibody which also recognized Lpp and to a lesser extent OmpA. Under constitutive expression in LB medium, we observed that JC892pPal cells produced more Pal than the wild-type (wt) strain, while KS303pLpp cells have similar levels of Lpp to that of lpp+ KS272 cells. Whatever the strain used, the levels of the two lipoproteins increased when they were produced simultaneously from the chromosome and the multicopy plasmid.
TABLE 2.
Outer membrane defects of lpp and pal cells
| Strains | OMVa | RNaseb | β-Lacc | SDSd | Vand |
|---|---|---|---|---|---|
| KS272pT7 | 0 | 0 | 9 | >2 | 160 |
| KS303 pT7 | ++ | ++ | 70 | <0.1 | 100 |
| JC8056 pT7 | 0 | 0 | 11 | >2 | 180 |
| JC892pT7 | + | ++ | 71 | <0.1 | 25 |
| KS303pLpp | 0 | 0 | 25 | 1.0 | 170 |
| KS303pPal | 0 | 0 | 17 | >2 | >200 |
| JC892pLpp | + | ++ | 68 | 0.2 | 25 |
| JC892pPal | 0 | 0 | 28 | >2 | 200 |
Amounts of outer membrane vesicles observed by electron microscopy after negative staining of cells grown on agar plate. ++, many vesicles on all the cells; +, some vesicles on most cells; 0, no vesicle on most cells.
RNase I leakage. The clear zone of RNA hydrolysis was larger (++) or smaller (+) than the size of the colony. 0, no leakage.
β-Lactamase activity present in the supernatant indicated in percent of the total activity (average values from duplicate experiments ± 10%).
SDS (% [wt/vol]) and vancomycin (μg/ml) values correspond to a 50% decrease of cell turbidity measured after 3 h of culture (average values from triplicate experiments ± 10%).
FIG. 1.
Comparison of production levels of Lpp and Pal. lpp+ KS272 (wt1), lpp KS303, pal+ JC8056 (wt2), and pal JC892 cells were used in the presence of the indicated plasmids. (A) Autoradiogram of immunoprecipitated Pal and Lpp from labeled membrane extracts using anti-Pal antibodies. (B) Western blot immunodetection of Pal and Lpp. Prestained (A) and unstained (B) size markers are indicated in kilodaltons.
The cell envelope defects were analyzed in different strains transformed by these plasmids (Table 2). Plasmids carrying pal or lpp were able to complement the corresponding chromosomal mutations. However, when reciprocal complementations were performed, we observed that overproduced Pal stabilizes the lpp strain while the absence of Pal cannot be balanced by overexpressing Lpp. Similarly, the overexpression effect of TolA has previously been found to stabilize the lpp but not the pal cells (8), while overproduction of either Pal or Lpp in a tolA strain had no stabilizing effects (not shown). Thus, the link between the minor inner membrane TolA protein with Pal seems to be functionally as important for cell integrity as the link between an abundant outer membrane protein with the peptidoglycan. Moreover, we showed that overproduced Pal can complement the absence of Lpp and that Pal is specifically required to maintain the outer membrane integrity in association with Tol proteins.
Determination of copy number of Pal in bacterial cells and relation with outer membrane integrity.
Pal was purified from the lpp-ompA strain harboring pPal in order to avoid contamination with major proteins interacting with the peptidoglycan. The number of Pal molecules per cell and per surface area was determined. Previous results indicate that the number of TolA (400 to 800 copies [26]) and TolR (2,000 to 3,000 copies [31]) molecules by cell is relatively low. This contrast with the abundance of Lpp present at about 7 × 105 molecules per cell increases the interest to quantify Pal. The quantitative estimations of Pal were performed by Western blot immunodetection with anti-Pal antibody. Serial dilutions of purified Pal were compared to those from total-cell extracts (not shown). The quantification of Pal was obtained by scanning densitometry of the Western blotting which was further related to the number of cells. We found between 30,000 to 40,000 copies of Pal in wt JC8056 cells and between 8,000 to 10,000 copies in lpp KS303 cells. The amount of overproduced Pal in the pal JC892 strain harboring pPal plasmid was determined by the same method and found to correspond to about 120,000 copies by cell. Statistical microscopic analyses (average of 500 to 800 cells analyzed by bright-field microscopy after fixation and staining with AZURII) indicated that wt JC8056 cells are larger and more elongated than lpp KS303 cells. From the size determinations (mean [± standard deviation]) of JC8056 and KS303 (4.6 [±0.5] μm by 1.1 [±0.1] μm and 1.7 [±0.1] μm by 0.6 [±0.1] μm, respectively), the average cell surface areas were found to correspond to 16.0 and 3.4 μm2, respectively. In the resulting estimation, a similar density of Pal was found in both strains which corresponds to about 2,000 to 3,000 molecules per μm2. Thus, the cell surface density of Pal contrasts with the abundance of Lpp, of about 100,000 copies per μm2 (32). Is the formation of large or small amounts of outer membrane vesicles, found in lpp or pal cells, respectively, related to their presence in the outer membrane? Outer membrane defects do not seem to depend on the abundance of a stabilizing protein since the absence of TolA yielded phenotypic effects similar to those observed with the KS303 (lpp) strain (2). Here, we observed in C600* cells that the deletion of the whole operon ybgC-tolQRA yielded defects similar to tolA deletion. C600*tolB cells formed small amounts of vesicles (as previously found [2]), while C600*ybgF cells did not form any vesicles (not shown). All these stabilizing effects may depend on the interaction of Pal with TolA requiring the whole Tol-Pal system (8. 9).
(ii) Pal interacts with Lpp and OmpA.
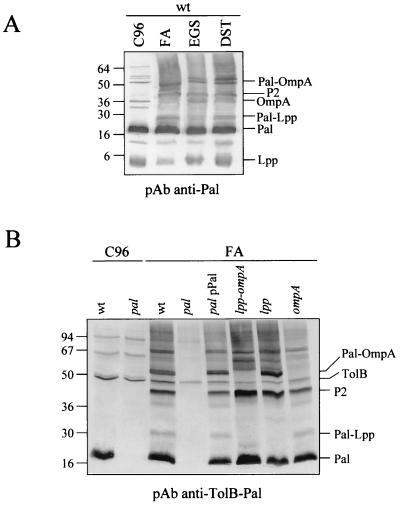
In vivo cross-linking experiments were performed with wt cells by using different cross-linkers to analyze Pal complexes (Fig. 4A). The anti-Pal antibodies which cross-reacted with Lpp, OmpA, and, to a lesser extent, with several unidentified proteins made the identification of the different complexes difficult. To characterize accurately the nature of these complexes, the same experiments were carried out with pal, lpp, or ompA strains by using anti-Pal or anti-TolB-Pal antibodies (4) (Fig. 4B). TolB, Pal, and its dimeric form (P2) were clearly detected in wt and lpp-ompA cells, while in pal cells, only TolB was immunodetected. Using ompA and lpp-ompA cells as a negative control, the Pal-OmpA complex previously described (11, 34) was clearly detected in the wt and lpp strains. Analyzing the cross-linked profiles, a new complex corresponding to Pal-Lpp was detected except in the pal, lpp, and lpp−ompA strains. The Pal-OmpA and Pal-Lpp complexes were also identified using the anti-Pal antibody (not shown). Thus, it appeared that Pal interacts with Lpp.
FIG. 4.
Pal interacts in vivo with Lpp and OmpA. (A) In vivo cross-linking of JC8056 cells (wt) treated with FA, EGS, or DST. (B) In vivo cross-linking of the indicated strains with FA. The antibodies used for immunodetections and the different proteins and complexes are indicated.
The numerous interaction of Pal with outer membrane components, such as OmpA and Lpp, could be attributed to the abundance of these interactants (OmpA is present at about 105 molecules per cell [22]). To check this hypothesis, the complexes obtained after DST cross-linking were heat denatured and analyzed by Western blot immunodetections using anti-Pal, anti-OmpA, and anti-OmpF/C antibodies. The results did not permit any detection of OmpF-C interacting with Pal, while anti-OmpA antibody immunodetected the OmpA-Pal complex (not shown). Since OmpF-C are almost as abundant as OmpA, this result argues in favor of a specificity of the OmpA-Pal complex. Moreover, the overexpression effects of OmpA checked in KS303 transformed with pTacompA plasmid (33) indicated that SDS sensitivity and β-lactamase release were restored to the level of the lpp+ KS272 cells while JC892pTacompA was not complemented. These results indicated that in the presence of Pal, overproduced OmpA is able to complement the lpp strain, as does overproduced Pal, while no complementation occurs in the absence of Pal.
According to the larger amounts of Lpp molecules compared to those of OmpA, the absence of a protein control as abundant as Lpp, and the lack of a functional interaction, the observed Pal-Lpp complex could simply depend on statistical interaction of two molecules present in the outer membrane bilayer. Previous data concerning the sensitivity to colicins indicated that the absence of Pal has no effect on colicin A, E2, and E3 lethal action, while some Pal point mutations confer some resistance (11). Similarly, colicin sensitivity is not affected in lpp cells (2). We further checked the colicin A and E1 sensitivities by spotting serial dilutions on lawns of JC892, JC892pPal, JC892pLpp, KS303, KS303pPal, and KS303pLpp cells. It appears that the only difference reproducibly observed corresponded to a 10-fold increase in tolerance to colicin A of JC892pPal. In all cases, colicin E1 was identically active. Thus, in the presence of Lpp, overproduced Pal interferes with colicin import while in the absence of Lpp, overproduced Pal has no effect. It remains to be understood how the presence of Lpp affects Pal or TolB-Pal complexes, since as previously shown in the absence of Pal, TolB does not interact with Lpp (11). However, our results on the dimerization of Pal and previous data on the trimerization of Lpp established by cross-linking and crystallographic experiments (10, 39) indicated that complexes between Pal dimers, Lpp trimers, TolB, or OmpA should have been detected in our analyses. This was not the case, and we hope to gain more information concerning these complexes using cross-linking and immunoprecipitation techniques with functional tagged Tol-Pal proteins as well as mutated proteins.
(iii) Pal oligomerization and Pal complexes did not require other Tol protein.
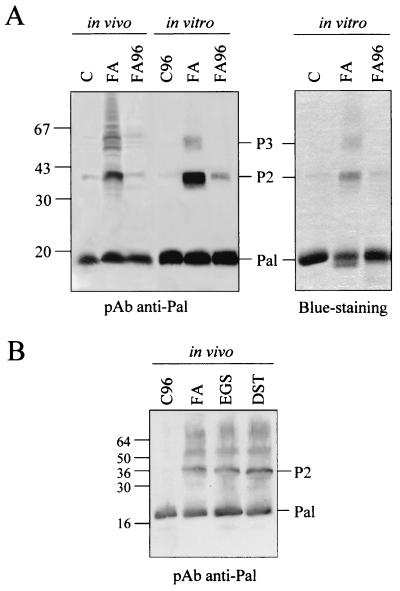
We previously showed that Pal dimerization was independent of Lpp and OmpA proteins (Fig. 2A). Similarly, the potential effect of the Tol proteins on Pal oligomerization and interactions was investigated. In vivo cross-linking with formaldehyde was carried out in ybgC-tolQRA (corresponding to the deletion of the first tol operon), tolB, tolA, and ybgF strains. All these mutations were constructed with a C600 derivative strain (C600*). The absence of either YbgC-TolQ-R-A, TolB, or YbgF proteins was found to have no effect on Pal dimerization or on Pal-Lpp and Pal-OmpA interactions (not shown). Thus, the oligomerization process of the Lpp and Pal lipoproteins is unknown and remains to be elucidated.
FIG. 2.
In vivo and in vitro oligomerization of Pal. In vivo experiments were carried out with the lpp-ompA JC8963 strain. Western blotting using anti-Pal antibodies or Coomassie blue staining are shown. (A) In vivo and in vitro cross-linking with FA cross-linked samples were treated at 37°C (FA) and 96°C (FA96). (B) In vivo cross-linking of lpp-ompA cells with FA, EGS, or DST. Control cells were treated at 37°C (C) and 96°C (C96). Pal multimers (P2 and P3) and size markers (in kilodaltons) are indicated.
FIG. 3.
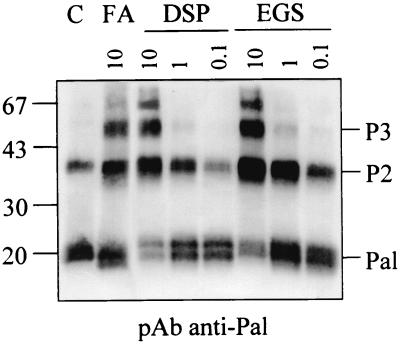
Purified Pal forms dimers. Serial dilutions of native Pal (indicated in μM) were cross-linked with FA, DSP, and EGS, and samples were analyzed by Western blot immunodetection with anti-Pal antibody in the absence of heat denaturation. Size markers and Pal multimers (P2 and P3) are indicated.
Acknowledgments
We thank J. Bonicel for MALDI-TOF experiments, D. Cavard, E. Bouveret, and J. Sturgis for careful reading of the manuscript, and F. Pattus and J. Rosenbush for the generous gifts of purified OmpA and OmpF, respectively.
This work was supported by the CNRS and by a grant from MENRT.
REFERENCES
- 1.Abergel, C., A. Walburger, S. Chenivesse, and C. Lazdunski. 2001. Crystallization and preliminary crystallographic study of the peptidoglycan-associated lipoprotein from Escherichia coli. Acta Crystallogr. 57:317–319. [DOI] [PubMed] [Google Scholar]
- 2.Bernadac, A., M. Gavioli, J. C. Lazzaroni, S. Raina, and R. Lloubès. 1998. Escherichia coli tol-pal mutants form outer membrane vesicles. J. Bacteriol. 180:4872–4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blair, D., D. Kim, and H. Berg. 1991. Mutant MotB proteins in Escherichia coli. J. Bacteriol. 173:4049–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouveret, E., R. Derouiche, A. Rigal, R. Lloubès, C. Lazdunski, and H. Bénédetti. 1995. Peptidoglycan-associated lipoprotein-TolB interaction. J. Biol. Chem. 270:11071–11077. [DOI] [PubMed] [Google Scholar]
- 5.Bouveret, E., H. Bénédetti, A. Rigal, E. Loret, and C. Lazdunski. 1999. In vitro characterization of peptidoglycan-associated lipoprotein (Pal)-peptidoglycan and Pal-TolB interactions. J. Bacteriol. 181:6306–6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braun, V. 1975. Covalent lipoprotein from the outer membrane of E. coli. Biochim. Biophys. Acta 415:335–377. [DOI] [PubMed] [Google Scholar]
- 7.Braun, V., and K. Rehn 1969. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J. Biochem. 10:426–438. [DOI] [PubMed] [Google Scholar]
- 8.Cascales, E., M. Gavioli, J. N. Sturgis, and R. Lloubès. 2000. Proton motive force drives the interaction of the inner membrane TolA and outer membrane pal proteins in Escherichia coli. Mol. Microbiol. 38:904–915. [DOI] [PubMed] [Google Scholar]
- 9.Cascales, E., R. Lloubès, and J. N. Sturgis. 2001. The TolQ-TolR proteins energise TolA and share homologies with the flagellar motor proteins MotA-MotB. Mol. Microbiol. 42:795–807. [DOI] [PubMed] [Google Scholar]
- 10.Choi, D. S., H. Yamada, T. Mizuno, and S. Mizushima 1986. Trimeric structure and localization of the major lipoprotein in the cell surface of E. coli. J. Biol. Chem. 261:8953–8957. [PubMed] [Google Scholar]
- 11.Clavel, T., P. Germon, A. Vianney, R. Portalier, and J. C. Lazzaroni 1998. TolB protein of E. coli K-12 interacts with the outer membrane peptidoglycan-associated proteins Pal, Lpp and OmpA. Mol. Microbiol. 29:359–367. [DOI] [PubMed] [Google Scholar]
- 12.De Mot, R., and J. Vanderleyden. 1994. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both gram-positive and gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol. Microbiol. 12:333–334. [DOI] [PubMed] [Google Scholar]
- 13.Derouiche, R., H. Bénédetti, J. C. Lazzaroni, C. Lazdunski, and R. Lloubès. 1995. Protein complex within E. coli inner membrane. J. Biol. Chem. 270:11078–11084. [DOI] [PubMed] [Google Scholar]
- 14.Derouiche, R., M. Gavioli, H. Bénédetti, A. Prilipov, C. Lazdunski, and R. Lloubès. 1996. TolA central domain interacts with E. coli porins. EMBO J. 15:6408–6415. [PMC free article] [PubMed] [Google Scholar]
- 15.Gaspar, J., J. Thomas, C. Marolda, and M. Valvano. 2000. Surface expression of O-specific lipopolysaccharide in Escherichia coli requires the function of the TolA protein. Mol. Microbiol. 38:262–275. [DOI] [PubMed] [Google Scholar]
- 16.Gennity, J., J. Goldstein, and M. Inouye. 1990. Signal peptide mutants of Escherichia coli. J. Bioenerg. Biomembr. 22:233–269. [DOI] [PubMed] [Google Scholar]
- 17.Gennity, J. M., and M. Inouye. 1991. The protein sequence responsible for lipoprotein membrane localization in Escherichia coli exhibits remarkable specificity. J. Biol. Chem. 266:16458–16464. [PubMed] [Google Scholar]
- 18.Gennity, J. M., and M. Inouye. 1991. Protein secretion in bacteria. Curr. Opin. Biotechnol. 2:661–667. [DOI] [PubMed] [Google Scholar]
- 19.Germon, P., T. Clavel, A. Vianney, R. Portalier, and J.-C. Lazzaroni. 1998. Mutational analyses of the Escherichia coli K-12 TolA N-terminal region and characterization of its TolQ-interacting domain by genetic suppression. J. Bacteriol. 180:6433–6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Journet, L., A. Rigal, C. Lazdunski, and H. Bénédetti. 1999. Role of TolR N-terminal, central, and C-terminal domains in its dimerization and interaction with TolA and TolQ. J. Bacteriol. 181:4476–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koebnik, R. 1995. Proposal for a peptidoglycan-associating alpha-helical motif in the C-terminal regions of some bacterial cell-surface proteins. Mol. Microbiol. 16:1269–1270. [DOI] [PubMed] [Google Scholar]
- 22.Koebnik, R. 2000. Structure and function of bacterial outer membrane proteins: barrels in a nutshell. Mol. Microbiol. 37:239–253. [DOI] [PubMed] [Google Scholar]
- 23.Lazzaroni, J.-C., and R. Portalier. 1992. The excC gene of E. coli K-12 required for cell envelope integrity encodes the peptidoglycan-associated lipoprotein (Pal). Mol. Microbiol. 6:735–742. [DOI] [PubMed] [Google Scholar]
- 24.Lazzaroni, J.-C., A. Vianney, J.-L. Popot, H. Bénédetti, F. Samatey, C. Lazdunski, R. Portalier, and V. Geli. 1995. Transmembrane α-helix interactions are required for the functional assembly of the E. coli Tol complex. J. Mol. Biol. 246:1–7. [DOI] [PubMed] [Google Scholar]
- 25.Leduc, M., K. Ishidate, N. Shakibai, and L. Rothfield. 1992. Interactions of Escherichia coli membrane lipoproteins with the murein sacculus. J. Bacteriol. 174:7982–7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levengood, S., W. Beyer, and R. Webster. 1991. TolA, a membrane protein involved in colicin uptake contains an extended helical region. Proc. Natl. Acad. Sci. USA 88:5939–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lloubès, R., E. Cascales, A. Walburger, A. Bouveret, C. Lazdunski, A. Bernadac, and L. Journet. 2001. The Tol-Pal proteins of the E. coli cell envelope: an energised system required for the outer membrane integrity? Res. Microbiol. 152:523–529. [DOI] [PubMed] [Google Scholar]
- 28.Matsuyama, S., T. Tajima, and H. Tokuda. 1995. A novel periplasmic carrier protein involved in the sorting and transport of Escherichia coli lipoproteins destined for the outer membrane. EMBO J. 14:3365–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuyama, S., N. Yokota, and H. Tokuda. 1997. A novel outer membrane lipoprotein, LolB (HemM), involved in the LolA (p20)-dependent localization of lipoproteins to the outer membrane of Escherichia coli. EMBO J. 16:6947–6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizuno, T. 1979. A novel peptidoglycan-associated lipoprotein found in the cell envelope of Pseudomonas aeruginosa and Escherichia coli. J. Biochem. (Tokyo) 86:991–1000. [DOI] [PubMed] [Google Scholar]
- 31.Muller, M. M., A. Vianney, J. C. Lazzaroni, R. E. Webster, and R. Portalier. 1993. Membrane topology of the Escherichia coliI TolR protein required for cell envelope integrity. J. Bacteriol. 175:6059–6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikaido, H. 1996. Outer membrane, p.29–47. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 33.Nouwen, N., B. De Kruijff, and J. Tommassen. 1996. prlA suppressors in Escherichia coli relieve the proton electrochemical gradient dependency of translocation of wild-type precursors. Proc. Natl. Acad. Sci. USA 93:5953–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palva, E. 1979. Protein interactions in the outer membrane of E. coli. Eur. J. Biochem. 93:495–503. [DOI] [PubMed] [Google Scholar]
- 35.Raetz, C. R. H. 1996. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles, p.1035–1063. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd. ed. American Society for Microbiology, Washington, D.C.
- 36.Ray, M. C., P. Germon, A. Vianney, R. Portalier, and J.-C. Lazzaroni. 2000. Identification by genetic suppression of Escherichia coli TolB residues important for TolB-Pal interaction. J. Bacteriol. 182:821–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rigal, A., E. Bouveret, R. Lloubès, C. Lazdunski, and H. Bénédetti. 1997. The TolB protein interacts with the porins of E. coli. J. Bacteriol. 179:7274–7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. Fritsch, and T. Maniatis. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 39.Shu, W., J. Liu, H. Ji, and M. Lu. 2000. Core structure of the outer membrane lipoprotein from Escherichia coli at 1.9 Å resolution. J. Mol. Biol. 299:1101–1112. [DOI] [PubMed] [Google Scholar]
- 40.Strauch, K., and J. Beckwith. 1988. An E. coli mutation preventing degradation of abnormal periplasmic proteins. Proc. Natl. Acad. Sci. USA 85:1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sturgis, J. N. 2001. Organization and evolution of the tol-pal gene cluster. J. Mol. Microbiol. Biotechnol. 3:113–122. [PubMed] [Google Scholar]
- 42.Suzuki, H., Y. Nishimura, S. Yasuda, A. Nishimura, M. Yamada, and Y. Hirota. 1978. Murein-lipoprotein of E. coli: a protein involved in the stabilization of bacterial cell envelope. Mol. Gen. Genet. 167:1–9. [DOI] [PubMed] [Google Scholar]
- 43.Tabor, S., and C. C. Richardson. 1985. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc. Natl. Acad. Sci. USA 82:1074–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tajima, T., N. Yokota, S. Matsuyama, and H. Tokuda. 1998. Genetic analyses of the in vivo function of LolA, a periplasmic chaperone involved in the outer membrane localization of Escherichia coli lipoproteins. FEBS Lett. 439:51–54. [DOI] [PubMed] [Google Scholar]
- 45.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vianney, A., M. M. Muller, T. Clavel, J. C. Lazzaroni, R. Portalier, R. E. Webster. 1996. Characterization of the tol-pal region of Escherichia coli K-12: translational control of tolR expression by TolQ and identification of a new open reading frame downstream of pal encoding a periplasmic protein. J. Bacteriol. 178:4031–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yakushi, T., K. Masuda, S. Narita, S. Matsuyama, and H. Tokuda. 2000. A new ABC transporter mediating the detachment of lipid-modified proteins from membranes. Nat. Cell Biol. 2:212–218. [DOI] [PubMed] [Google Scholar]
- 48.Yem, D., and H. Wu. 1978. Physiological characterization of an E. coli mutant altered in the structure of murein-lipoprotein. J. Bacteriol. 133:1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yokota, N., T. Koruda, S. Matsuyama, and H. Tokuda. 1999. Characterization of the LolA-LolB system as the general lipoprotein localization mechanism of E. coli. J. Biol. Chem. 274:30995–30999. [DOI] [PubMed] [Google Scholar]
- 50.Zlotnick, G., V. Sanfilippo, J. Mattler, D. Kirkley, R. Boykins, and J. Seid. 1988. Purification and characterization of a peptidoglycan-associated lipoprotein from Haemophilus influenzae. J. Biol. Chem. 263:9790–9794. [PubMed] [Google Scholar]