Abstract
In several diazotrophic species of Proteobacteria, PII signal transduction proteins have been implicated in the regulation of nitrogen fixation in response to NH4+ by several mechanisms. In Azotobacter vinelandii, expression of nifA, encoding the nif-specific activator, is constitutive, and thus, regulation of NifA activity by the flavoprotein NifL appears to be the primary level of nitrogen control. In vitro and genetic evidence suggests that the nitrogen response involves the PII-like GlnK protein and GlnD (uridylyltransferase/uridylyl-removing enzyme), which reversibly uridylylates GlnK in response to nitrogen limitation. Here, the roles of GlnK and GlnK-UMP in A. vinelandii were studied to determine whether the Nif − phenotype of glnD strains was due to an inability to modify GlnK, an effort previously hampered because glnK is an essential gene in this organism. A glnKY51F mutation, encoding an unuridylylatable form of the protein, was stable only in a strain in which glutamine synthetase activity is not inhibited by NH4+, suggesting that GlnK-UMP is required to signal adenylyltransferase/adenylyl-removing enzyme-mediated deadenylylation. glnKY51F strains were significantly impaired for diazotrophic growth and expression of a nifH-lacZ fusion. NifL interacted with GlnK and GlnKY51F in a yeast two-hybrid system. Together, these data are consistent with those obtained from in vitro experiments (Little et al., EMBO J., 19:6041–6050, 2000) and support a model for regulation of NifA activity in which unmodified GlnK stimulates NifL inhibition and uridylylation of GlnK in response to nitrogen limitation prevents this function. This model is distinct from one proposed for the related bacterium Klebsiella pneumoniae, in which unmodified GlnK relieves NifL inhibition instead of stimulating it.
Transcriptional control of nitrogenase synthesis is a common mechanism for the regulation of nitrogen fixation in many diazotrophic bacteria. In the Proteobacteria, regulation of nitrogen fixation genes (nif genes) occurs by controlling the expression and/or activity of the ς54-dependent activator, NifA. Within the γ subgroup of the Proteobacteria, which includes the aerobic diazotroph Azotobacter vinelandii and the facultative diazotroph Klebsiella pneumoniae, a second protein, NifL, controls NifA activity. The nifL gene is cotranscribed with nifA, and together, the two encoded proteins comprise an unusual two-component system in which NifL inhibits NifA activity stoichiometrically by the formation of an inactive complex (17, 21); inactivation of nifL can lead to overexpression of nif genes (6, 32). Inhibition of NifA by NifL occurs in response to increased oxygen tension or fixed-nitrogen excess (13); ADP also increases the inhibitory properties of NifL in vitro, suggesting that NifL may respond to the energy state of the cell (16). Oxygen control of NifL activity involves the oxidation and reduction of a flavin moiety bound to the N-terminal PAS domain of NifL (22, 39). The nitrogen response is thought to involve components of a nitrogen-sensing system homologous to those best characterized in the nondiazotrophic organism Escherichia coli and is less well understood in A. vinelandii.
Nitrogen sensing in E. coli and other bacteria involves the concerted activities of the glutamine sensor, GlnD (uridylyltransferase/uridylyl-removing enzyme), and one or more trimeric signal transduction components generally referred to as PII proteins (2, 36). In E. coli, nitrogen limitation, sensed as an internal glutamine deficiency (24), results in the uridylylation of GlnB (PII) by GlnD and also in expression and uridylylation of a second PII-like protein, GlnK (44, 45). Uridylylation of GlnB and GlnK prevents activation of the phosphatase activity of NtrB by unmodified GlnB, leading to the phosphorylation of NtrC and an Ntr response wherein phosphorylated NtrC (NtrC-P) activates a subset of genes required for growth under these conditions (33). Importantly, the expression of glnK is controlled by NtrC-P, which has implications for using E. coli as a heterologous system to study regulation of NifA activity. Another key target of the Ntr response is the ammonia-assimilatory enzyme glutamine synthetase (GS), encoded by glnA. When PII and GlnK are uridylylated, both the expression, activated by NtrC-P, and activity of GS are high (33, 44). The activity of GS is controlled by GlnE, which catalyzes its reversible adenylylation (inactivation) in response to nitrogen excess (1).
In K. pneumoniae, the expression of nifLA is tightly controlled by NtrC-P, which limits expression to conditions of low fixed-N supply (14). K. pneumoniae, like E. coli, has both GlnB and GlnK, and expression of glnK requires NtrC-P (23). Recently, it was also discovered that GlnK, but not GlnB (PII), modulates NifA activity (20, 23). Moreover, the uridylylation state of GlnK is apparently irrelevant with respect to NifA activity in experiments using a glnKY51N allele, which encodes a protein that cannot be uridylylated, or in a glnD background of E. coli (20) or K. pneumoniae (15). These results indicate that in K. pneumoniae, effective transcriptional control of glnK and nifLA and modulation of NifL inhibition by GlnK are important factors for the regulation of nitrogenase biosynthesis.
There are several differences between regulation of nitrogenase expression in A. vinelandii and in K. pneumoniae. For example, in A. vinelandii, nifLA expression is not activated by NtrC-P, nor does glnK expression appear to be N regulated (8, 31); hence, control of NifA activity appears to be the main mechanism regulating nitrogenase expression in this organism. Early mutagenesis experiments in A. vinelandii identified GlnD, previously named NfrX, as a key regulator of NifA activity because mutations in the 3′ end of glnD resulted in a Nif− phenotype that could be suppressed to Nif+ by deletion of nifL (12, 38). This result suggested that NifA activity depends on the uridylylation of a PII-like protein. Complicating these results, recent experiments have shown that glnD null mutations are conditionally lethal in the wild-type A. vinelandii background because homogenous replacement of the wild-type glnD allele with a null allele does not occur in the absence of extragenic suppressors which elevate GS activity. These stabilizing suppressor mutations include (i) a site-directed mutation of GS (Y407F) preventing adenylylation and (ii) unlinked suppressor mutations (11). The inability to homogenously replace the wild-type glnD allele supports the hypothesis that in cells lacking glnD, GS is always inactivated by adenylylation. Since in A. vinelandii GS is thought to be the sole ammonia-assimilatory pathway and glnA (encoding GS) null mutants cannot be isolated (43), an inability to deadenylylate GS represents a lethal event. One spontaneous glnD null suppressor, gln-71, is probably in the glnE gene, encoding adenylyltransferase/adenylyl-removing enzyme (ATase/AR), because introduction of a wide-host-range plasmid carrying the E. coli glnE gene into gln-71 glnD+ strains results in reestablishment of normal GS regulation (GS adenylylation) by NH4+ (11). Nevertheless, glnD null mutants are Nif−, as were the original nfrX isolates, and fail to uridylylate a PII-like protein, indicating that, unlike in K. pneumoniae, uridylylation of a PII-like protein may be required for NifA activity as well as GS deadenylylation.
Only one PII-like protein has been identified in A. vinelandii, and it is named GlnK because the gene encoding this protein is linked to the methylammonium transporter gene amtB, as occurs in many other bacteria (42). Unfortunately, efforts to demonstrate a role for this protein in A. vinelandii have been hampered because glnK, but not amtB, is an essential gene (31). Therefore GlnK has been studied in vitro and in a heterologous E. coli system (28, 37, 40). In E. coli cells carrying a K. pneumoniae nifH-lacZ reporter and expressing A. vinelandii nifLA in trans, the NifL-NifA system responds to both oxygen and fixed nitrogen (40). In this system, E. coli PII (GlnB) and not GlnK is required for NifL-mediated inhibition in response to fixed nitrogen (37). This result is in contrast to what occurs in K. pneumoniae, where NifL inhibition is relieved and not stimulated by GlnK and not GlnB (20, 23). For the A. vinelandii heterologous system, NtrC also appears to have some role in limiting NifA activity in response to excess fixed N; how this occurs was not examined (37). In vitro, formation of an inactive A. vinelandii NifL-NifA complex, as measured by a decrease in open complex formation at the nifH promoter, is stimulated by E. coli PII and A. vinelandii GlnK in their unuridylylated forms. The corresponding decrease in NifA activity depends on the presence of NifL, demonstrating that NifL, and not NifA, responds to these PII-like proteins. Interestingly, 2-oxoglutarate, at physiological concentrations, was shown to favor dissociation of the NifL-NifA complex, possibly indicating an integrated role for carbon sensing (28).
In this report, the following two questions are addressed: is GlnD-mediated uridylylation of GlnK required for A. vinelandii NifA activity in vivo, and does GlnK potentiate NifL inhibition directly? These questions were addressed in order to compare nitrogen regulation of NifA activity in A. vinelandii to that in K. pneumoniae and also to study the consequence of GlnK uridylylation under natural gene dosage. To this end, the glnD suppressor strain MV72 (gln-71) was used to construct a glnKY51F mutation, encoding a protein that cannot be uridylylated. This mutation was not stable in a wild-type strain, indicating a requirement for GlnK-UMP for deadenylylation of GS. Strains carrying glnKY51F failed to derepress nitrogenase expression in response to nitrogen limitation, and GlnK protein-protein interactions with NifL were examined in a yeast two-hybrid system. Together, results from these experiments support a model for regulation of NifA activity involving GlnK where uridylylation prevents unmodified GlnK from stimulating the inhibitory properties of NifL, a model that is clearly distinct from that proposed for the related K. pneumoniae.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. A. vinelandii strains were grown in Burk’s medium supplemented with 1% sucrose (BS) at 30°C (35). Liquid cultures were grown with shaking in air (200 rpm). Strains carrying gln-71 were grown with 5 or 10 mM ammonium acetate (BSN5 and BSN10); higher concentrations decrease the growth rate, probably due to depletion of glutamate pools, as observed for a glnE strain of Salmonella enterica serovar Typhimurium (26). Growth of A. vinelandii strains for determination of β-galactosidase activity is described below. A. vinelandii was made competent by plating on BS lacking Fe and Mo (competence medium [CM]) (6). Cells were resuspended in liquid CM containing 16 mM MgSO4 prior to transformation. E. coli strains were grown in Luria-Bertani medium (LB) at 37°C with shaking (250 rpm). P1vir transduction was performed as described in reference 30. E. coli was transformed by standard techniques (29). Saccharomyces cerevisiae strains were maintained or grown in or on rich yeast extract-peptone-dextrose medium or minimal SD medium (yeast nitrogen base, dextrose, ammonium sulfate) with shaking for selection of strains carrying plasmids encoding prototrophy markers according to the manufacturer’s protocol book for the Matchmaker two-hybrid system (Clontech, Palo Alto, Calif.). Liquid cultures of yeast were grown at 30°C with shaking (200 rpm). Yeast transformations were conducted by the lithium acetate method (Clontech). Media were supplemented with antibiotics where appropriate: for A. vinelandii, ampicillin (25 μg/ml), chloramphenicol (50 μg/ml), kanamycin (1 μg/ml), streptomycin (1 μg/ml), and tetracycline (5 μg/ml); for E. coli, ampicillin (100 μg/ml), chloramphenicol (30 μg/ml), kanamycin (50 μg/ml), spectinomycin (30 μg/ml), streptomycin (50 μg/ml), and tetracycline (10 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and/or characteristics | Reference or source |
|---|---|---|
| Strains | ||
| A. vinelandii | ||
| UW136 | Rifr derived from strain UW (ATCC 13705) | 7 |
| UW136Ha | nifH1-lacZ/KSS | This work; 46 |
| MV71 | gln-71 glnD::Ω | 11 |
| MV72 | gln-71 (spontaneous glnD1::Ω suppressor) | 11 |
| MV378 | nifL1::KIXX nifH1-lacZ/KSS | 6 |
| MV577 | gln-71 glnKY51F amtB3::Tetr | This work |
| MV577H | gln-71 glnKY51F amtB3::TetrnifH1-lacZ/KSS | This work |
| MV578 | gln-71 amtB3::Tetr | This work |
| MV578H | gln-71 amtB3::TetrnifH1-lacZ/KSS | This work |
| MV579 | gln-71 glnKY51F amtB3::TetrnifL1::KIXX | This work |
| MV579H | gln-71 glnKY51F amtB3::TetrnifL1::KIXX nifH1-lacZ/KSS | This work |
| MV580 | gln-71 amtB3::TetrnifL1::KIXX | This work |
| MV580H | gln-71 amtB3::TetrnifL1::KIXX nifH1-lacZ/KSS | |
| MV581 | gln-71 nifL1::KIXX | This work |
| MV581H | gln-71 nifL1::KIXX nifH1-lacZ/KSS | |
| E. coli | ||
| DH5α | supE44 hsdR17 recA1 thi-1 ΔlacU169 (φ80lacZΔM15) endA1 gyrA96 relA1 | 18 |
| MC4100 | [Δ(lac)U169 araD139 rpsL thi] | 10 |
| CK1005 | MC4100 with (ΔglnB)::Camr | |
| CK1007 | MC4100 with (ΔglnB)::CamrglnK::Spcr | This work |
| CK1008 | MC4100 with (ΔglnB)::CamrglnK::SpcrglnD99::Tn10 | This work |
| NCM1971 | glnK::Spcr | 20 |
| NCM1686 | glnD99::Tn10 | 19 |
| NCM1736 | (ΔglnB)::Camr | 19 |
| S. cerevisiae | ||
| SFY526 | UAS-GAL1-lacZ reporter (His− Trp− Leu−) | Clontech |
| HF7c | UASG 17-mer(X3)CYC1-lacZ UAS-GAL1-HIS3 | Clontech |
| Plasmids | ||
| pAB29 | pTZ19 derivative carrying carrying nifL1::KIXX | 6 |
| pBR322 | Tetr vectors source of Tetr gene | 9 |
| pGAD424b | GAL4 activation domain fusion two-hybrid vector | Clontech |
| pGBT9b | GAL4 binding domain fusion two-hybrid vector | Clontech |
| pJAW2 | Suicide plasmid carrying nifH1-lacZ/KSS | 46 |
| pPR101 | pTZ19 carrying A. vinelandii glnK amtB | 31 |
| pPR102 | pTZ19 carrying A. vinelandii glnKY51F amtB | This work |
| pPR113 | Plasmid encoding GlnKHis6 in pTrcHisB | This work |
| pPR115 | Plasmid encoding GlnKY51FHis6 in pTrcHisB | This work |
| pPR118 | pPR102 with a 1.4-kb EcoRI/AvaI Tetr gene cloned into amtB | This work |
| pTrcHis B | His tag cloning vector | Invitrogen |
| pTD1 | Activation domain control fusion for two-hybrid system | Clontech |
| pVA3 | Binding domain control fusion for two-hybrid system | Clontech |
Strain UW136H is a remake of strain MV101 (46).
For descriptions of two-hybrid fusion constructs, see Materials and Methods.
Construction of a plasmid carrying glnKY51F.
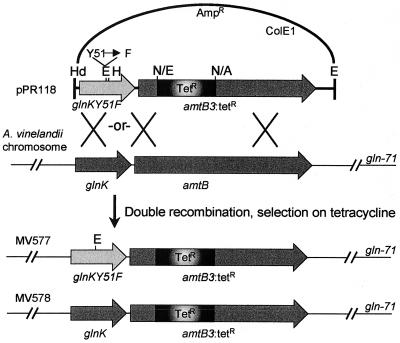
Forward primer glnB F H3 (5′-TCCATAACCACAAGCTTGATAGGGG-3′), which introduces a HindIII site (underlined) for cloning, and reverse mutagenic primer glnB Y-F (5′-GCAGAAAGTCGACTACGAATTCCG-3′), which introduces an EcoRI site at tyrosine 51 (TAC → TTC) and contains a recognition site for HincII (both underlined), were used to amplify the 5′ half of glnK from pPR101 with cloned Pfu polymerase (Stratagene, La Jolla, Calif.). pPR101 was then digested with HindIII, which cuts in the vector, and HincII and ligated to a similarly digested glnKY51F PCR product to give pPR102. The presence of the glnKY51F mutation and the absence of other mutations was verified by sequencing of the pPR102 (LMSE sequencing facility, University of Arizona). Standard recombinant techniques were performed essentially as described by Maniatis et al. (29). To construct pPR118 (Fig. 1), a 1.4-kb EcoRI/AvaI fragment containing the Tetr gene from pBR322 was blunt-ended with the addition of deoxynucleoside triphosphates and the Klenow fragment of DNA polymerase I and cloned into the single NspV site of the amtB gene in pPR102. glnKY51F was verified in this clone by sequencing prior to transformation of A. vinelandii. A clone carrying the tetracycline resistance gene in the forward orientation, pPR118, was isolated to be used for mutagenesis of the A. vinelandii chromosome (Fig. 1).
FIG. 1.
Map of the glnK amtB region and construction of glnKY51F. To construct the glnKY51F mutation, the 5′ half of glnK was amplified with a mutagenic primer that contained the TAC → TTC base change encoding residue 51. The mutation of Y51 prevents uridylylation of the encoded protein and introduces a new EcoRI site. For selection of A. vinelandii transformants carrying the point mutation, a tetracycline resistance gene was introduced into the 5′ end of the downstream amtB gene carried on the plasmid. A. vinelandii mutants were selected on the basis of Tetr and screened for sensitivity to vector-borne Ampr. Of these, glnK was amplified and digested with EcoRI to identify mutants carrying the glnKY51F allele. The genetic structure of the stable gln-71 transformants MV577 and MV578 is shown. A, AvaI; E, EcoRI; H, HincII; Hd, HindIII; N, NspV; P PstI.
Construction of plasmids expressing GlnKHis6 and GlnKY51FHis6.
pPR113 was constructed by PCR amplification of glnK from pPR101 with the primers GLNK FB (5′-GAGATGGATCCGATGAAGCTAGT-3′) and GLNK RK (5′-AATCCACGGTACCCTCCTG-3′), which introduce a BamHI and a KpnI site (underlined), respectively, for directional cloning into the His tag vector pTrcHisB (Invitrogen, Carlsbad, Calif.). The PCR product was first cloned into the T-A cloning vector pGEM-T EASY as an intermediate (Promega, Madison, Wis.). The BamHI/KpnI fragment was then cloned into similarly digested pTrcHisB to give pPR113. pPR115 was constructed in essentially the same manner, except that glnKY51F was amplified from plasmid pPR102 and cloned directly into pTrcHisB following digestion. pPR113 and pPR115 have N-terminal translational fusions that contains six consecutive histidine residues, encoded by pTrcHisB.
Construction of yeast two-hybrid plasmids.
Plasmid vectors used in the yeast two-hybrid assays were pGAD424 (activation domain fusion) and pGBT9 (binding domain fusion) from the Matchmaker two-hybrid system. For cloning of nifL, glnK, or glnKY51F, PCR products were amplified from plasmid or chromosomal template DNA and ligated to pGEM-T EASY, again, as an intermediate step. For each of these genes, the entire reading frame was cloned by the introduction of restriction site compatible with sites in the two-hybrid vectors to generate in-frame fusions. nifL was amplified with the primers NifL.For.ApoI (5′-TCGCCGAATTTCTTGGATCGACGAGG-3′) and NifL.Rev.BamHI (5′-GGTTGGATCCATGGGCATTCAT-3′) from chromosomal DNA as the template; glnK and glnKY51F were amplified from pPR101 and pPR102, respectively. Amplification of glnK or glnKY51F was performed with the primers GlnK.For.EcoRI (5′-TTACACGGAATTCTGTTTCATGAA-3′) and GlnK.R.BamHI (5′-CGGGGGGGATCCTGGGCT-3′). The cloned products were fully or partially digested with EcoRI and cloned into either pGBT9 or pGAD424. All plasmids were verified by sequencing (LMSE sequencing facility, The University of Arizona).
Recombination of glnKY51F onto the A. vinelandii chromosome.
The suicide plasmid pPR118 was used to transform A. vinelandii strains UW136 and MV72 followed by selection on tetracycline. To screen for allelic replacement mutants, Tetr transformants were patched onto BSN plates supplemented with ampicillin. Tetr Amps clones were purified and analyzed for the presence of the glnKY51F mutation by PCR amplification of the ∼350-bp glnK gene using primers K101U (5′-ACTTGAATCGGGATCGTTT-3′) and K101D (5′-GCCTTTGCGCAGCGTCAT-3′) from single colonies as templates followed by EcoRI digestion. nifL1::KIXX strains were constructed by transformation with pAB29 (6) followed by selection on kanamycin and screening for sensitivity to ampicillin.
Construction of nifH1-lacZ/KSS reporter strains.
A. vinelandii strains UW136, MV72, MV577, MV578, and MV579 were transformed with pJAW2 (47) to generate nifH1-lacZ/KSS transcriptional fusions on the chromosome. pJAW2 is a suicide vector carrying the nifH1-lacZ/KSS fusion, which carries a nifH-lacZ fusion followed by the kanamycin and streptomycin marker genes from Tn5. Transformants were selected on streptomycin and screened for sensitivity to chloramphenicol to identify allelic exchange mutations. The fusions in Smr Cams isolates were verified by PCR amplification of a nifH-lacZ product from the chromosome (not shown).
Construction of E. coli strains.
To construct an E. coli strain devoid of both glnB and glnK in strain MC4100, ΔglnB::Camr was moved from strain NCM1736 (19) by P1 transduction to generate CK1005. CK1005 was then transduced with glnK::Spcr with a lysate grown on strain NCM1971 (20) to generate strain CK1007. A triple glnB glnK glnD mutant strain was constructed by transducing glnD99::Tn10 from NCM1686 (19) followed by selection on tetracycline and screening for glutamine bradytrophy.
Growth curve and β-galactosidase assays.
Cells used for growth curves were first grown overnight in 10-ml starter cultures in BSN5. The next morning, the cultures were diluted to 10 Klett units (optical density at 600 nm [OD600] ≈ 0.1) in either BS or BSN10 and grown for 27 h. Samples were read periodically on a Klett-Summerson colorimeter through a no. 54 green filter measuring the OD526 in sidearm flasks, and values were plotted on a semilog graph. For analysis of nifH1-lacZ fusions in A. vinelandii, 10-ml overnight cultures were grown in BS supplemented with 5 mM urea (BSU5) and the appropriate antibiotics. The following day, the cultures were diluted to approximately 20 Klett units in fresh BSN10 for repression or BSU2 for derepression. Five samples were taken throughout a 12-h period, at which time the OD600 was recorded for each sample. Samples were immediately frozen and stored at −20°C. β-Galactosidase activities were determined for each sample in units per milliliter according to the following calculation, which has been modified for an enzyme-linked immunosorbent assay microtiter plate reader: 1,000[OD414 − (1.75 × OD540)]/(Δt × v), where ΔT is the time of the reaction and v is the volume of extract. Each time point was assayed in duplicate, and the experiment was repeated for each strain at least three times (34). The activities for each time point were then plotted against OD600. This analysis indicated that the differential rate of β-galactosidase synthesis was constant throughout the growth curve and therefore the slope of each line was approximated to give rates, which are reported in Table 2. β-Galactosidase assays of yeast strains were performed by the protocols supplied by the manufacturer (Clontech). Briefly, single colonies of strains carrying the appropriate plasmids were inoculated into SD selective media and grown to saturation at 30°C. Cultures were then diluted into 8 ml of yeast extract-peptone-dextrose and grown to an OD600 of 0.5 to 0.8, and 1.5 ml of culture was pelleted, washed, concentrated, and assayed for determination of β-galactosidase activity.
TABLE 2.
Expression of nifH1-lacZ in glnKY51F strains of A. vinelandii
| Strainb | Relevant genotype | β-Galactosidase activity (U/ml/OD600 unit)a
|
|
|---|---|---|---|
| +Nc | −Nd | ||
| UW136H | Wild type | 200 | 16,100 |
| MV72H | gln-71 | 240 | 15,130 |
| MV71H | gln-71 glnD1::Ω | 160 | 160 |
| MV577H | gln-71 amtB3::TetrglnKY51F | 260 | 860 |
| MV578H | gln-71 amtB3::Tetr | 191 | 12,010 |
| MV579H | gln-71 amtB3::TetrglnKY51F nifL1::KIXX | 4,880 | 14,610 |
| MV580H | gln-71 amtB3::TetrnifL1::KIXX | NTe | 5,870 |
| MV581H | gln-71 nifL1::KIXX | 5,260 | 9,570 |
See Materials and Methods for determination of β-galactosidase activities.
All strains carry a nifH1-lacZ/KSS transcriptional fusion (46).
BS medium supplemented with 10 mM ammonium acetate.
BS containing 2 mM urea as a nonrepressive nitrogen source.
NT, not tested.
Preparation of cell extracts, in vitro uridylylation assays, and Western blotting.
For preparation of cell extracts, CK1007 or CK1008 carrying plasmid pPR113 or pPR115 was grown in 25 ml of LB in a 125-ml flask to an OD600 of ∼0.5, at which time the cultures were induced with 500 μM IPTG (isopropyl-β-d-galactopyranoside) for 2 to 3 h. Strain CK1008 grew markedly slower than CK1007; plasmid pPR115 inhibited growth of CK1007, CK1008, and MC4100, with greater inhibition observed after induction. The cells were harvested, washed once in an equal volume of uridylylation buffer (50 mM Tris-Cl, 100 mM KCl, 10 mM MgCl2 [pH 7.5]), and resuspended in 0.5 or 1.0 ml of the same buffer (4). The cell suspensions were lysed with three 5-s bursts on a Branson sonicator on medium-high power followed by three cycles of rapid freezing in liquid N2 and rapid thawing at 37°C. Cell debris was then pelleted by centrifugation at 12,000 × g for 15 min at 4°C, and the soluble proteins were recovered in the supernatant. All extracts were quantitated for protein content by a modified Bradford method (Bio-Rad, Hercules, Calif.). Uridylylation of GlnKHis6 was carried out in vitro basically as described in reference 25. Briefly, 30 μg of soluble protein containing GlnKHis6 or GlnKY51FHis6 was incubated in uridylylation buffer plus 0.2 mM ATP. [α32-P]UTP (1 μl; 800Ci/mmol) and 1 mM 2-oxoglutarate (pH 7.5) were added to initiate labeling of GlnK. The reaction mixture was incubated for 30 min. at 30°C. Duplicate samples were incubated with unlabeled UTP for Western analysis and were processed in parallel. All samples were mixed in 1× sodium dodecyl sulfate sample buffer and boiled for 5 min, and half of the reaction mixture was separated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (5). The gel containing labeled proteins was dried overnight between cellulose sheets and exposed to Kodak Biomax X-ray film for approximately 30 min. The nonradioactive gel was blotted to a nitrocellulose membrane with a semidry transblotter (Bio-Rad) following equilibration in Bjerrum and Schafer-Nielson transfer buffer (48 mM Tris, 39 mM glycine, 20% methanol [pH 9.2]) (Bio-Rad electroblotter manual). Polyclonal E. coli PII rabbit antiserum was applied as the primary antibody. Alkaline phosphatase conjugate was then used to detect the proteins, by using an AP substrate pack for colorimetric development (Bio-Rad).
RESULTS
Uridylylation of GlnK is essential for growth of wild-type strain UW136.
One hypothesis for the lethality of glnD null mutations in A. vinelandii is that uridylylation of GlnK is required for deadenylylation of GS. Inactivation of GS in A. vinelandii by adenylylation would be lethal, as are glnA null mutations in this organism, attributed to the lack of an alternate ammonia-assimilatory mechanism and an inability to transport glutamine (43). This is apparently the case, since suppressors of glnD mutations (i.e., gln-71) resemble glnE strains of other organisms, and E. coli glnE complements this suppressor strain for restoration of normal regulation of GS activity by NH4+ (11). Therefore, attempts were made to construct glnK null mutations in a gln-71 strain (data not shown). These attempts failed, which indicates that GlnK is essential for at least two functions in A. vinelandii: deadenylylation of GS and another, unknown function. Nevertheless, if the second essential function of GlnK does not require the uridylylated form but only the unmodified form, then it might be possible to construct glnK zmutations altered only at the site of uridylylation. This protein would perform the functions of the unmodified form but lack the activities of GlnK-UMP, including those that may include regulation of NifA activity. To test this hypothesis, a glnKY51F mutation was constructed by elongation of a mutagenic primer (see Materials and Methods). The point mutation at Y51 of glnK created a new EcoRI site, which was used to screen for the presence of the mutation. To select for A. vinelandii strains carrying this mutation, a Tetr cassette was cloned into the 5′ end of the adjacent amtB gene to give pPR118 (Fig. 1). Previous studies in this organism, K. pneumoniae, and heterologous E. coli systems have determined that amtB mutations have little or no effect on the regulation of nitrogenase expression (23, 31, 37). Therefore, we expected these two mutations together to reflect the effects of the glnKY51F allele. However, since the complete function of AmtB has not been defined, it cannot be ruled out that loss of amtB may cause differences in nif expression in response to ammonium in the growth medium (41). Therefore, glnK+ strains carrying amtB3::Tetr were examined in parallel.
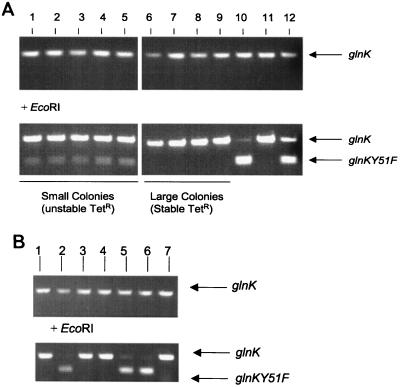
To place the glnKY51F mutation on the chromosome, suicide plasmid pPR118 was used to transform the wild-type A. vinelandii strain UW136. Transformation gave rise to small and large Tetr Amps (allelic exchange mutants) colony types, suggesting that the glnKY51F mutation, perhaps present in the smaller of the two colony types, might be unstable or have a dominant negative effect on colony growth, since amtB mutations do not impair growth on the selective medium (31). To determine if the glnKY51F mutation had recombined into the chromosome along with amtB3::Tetr, glnK was PCR amplified from several transformants. Following amplification, the PCR products were digested with EcoRI, which cuts the glnKY51F allele in half, while glnk+ is EcoRI resistant. This analysis revealed that cells in the larger of the two colony types, as hypothesized, were glnK+ (Fig. 2A, lanes 6 to 9). The smaller colonies contained both alleles, demonstrating an inability of glnKY51F to homogeneously replace glnK in these cells (lanes 1 to 5) and consistent with other presumed lethal mutations in A. vinelandii reported by this and other laboratories (11, 31, 43, 49). The controls indicate that the enzyme digestion was specific for glnKY51F and was nearly complete (lanes 10 and 11). Lane 12 contains glnK amplification products from a Tetr Ampr transformant carrying and integrated copy of pPR118. When these products are digested, the concentration of the smaller glnKY51F band appears to be higher than in the unstable small-colony transformants. This result indicates that the number of copies of the glnKY51F-containing chromosome in the unstable colonies may be lower than that obtained by integrating a second copy of glnK on the chromosome by single recombination.
FIG. 2.
PCR analysis of glnKY51F amtB3::Tet transformants. (A) Large- and small-colony Tetr Amps transformants of wild-type strain UW136. (Top) amplified PCR products; (bottom) products after EcoRI digestion. EcoRI cleaves glnKY51F in half, while glnK is EcoRI resistant. Lanes: 1 to 5, small-colony transformants; 6 to 9, large-colony isolates; 10, glnKY51F PCR product amplified from plasmid pPR118; 11, glnK amplified from plasmid pPR101. 12, Ampr Tetr isolate resulting from integration of plasmid pPR118 carrying both alleles in tandem. (B) Transformation of glnD1::Ω suppressor strain MV72. Lanes: 1 to 5, Tetr Amps isolates; 6, glnKY51F amplified from plasmid pPR118; 7, wild-type glnK from pPR101.
In cases of unstable alleles, the drug resistance carried on the drug cassette is lost when the selective pressure for the mutated copy is removed. Therefore, UW136 glnKY51F transformants were tested for stability of the tetracycline resistance marker by plating on BSN10 in the absence of tetracycline followed by reculturing on tetracycline-containing medium. This experiment revealed that the antibiotic resistance in strains carrying glnKY51F amtB3::Tetr was unstable in the wild-type UW136 background; cells from the small colonies did not grow when recultured on tetracycline, in contrast to the large glnk+ transformants. These analyses indicate that the glnKY51F mutation is lethal in the wild-type background.
Strains carrying the gln-71 suppressor mutation stabilize glnKY51F.
Recent work on the glnD gene of A. vinelandii revealed that null mutations, where the 5′ or central region is removed or replaced by gene cassettes (Ω or KIXX), are lethal and cannot be stabilized in the wild-type background. One of these alleles (glnD1::Ω) was stabilized by a suppressor mutation, gln-71, identified after spontaneous appearance of a large, stably antibiotic-resistant (Spcr) colony, in which GS activity is not regulated by ammonium. The gln-71 mutation is apparently in the glnE gene, encoding ATase/AR, because GS activity becomes regulated in MV72 (a glnD+ derivative of MV71 carrying only gln-71) (i.e., is inactivated by ammonium) after introduction of a plasmid carrying the E. coli glnE gene (11). To determine whether the glnKY51F allele could be stabilized by the gln-71 suppressor, MV72 was transformed with pPR118. Transformants were selected on BSN5 with tetracycline, and all were of a uniform large colony size. About 60% of the Amps Tetr transformants carried only the glnKY51F allele; the others were glnk+, as determined by PCR amplification followed by EcoRI digestion of the PCR products (Fig. 2B, lanes 1 to 5). In contrast to the wild-type A. vinelandii pPR118 transformants, none of the MV72 transformants carried both the mutated and wild-type glnK alleles. In addition, Tetr in the glnKY51F amtB3::Tetr transformants was stable and not lost after subculturing on antibiotic-free medium (data not shown). One gln-71 glnKY51F amtB3::Tetr transformant was named MV577, and one corresponding glnk+ transformant was named MV578 (Fig. 1). These results support the hypothesis that GlnK-UMP is required for control of GS activity, almost certainly by signaling deadenylylation of GS by ATase/AR. Importantly, they also indicate that MV72 could probably be used as a host strain to study the role of GlnK in the regulation of NifA activity in A. vinelandii.
glnKY51F encodes a protein which cannot be uridylylated.
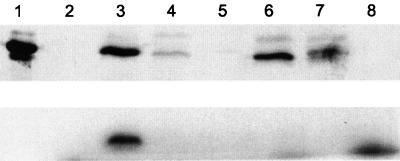
To test whether the GlnKY51F protein could be modified by uridylylation, glnKY51F and wild-type glnK were expressed in E. coli from plasmids pPR113 and pPR115 (see Materials and Methods). These two plasmids express the complete GlnK or GlnKY51F proteins fused to a six-residue N-terminal His tag. Since E. coli expresses GlnB and GlnK which can be uridylylated, a strain was constructed that lacked both glnB and glnK (CK1007) or glnB glnK and glnD (CK1008) (see Materials and Methods) (Table 1). For the uridylylation experiments, strains CK1007 and CK1008 carrying either pPR113 or pPR115 were grown to mid-log phase in LB or LB plus glutamine (100 μg/ml), and expression of GlnK or GlnKHis6 was induced from the plasmid with 0.5 mM IPTG. In extracts, GlnKHis6 could be uridylylated in a GlnD-dependent fashion while GlnKY51FHis6 could not (Fig. 3, bottom). Western blotting of the extracts used in the uridylylation experiments indicated that both plasmids expressed stable proteins which cross-reacted with E. coli PII antisera (Fig. 3, top) (gift from W. vanHeeswijk). In the upper panel of Fig. 3 it is also evident that GlnKY51F is not detected at the same level as GlnK. This may be because the level of expression from pPR115 is lower or that the Y51F mutation disrupts a region of important antigenicity.
FIG. 3.
Western analysis and uridylylation of A. vinelandii GlnK and GlnKY51F in E. coli. (Top) Western blot of E. coli extracts incubated with 2-oxoglutarate and UTP for 30 min. (Bottom) Autoradiograph of the same extracts labeled with [α32-P]UTP. Lanes: 1, purified GlnKHis6 as a size standard; 2, CK1007 (glnB glnK); 3, CK1007(pPR113); 4, CK1007(pPR115); 5, CK1008 (glnB glnK glnD); 6, CK1008(pPR113); 7, CK1008(pPR115); 8, no protein.
Uridylylated GlnK is required for growth in N-free media and for NifA activity.
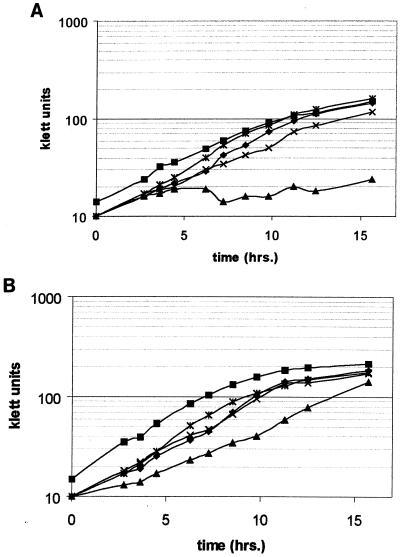
MV577 (glnKY51F amtB3::Tetr gln-71) formed only very small colonies on N-free medium, indicating that the glnKY51F mutation affected nitrogen fixation and/or assimilation (data not shown). Growth rates were determined for strains MV577, MV578, and MV579 and control strains in order to quantitate the effects of the glnKY51F mutation on growth in the presence and absence of fixed nitrogen (Fig. 4). In these experiments, the doubling time of MV577 was greater than 11 h in N-free media. MV578, the corresponding glnk+ strain, doubled in about 2.5 h (Fig. 4A). An increase in the growth rate of MV577 was observed with the addition of 10 mM ammonium acetate to the cultures (Fig. 4B), although there was no increase in the rate of MV578, which may indicate effects of GlnKY51F on other cellular functions in N-rich medium. Nonetheless, the glnKY51F mutation severely impaired growth under diazotrophic conditions. Also in these experiments, a nifL mutation was epistatic to the glnKY51F mutation (MV579). This result is consistent with the hypothesis that the unmodified GlnK positively influences NifL inhibition and GlnK does not effect nitrogenase activity per se, as measured by diazotrophic growth.
FIG. 4.
Growth of glnKY51F strains in BS. (A) N-free growth; (B) growth with the addition of 10 mM ammonium acetate. ▪, UW136 (wild-type strain); ⧫, MV72 (gln-71); ▴, MV577 (gln-71 glnKY51F amtB3::Tetr); ×, MV578 (gln-71 amtB3::Tetr); (×|), MV579 (gln-71 glnKY51F amtB3::Tetr nifL1:: KIXX).
To examine the effect of the glnKY51F mutation on NifA activity, nifH1-lacZ reporter strains were constructed (Table 1) and the differential rates of β-galactosidase synthesis were determined (see Materials and Methods). These data indicate that the gln-71 suppressor mutation, used to stabilize the glnKY51F mutation, had little effect on nifH expression under derepressed conditions (MV72H), as did the combined gln-71 and amtB3::Tetr mutations in strain MV578H. Therefore, any effects in the isogenic glnKY51F strains are a consequence of the glnK mutation and not other mutations in these strains. For comparison, a nifH1-lacZ fusion in MV71 (glnD1::Ω gln-71) was constructed and assayed. Compared to MV578H (glnK+), strain MV577H (glnKY51F) expressed about 5% of the amount of β-galactosidase under derepressing conditions but still more than the glnD strain (compare MV577H and MV71H), indicating that uridylylated GlnK is required for full activation of the nifH promoter in vivo (Table 2).
To determine if the effect of GlnK on NifA activity was mediated through NifL, nifL in MV577 was disrupted with a KIXX cassette encoding kanamycin resistance to give strain MV579 and the corresponding nifH1-lacZ reporter strain, MV579H. Both growth on N-free medium (Fig. 4A) and nifH1-lacZ expression (Table 2) were restored to wild-type levels under derepressing conditions by mutation of nifL, and as observed with other nifL strains, expression of nifH1-lacZ was not fully repressed in the presence of NH4+ (Table 2) (6). As controls, nifL1::KIXX was introduced into strains MV72H and MV578H for comparison (MV580H and MV581H). Taken together, these data indicate that unmodified GlnK negatively regulates NifA activity by a mechanism involving NifL.
GlnK and GlnKY51F interact with NifL in a yeast two-hybrid assay.
To assess if GlnK interacts directly with NifL, translational fusions to the GAL4 DNA binding and activation domains were made using nifL, glnK, and glnKY51F. The resulting plasmids and pairs of plasmids were used to transform yeast strain SFY526, carrying a lacZ reporter downstream of the GAL1 upstream activation sequence (Matchmaker system). In this system, activation of the reporter is dependent on the fusion proteins coming into proximity long enough to activate transcription, a function that is dependent on interaction of the fused target proteins. The results indicate that none of the plasmids carrying fusions activated expression when paired with either vector alone; in these strains, β-galactosidase activity was less than 2 U (Table 3). As a positive control, p53 and simian virus 40 large T antigen (supplied with the kit) together activated transcription sufficiently to give ∼100 β-galactosidase units. In test assays, GlnK and NifL interacted in both vector combinations (16 to 49 β-galactosidase units). GlnKY51F interacted with NifL, but only when NifL was fused to the GAL4 DNA-binding domain (Table 3). These results indicate that NifL and unuridylylated GlnK may physically interact in vivo, demonstrating a mechanism for activating the inhibitory properties of NifL in response to nitrogen sufficiency.
TABLE 3.
GlnK interactions in a yeast two-hybrid assay
| Straina | Fusionb
|
β-Galactosidase activity (U/ml/OD600 unit)c | |
|---|---|---|---|
| BD | AD | ||
| S122de | p53 | SV40 large T-antigen | 110 |
| S112a | NifL | GlnK | 18 |
| S113e | GlnK | NifL | 49 |
| S114e | NifL | GlnKY51F | 16 |
| S124 | GlnKY51F | NifL | <2 |
| S103 | Vector only | Vector only | <2 |
| S123 | GlnK | Vector only | <2 |
| S125 | GlnKY51F | Vector only | <2 |
| S132 | Vector only | NifL | <2 |
| S134 | NifL | Vector only | <2 |
| S135 | Vector only | GlnK | <2 |
| S136 | Vector only | GlnKY51F | <2 |
All strains are derivatives of yeast strain SFY526 carrying two plasmids.
BD, fusion to GAL4 DNA-binding domain; AD, fusion to GAL4 transcriptional activation domain.
See Materials and Methods for determination of β-galactosidase activities.
Strain S122 carries plasmid pVA3 and pTD1 from the Clontech kit as positive controls.
This strain also gives sufficient expression of a GAL4-his reporter in host strain HF7c to allow growth on His− DO (Drop Out) medium (Clontech, Palo Alto, Calif.).
DISCUSSION
GlnK-UMP is essential for activation of GS in A. vinelandii.
In this work, we proposed that gln-71, a likely allele of glnE, would stabilize glnKY51F, as it did glnD1::Ω, because either glnD or a glnKY51F mutation would create a state in which GS would be irreversibly inactivated by adenylylation, if GlnK positively influences the adenylylation of GS (11). The allelic instability of these mutations mutation, due to the effects on GS, might be expected because A. vinelandii does not transport glutamine and lacks a glutamate dehydrogenase activity as an alternate ammonia-assimilatory pathway (43). The gln-71 mutation did stabilize the glnKY51F allele, suggesting a dependence on the uridylylated form of GlnK for deadenylylation of GS. However, the effects of the glnKY51F are probably not restricted to those of GS, because a significant decrease in growth rate of the glnKY51F strain (MV577) over the glnk+ strain (MV578) was noted when fixed N was present in the culture medium (Fig. 4B). This result illustrates the complexity of the mutation and suggests the possible occurrence of other GlnK targets in A. vinelandii and in other organisms.
To determine whether the only reason for the lethality of glnK mutations was this requirement, the null allele glnK::KIXX (31) was used to transform MV72 (gln-71). Kanr Amps transformants were unstable (high-frequency loss of Kanr after growth without kanamycin), indicating that GlnK in its unmodified form is required for an as-yet-unidentified function in A. vinelandii. The reason why glnK null mutants cannot be isolated remains unknown, although A. vinelandii is well suited for a study that may reveal potentially new regulatory targets for this class of proteins, possibly involved in carbon or energy metabolism or DNA processing, as may occur in Rhodobacter capsulatus, where new PII targets, including dinitrogenase reductase ADP-ribosyl transferase (DRAT), have been identified by using a yeast two-hybrid system (Werner Klipp, personal communication). In these experiments, as in other two-hybrid screens, caution should be used before any conclusions can be made concerning the validity of these new targets, and each will have to be verified in vivo or in vitro. On a positive note, however, an interaction with DRAT in this organism might be predicted from genetic evidence in the related organism Rhodospirillum rubrum (48).
GlnKY51F cannot relieve the inhibitory properties of NifL.
An advantage of these in vivo experiments over other approaches is that in assays for NifA activity, gene expression was limited by both the chromosomal location and the preservation of native promoters for the regulatory proteins. These results should therefore reflect natural changes in nitrogenase expression with respect to growth phase and fixed nitrogen content without the ambiguity that might arise from the use of heterologous or in vitro systems or highly expressed promoters. The most significant finding of this work is that, in contrast to what occurs in K. pneumoniae, uridylylation of A. vinelandii GlnK is apparently required for relief of NifL inhibition of NifA activity. However, this requirement is not absolute because significant nifH-lacZ expression was detected with strain MV577H (glnKY51F) under N-limited conditions. The residual activity might indicate that GlnKY51F may not form as tight a complex with NifL as does unmodified wild-type GlnK, such that NifL inhibition can be partially relieved by a reduction in fixed nitrogen, perhaps by the binding of 2-oxoglutarate (28) or other mechanisms affecting GlnK activity.
As has been proposed for A. vinelandii and other organisms which probably have only one PII-like protein, the composition of A. vinelandii GlnK may be an amalgam of residues such that a single protein can function with all regulatory targets (2). In light of this, it is also not surprising that while GlnK of A. vinelandii and GlnK of E. coli are more similar (83% identity) than are A. vinelandii GlnK and E. coli GlnB (75% identity), the T-loop region of A. vinelandii GlnK containing Y51 is more characteristic of GlnB proteins (3). This may be why E. coli GlnB and A. vinelandii GlnK apparently interact with NifL while E. coli GlnK does not (28). If GlnK is an essential protein in A. vinelandii, its rapid uridylylation in response to nitrogen flux mediates protein activities and would rely less on changes in the level of GlnK by transcriptional control. This is consistent with the observation that neither glnA (encoding GS) nor nifL gene expression is regulated by NH4+ in A. vinelandii (8, 43). In contrast, K. pneumoniae harbors two PII-like proteins, probably because they provide a selective advantage over a single protein. In this organism, PII (GlnB), whose expression is not regulated by nitrogen status, may be specific for targets involved in more subtle environmental tuning. GlnK, on the other hand, may be specific to nitrogen fixation and/or severe N starvation responses. In this scenario, it would seem sufficient to express glnK in the absence of combined nitrogen to activate NifA, which has been suggested for K. pneumoniae (20, 23) and for E. coli (44).
Unmodified GlnK interacts with NifL in a yeast two-hybrid assay.
In this work it was proposed that A. vinelandii GlnK might exert its effects directly on the NifL. While a GlnK NifL-NifA interaction has been observed in vitro (28), it was important to study the interaction of the proteins in vivo and to determine which protein GlnK might target. In the yeast two-hybrid system, both GlnK and GlnKY51F interacted significantly with A. vinelandii NifL; that NifL and NifA interact in yeast was shown previously (27). The possibility that GlnK interacts with NifA as well could not be ruled out. This, however, seems unlikely based on in vitro data (28). Nevertheless, the demonstration of interaction between GlnK and NifL is further evidence that these two proteins interact in such a way that GlnK stabilizes the NifL-NifA nonactivating complex or promotes an inhibitory conformation of NifL and uridylylation prevents either function.
Since no glnK null mutants of this organism are available, it is difficult to predict what may be the result of loss of GlnK with respect to NifA activity and to other cellular functions in A. vinelandii. However, experiments with E. coli indicate that NifL is less inhibitory to NifA activity in the absence of both GlnB and GlnK than in their presence (37). This suggests that in A. vinelandii, GlnK may act solely as a negative regulator of NifA activity by stimulating the inhibitory properties of NifL and that in its absence, nif gene expression would occur constitutively, regardless of N status. It will be important to learn if interaction of GlnK with NifL is dependent on the uridylylation state of GlnK and which residues are important for such an interaction. This question can be addressed either in vitro or by using a two-hybrid approach. It is also of interest to discover new regulatory targets for A. vinelandii GlnK. Finding these targets may explain the apparent essentiality of this protein and have implications for all other organisms whose genomes contain one or more homologs of this interesting group of signal transduction proteins.
Acknowledgments
We thank Luhong He and Sydney Kustu for E. coli strains and Wally van Heeswijk for the generous gift of PII antibody. We also thank Stephen Billington and B. Helen Jost for a critical reading of the manuscript and Stefani Gilbert for technical assistance and critical comments.
This work was supported by a grant from USDA-NRI (95-37305-2067) to C.K.
REFERENCES
- 1.Adler, S. P., D. Purich, and E. R. Stadtman. 1975. Cascade control of Escherichia coli glutamine synthetase: properties of the PII regulatory protein and the uridylyltransferase-uridylyl removing enzyme. J. Biol. Chem. 250:6264–6272. [PubMed] [Google Scholar]
- 2.Arcondeguy, T., R. Jack, and M. Merrick. 2001. P(II) signal transduction proteins, pivotal players in microbial nitrogen control. Microbiol. Mol. Biol. Rev. 65:80–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arcondeguy, T., D. Lawson, and M. Merrick. 2000. Two residues in the T-loop of Klebsiella pneumoniae GlnK determine NifL-dependent nitrogen control of nif gene expression. J. Biol. Chem. 275:38452–38456. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson, M. R., E. S. Kamberov, R. L. Weiss, and A. J. Ninfa. 1994. Reversible uridylylation of the Escherichia coli PII signal transduction protein regulates its ability to stimulate the dephosphorylation of the transcription factor nitrogen regulator I (NRI or NtrC). J. Biol. Chem. 269:28288–28293. [PubMed] [Google Scholar]
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, K. Struhl, P. Wang-Iverson, and S. G. Bonitz. 1989. Short protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 6.Bali, A., G. Blanco, S. Hill, and C. Kennedy. 1992. Excretion of ammonium by a nifL mutant of Azotobacter vinelandii fixing nitrogen. Appl. Environ. Microbiol. 58:1711–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop, P. E., and W. J. Brill. 1977. Genetic analysis of Azotobacter vinelandii mutant strains unable to fix nitrogen. J. Bacteriol. 130:954–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco, G., M. Drummond, P. Woodley, and C. Kennedy. 1993. Sequence and molecular analysis of the nifL gene of Azotobacter vinelandii. Mol. Microbiol. 9:869–879. [DOI] [PubMed] [Google Scholar]
- 9.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95–113. [PubMed] [Google Scholar]
- 10.Casadaban, M. J. 1976. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J. Mol. Biol. 104:541–555. [DOI] [PubMed] [Google Scholar]
- 11.Colnaghi, R., P. Rudnick, L. He, A. Green, D. Yan, E. Larson, and C. Kennedy. 2001. Lethality of glnD null mutations in Azotobacter vinelandii is suppressible by prevention of glutamine synthetase adenylylation. Microbiology 147:1267–1276. [DOI] [PubMed] [Google Scholar]
- 12.Contreras, A., M. Drummond, A. Bali, G. Blanco, E. Garcia, G. Bush, C. Kennedy, and M. Merrick. 1991. The product of the nitrogen fixation regulatory gene nfrX of Azotobacter vinelandii is functionally and structurally homologous to the uridylyltransferase encoded by glnD in enteric bacteria. J. Bacteriol. 173:7741–7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon, R. 1998. The oxygen-responsive NIFL-NIFA complex: a novel two-component regulatory system controlling nitrogenase synthesis in gamma-proteobacteria. Arch. Microbiol. 169:371–380. [DOI] [PubMed] [Google Scholar]
- 14.Drummond, M., J. Clements, M. Merrick, and R. Dixon. 1983. Positive control and autogenous regulation of the nifLA promoter in Klebsiella pneumoniae. Nature 301:302–307. [DOI] [PubMed] [Google Scholar]
- 15.Edwards, R., and M. Merrick. 1995. The role of uridylyltransferase in the control of Klebsiella pneumoniae nif gene regulation. Mol. Gen. Genet. 247:189–198. [DOI] [PubMed] [Google Scholar]
- 16.Eydmann, T., E. Soderback, T. Jones, S. Hill, S. Austin, and R. Dixon. 1995. Transcriptional activation of the nitrogenase promoter in vitro: adenosine nucleotides are required for inhibition of NIFA activity by NIFL. J. Bacteriol. 177:1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govantes, F., J. A. Molina-Lopez, and E. Santero. 1996. Mechanism of coordinated synthesis of the antagonistic regulatory proteins NifL and NifA of Klebsiella pneumoniae. J. Bacteriol. 178:6817–6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580. [DOI] [PubMed] [Google Scholar]
- 19.He, L., E. Soupene, and S. Kustu. 1997. NtrC is required for control of Klebsiella pneumoniae NifL activity. J. Bacteriol. 179:7446–7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He, L., E. Soupene, A. Ninfa, and S. Kustu. 1998. Physiological role for the GlnK protein of enteric bacteria: relief of NifL inhibition under nitrogen-limiting conditions. J. Bacteriol. 180:6661–6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson, N., S. A. Austin, and R. A. Dixon. 1989. Role of metal ions in negative regulation of nitrogen fixation by the nifL gene product from Klebsiella pneumoniae. Mol. Gen. Genet. 216:484–491. [Google Scholar]
- 22.Hill, S., S. Austin, T. Eydmann, T. Jones, and R. Dixon. 1996. Azotobacter vinelandii NIFL is a flavoprotein that modulates transcriptional activation of nitrogen-fixation genes via a redox-sensitive switch. Proc. Natl. Acad. Sci. USA 93:2143–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jack, R., M. De Zamaroczy, and M. Merrick. 1999. The signal transduction protein GlnK is required for NifL-dependent nitrogen control of nif gene expression in Klebsiella pneumoniae. J. Bacteriol. 181:1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang, P., J. A. Peliska, and A. J. Ninfa. 1998. Enzymological characterization of the signal-transducing uridylyltransferase/uridylyl-removing enzyme (EC 2.7.7.59) of Escherichia coli and its interaction with the PII protein. Biochemistry 37:12782–12794. [DOI] [PubMed] [Google Scholar]
- 25.Kamberov, E. S., M. R. Atkinson, and A. J. Ninfa. 1995. The Escherichia coli PII signal transduction protein is activated upon binding 2-oxoglutarate and ATP. J. Biol. Chem. 270:17797–17807. [DOI] [PubMed] [Google Scholar]
- 26.Kustu, S., J. Hirschman, D. Burton, J. Jelesko, and J. C. Meeks. 1984. Covalent modification of bacterial glutamine synthetase: physiological significance. Mol. Gen. Genet. 197:309–317. [DOI] [PubMed] [Google Scholar]
- 27.Lei, S., L. Pulakat, and N. Gavini. 1999. Genetic analysis of nif regulatory genes by utilizing the yeast two-hybrid system detected formation of a NifL-NifA complex that is implicated in regulated expression of nif genes. J. Bacteriol. 181:6535–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little, R., F. Reyes-Ramirez, Y. Zhang, W. C. van Heeswijk, and R. Dixon. 2000. Signal transduction to the Azotobacter vinelandii NIFL-NIFA regulatory system is influenced directly by interaction with 2-oxoglutarate and the PII regulatory protein. EMBO J. 19:6041–6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Manoil, C., B. Bassler, and J. Slauch. 1999. Advanced bacterial genetics laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 31.Meletzus, D., P. Rudnick, N. Doetsch, A. Green, and C. Kennedy. 1998. Characterization of the glnK-amtB operon of Azotobacter vinelandii. J. Bacteriol. 180:3260–3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merrick, M., S. Hill, H. Hennecke, R. Hahn, R. Dixon, and C. Kennedy. 1982. Repressor properties of the nifL gene product in Klebsiella pneumoniae. Mol. Gen. Genet. 185:75–81. [Google Scholar]
- 33.Merrick, M. J., and R. A. Edwards. 1995. Nitrogen control in bacteria. Microbiol. Rev. 59:604–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 35.Newton, W. E., P. W. Wilson, and R. H. Burris. 1953. Direct demonstration of ammonia as an intermediate in nitrogen fixation by Azotobacter. J. Biol. Chem. 204:445–451. [PubMed] [Google Scholar]
- 36.Ninfa, A. J., and M. R. Atkinson. 2000. PII signal transduction proteins. Trends Microbiol. 8:172–179. [DOI] [PubMed] [Google Scholar]
- 37.Reyes-Ramirez, F., R. Little, and R. Dixon. 2001. Role of Escherichia coli nitrogen regulatory genes in the nitrogen response of the Azotobacter vinelandii NifL-NifA complex. J. Bacteriol. 183:3076–3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Santero, E., A. Toukdarian, R. Humphrey, and C. Kennedy. 1988. Identification and characterization of two nitrogen fixation regulatory regions, nifA and nfrX, in Azotobacter vinelandii and Azotobacter chroococcum. Mol. Microbiol. 2:303–314. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz, R. A. 1997. NifL of Klebsiella pneumoniae carries an N-terminally bound FAD cofactor, which is not directly required for the inhibitory function of NifL. FEMS Microbiol. Lett. 157:313–318. [DOI] [PubMed] [Google Scholar]
- 40.Soderback, E., F. Reyes-Ramirez, T. Eydmann, S. Austin, S. Hill, and R. Dixon. 1998. The redox- and fixed nitrogen-responsive regulatory protein NifL from Azotobacter vinelandii comprises discrete flavin and nucleotide-binding domains. Mol. Microbiol. 28:179–192. [DOI] [PubMed] [Google Scholar]
- 41.Soupene, E., L. He, D. Yan, and S. Kustu. 1998. Ammonia acquisition in enteric bacteria: physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc. Natl. Acad. Sci. USA 95:7030–7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas, G., G. Coutts, and M. Merrick. 2000. The glnKamtB operon. A conserved gene pair in prokaryotes. Trends Genet. 16:11–14. [DOI] [PubMed] [Google Scholar]
- 43.Toukdarian, A., G. Saunders, G. Selman-Sosa, E. Santero, P. Woodley, and C. Kennedy. 1990. Molecular analysis of the Azotobacter vinelandii glnA gene encoding glutamine synthetase. J. Bacteriol. 172:6529–6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Heeswijk, W. C., S. Hoving, D. Molenaar, B. Stegeman, D. Kahn, and H. V. Westerhoff. 1996. An alternative PII protein in the regulation of glutamine synthetase in Escherichia coli. Mol. Microbiol. 21:133–146. [DOI] [PubMed] [Google Scholar]
- 45.van Heeswijk, W. C., B. Stegeman, S. Hoving, D. Molenaar, D. Kahn, and H. V. Westerhoff. 1995. An additional PII in Escherichia coli: a new regulatory protein in the glutamine synthetase cascade. FEMS Microbiol. Lett. 132:153–157. [DOI] [PubMed] [Google Scholar]
- 46.Walmsley, J., and C. Kennedy. 1991. Temperature-dependent regulation by molybdenum and vanadium of expression of the structural genes encoding three nitrogenases in Azotobacter vinelandii. Appl. Environ. Microbiol. 57:622–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walmsley, J., A. Toukdarian, and C. Kennedy. 1994. The role of regulatory genes nifA, vnfA, anfA, nfrX, ntrC, and rpoN in expression of genes encoding the three nitrogenases of Azotobacter vinelandii. Arch. Microbiol. 162:422–429. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, Y., E. L. Pohlmann, C. M. Halbleib, P. W. Ludden, and G. P. Roberts. 2001. Effect of PII and its homolog GlnK on reversible ADP-ribosylation of dinitrogenase reductase by heterologous expression of the Rhodospirillum rubrum dinitrogenase reductase ADP-ribosyl transferase-dinitrogenase reductase-activating glycohydrolase regulatory system in Klebsiella pneumoniae. J. Bacteriol. 183:1610–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng, L., and D. R. Dean. 1994. Catalytic formation of a nitrogenase iron-sulfur cluster. J. Biol. Chem. 269:18723–18726. [PubMed] [Google Scholar]