Abstract
Plus-strand RNA virus replication occurs via the assembly of viral replicase complexes involving multiple viral and host proteins. To identify host proteins present in the cucumber necrosis tombusvirus (CNV) replicase, we affinity purified functional viral replicase complexes from yeast. Mass spectrometry analysis of proteins resolved by two-dimensional gel electrophoresis revealed the presence of CNV p33 and p92 replicase proteins as well as four major host proteins in the CNV replicase. The host proteins included the Ssa1/2p molecular chaperones (yeast homologues of Hsp70 proteins), Tdh2/3p (glyceraldehyde-3-phosphate dehydrogenase, an RNA-binding protein), Pdc1p (pyruvate decarboxylase), and an unknown ∼35-kDa acidic protein. Copurification experiments demonstrated that Ssa1p bound to p33 replication protein in vivo, and surface plasmon resonance measurements with purified recombinant proteins confirmed this interaction in vitro. The double mutant strain (ssa1 ssa2) showed 75% reduction in viral RNA accumulation, whereas overexpression of either Ssa1p or Ssa2p stimulated viral RNA replication by approximately threefold. The activity of the purified CNV replicase correlated with viral RNA replication in the above-mentioned ssa1 ssa2 mutant and in the Ssa overexpression strains, suggesting that Ssa1/2p likely plays an important role in the assembly of the CNV replicase.
Viral replicases are the key enzymes in the replication of plus-stranded RNA viruses (1, 7). The viral replicase complexes, which are assembled on intracellular membranes, consist of virus-encoded proteins, such as the RNA-dependent RNA polymerase (RdRp) and auxiliary viral protein(s), as well as host-derived proteins and the viral RNA template (2, 19). Studies with a small number of plant RNA viruses identified several host factors which are likely involved in the targeting of the viral replication proteins to intracellular compartments and replicase assembly and/or function. For example, subunit 45 of eukaryotic initiation factor 3 was found in association with brome mosaic virus (BMV) RdRp (35), while subunit 56 of eukaryotic initiation factor 3 was detected in a tobacco mosaic virus replicase preparation (25). Moreover, a molecular chaperone (Ydj1p) is known to be essential for the activation of the BMV replicase (45). TOM1 and TOM3 integral membrane proteins of Arabidopsis were found to interact with the tobacco mosaic virus replicase proteins and cofractionated with the RdRp activity (46, 53).
Host factors interacting with and/or affecting viral replicase activities have also been identified in animal plus-stranded RNA viruses. For example, hepatitis C virus (HCV) RdRp is known to bind to nucleolin (an RNA binding protein) and initiation factor 4A (11, 15, 20). The HCV NS5A and NS5B replication proteins interacted with hVAP-33, a SNARE-like protein bound to cellular membranes (47). hVAP-33 has been suggested to serve as a docking site to anchor the HCV RdRp to the membrane during assembly of the replicase complex (12, 47). Also, host chaperones are involved in proteolytic processing of the HCV nonstructural proteins (44). The Hsp90 molecular chaperone has been shown to enhance Flock House virus replication (18). Poly(C) and poly(A) binding proteins were found to interact with both the poliovirus RNA and the viral polymerase precursor 3CD (14, 49). Nucleolin and Sam68, which are involved in virus replication, are relocalized in the cell during poliovirus infection (21, 48). In addition, the cellular COPII proteins are involved in the assembly of the poliovirus replication complex (39). hnRNP A1 was found to be part of the mouse hepatitis virus transcription/replication complex, and it is involved in subgenomic RNA synthesis and genome replication (42, 50). Overall, the above examples illustrate that host factors play complex and significant roles in the replication of many plus-stranded RNA viruses.
Tomato bushy stunt virus (TBSV) and the closely related cucumber necrosis virus (CNV) are nonsegmented plus-stranded viruses of plants. Among the five TBSV-encoded proteins, only p33 and p92 are required for replication (26, 30, 40, 52), whereas the other proteins are involved in cell-to-cell movement, encapsidation, and suppression of gene silencing (52). The p92 replication protein has the RdRp signature motifs in its unique C terminus, whereas the auxiliary p33, which overlaps with the N-terminal sequence of p92, plays a role in template selection, the recruitment of the viral RNA and p92 into replication (23, 27, 34), and replicase assembly (31, 32). p33 has been shown to interact with p92 and with the viral RNA both in vivo and in vitro (27, 30, 34, 36, 37). The CNV replicase proteins can replicate the TBSV-derived RNAs as efficiently as the homologous TBSV proteins in plant protoplasts (26).
Yeast (Saccharomyces cerevisiae) has recently been developed as a model host to facilitate studies on tombusvirus-host interactions (28, 33). Coexpression of CNV p33 and p92 proteins with a small TBSV RNA replicon, which is a based on a defective-interfering (DI) RNA, leads to assembly of functional replicase complexes and robust TBSV RNA replication (31, 32, 34) and recombination (41). Genome-wide screens for host factors affecting virus replication have been performed for TBSV, demonstrating the involvement (direct or indirect) of ∼100 host genes in the viral replication process (29).
In this paper, we used a proteomics approach based on the production of highly purified replicase complex via two-step affinity chromatography, two-dimensional (2D) gel electrophoresis and mass spectrometry. Altogether, we identified three host proteins that included the Ssa1p and Ssa2p molecular chaperones. Further analysis of possible roles of Ssa1/2p in tombusvirus replication revealed that both proteins interacted with p33 in vitro and in vivo. Moreover, the dual mutant strain inhibited replication of the TBSV replicon by fourfold in yeast, whereas the overexpression of either protein enhanced replication by approximately threefold. The in vitro activity of purified tombusvirus replicase from the yeast strains used in this study suggested that Ssa1/2p likely plays a role in the assembly of the viral replicase.
MATERIALS AND METHODS
Yeast strains.
Saccharomyces cerevisiae strain InvSc1 (Invitrogen) was used as the wild type (wt), whereas single deletion ssa1Δ (YAL005C) and ssa2Δ (YLL024C) strains were obtained from Open Biosystems (Huntsville, AL). The single deletion strains are based on BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0). The double mutant (ssa1 ssa2) strain MW123 (his3 leu2 lys2 Δtrp1 ura3 ssa1::HIS3 ssa2::LEU2) was kindly provided by Elizabeth A. Craig (University of Wisconsin) (51).
E. coli and yeast plasmids.
Full-length SSA1 and SSA2 sequences were cloned into Escherichia coli vector pMAL-c2x (a maltose-binding protein [MBP]-based vector) (New England Biolabs, Ipswich, MA) at SacI and BamHI sites after PCR amplification from yeast chromosomal DNA using primers #1436 (5′-GCGGAGCTCGTCAAAAGCTGTCGGTATTG-3′) and #1437 (5′-GCGGGATCCGCACCAATTGGCTTAATCAAC-3′) or #1438 (5′-GCGGGATCCCGATCGCTAAGCTTAATCAAC-3′) for SSA1 and SSA2, respectively.
The yeast expression plasmid pGBK-33HF (containing six-His- and FLAG-tagged p33) (Fig. 1A) has been constructed by PCR using primers #1210 (5′-CGGATCCGGGTACCTTATCGTCATCGTCCTTGTAATCCCGACCCA-3′) and #1211 (5′-CGTCATTGTTCTCGTTCCCT-3′) by use of plasmid pGBK-His33 (32). Both the PCR product and pGBK-His33 were digested with NcoI and BamHI before ligation. The same strategy was used to obtain pGAD-92HF from pGAD-His92 (32).
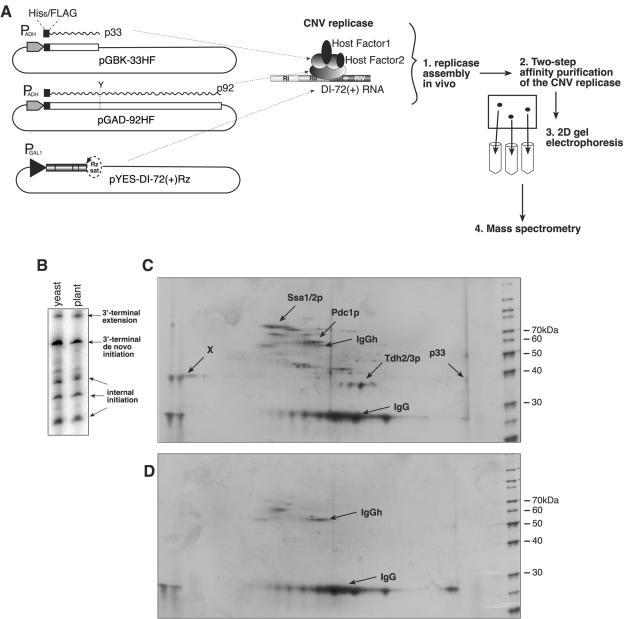
FIG. 1.
Proteomics analysis of the CNV replicase complex. (A) Plasmids used to express the CNV p33 and p92 replicase proteins (double-tagged with six-His and FLAG; represented by black boxes) and DI-72(+) RNA in yeast cells. p33 and p92 are expressed from PADH1 promoter, and DI-72(+) RNA is expressed from galactose-inducible (PGAL1) promoter. The translation termination codon of p33 was replaced with a tyrosine (Y) codon, allowing p92 expression from pGAD-92HF plasmid. There is a satTRSV(−) ribozyme (Rz sat) at the 3′ end of DI-72. The right side of the panel illustrates schematically the proteomics approach. (B) In vitro activity of the two-step affinity-purified CNV replicase preparation. The activity of the affinity-purified CNV replicase preparation (left lane) was compared to that of CNV replicase obtained from CNV-infected plants (right lane) (24). These replicases were tested in the presence of an exogenous RI/III(−) template, which contains the minus-stranded regions I and III of DI-72 in a standard CNV replicase assay (32). Radiolabeled RNA products from CNV replicase preparations were analyzed on denaturing 5% PAGE-8 M urea gels. (C and D) Silver-stained 2D gel images of the two-step affinity-purified CNV replicase preparations obtained from yeast expressing six-His- and FLAG-tagged p33/p92 (panel C) or six-His-tagged p33/p92 (control panel D). The consistently identified proteins (determined with mass spectrometry) as unique spots on the 2D gel are indicated.
The pGBK-33dN-HF-expressing six-His- and FLAG-tagged p33 fragment, missing amino acids (aa) 1 to 171 at the N terminus, was generated by treatment of pGBK-33HF with BamHI and NdeI followed by Klenow treatment and religation. pGAD-92HFT was obtained by PCR amplification of the region containing the TRP1 gene from pGBK-33HF by use of primers #1658 (5′-CCTTTCGAATCGCGCGTTTCGGTGATGACG-3′) and #1659 (5′-CACTGTACAGATCGGCAAGTGCACAAAC-3′). The PCR product was digested with Bsp119I and Bsp1407I and ligated together with the 700-bp XhoI-Bsp1407I fragment of pGAD-92HF and cloned into pGAD-92HF digested with Bsp119I and XhoI.
For construction of Ssa1p- or Ssa2p-overexpressing plasmids, pGBK-33HF was cut by NcoI, blunted, and then treated with BamHI. The resulting DNA fragment was ligated into pYC2/CT cloning vector (Invitrogen) digested with SspI/BamHI. The obtained pYC-HF was cut by Acc65I, blunted, and then digested with BamHI, which was followed by ligation of the SSA1 and SSA2 sequences from the pMAL-Ssa plasmids (digested with SacI, blunted, and then cleaved by BamHI).
Transformation and culturing of yeast.
Yeast strains were cotransformed with selected combination of plasmids using the standard lithium acetate method (13, 32). After transformation, yeast cells were plated on selective synthetic complete medium and incubated at 30°C for 3 to 4 days. Yeast colonies were grown as described earlier (32).
Proteomics analysis of CNV replicase composition.
The enriched membrane fraction of yeast containing the CNV replicase was prepared as described previously (32). Membrane was then solubilized in 10 ml extraction buffer (50 mM Tris-HCl [pH 7.5], 10% glycerol, 15 mM MgCl2, and 10 mM KCl supplemented with 0.5 M NaCl, 5 mM β-mercaptoethanol, 1% Brij35, 5% SB3-10 [caprylyl sulfobetaine] [Sigma] and 1% [vol/vol] yeast protease inhibitor cocktail) via gentle rotation for 2 h at 4°C. Then, the tube was incubated at 37°C for 5 min and centrifuged at 21,000 × g for 15 min at 4°C. Supernatant was combined with 1.5 ml ProBond resin (Invitrogen) in the extraction buffer supplemented with 0.5 M NaCl and 1% Brij35 and 2 mM imidazole, which was followed by gentle rotation for 1 h. Next, the unbound materials were removed by centrifugation at 800 × g, and the resin was washed twice with 6 ml washing buffer (extraction buffer supplemented with 0.5 M NaCl, 1% Nonidet P-40, and 1% SB3-10). The samples were eluted in a total of 2 ml washing buffer supplemented with 100 mM EDTA via repeated elution. The combined elution samples were then loaded onto an anti-FLAG M2-agarose affinity gel (Sigma) prepared from 0.2 ml slurry and prewashed twice with 0.6 ml 50 mM Tris-HCl, pH 7.5, containing 150 mM NaCl and 1% Nonidet P-40. After a 2-h rotation, the resin was washed three times with 0.6 ml of the above-described buffer. The final wash was with 0.6 ml of water containing 1% Nonidet P-40 and 1% SB3-10. The resin with the bound proteins was suspended in 0.25 ml rehydration buffer consisting of 8 M urea, 2 M thiourea, 1% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}, and 1% SB3-10 supplemented with 0.15 mg/ml FLAG peptide (Sigma) and 2 mM tributylphosphine, which was followed by incubation at room temperature for 30 min. The eluted proteins were collected by centrifugation at 1,000 × g. The elution step was repeated, and the resulting ∼0.35-ml sample was loaded for rehydration on 11-cm ReadyStrip IPG (immobilized pH gradient from pH 3 to pH 10) strips as recommended by the manufacturer (Bio-Rad Laboratories, Hercules, CA). The conditions of isofocusing and 2D gel electrophoresis will be published elsewhere (available upon request). Briefly, the isofocusing was performed under rapid ramp conditions for a 50,000 to 90,000 V · h. The strips were equilibrated in a solution of 6 M urea, 0.375 M Tris-HCl (pH 8.8), 2% sodium dodecyl sulfate (SDS), 20% glycerol, and 2% (wt/vol) dithiothreitol for 10 min, and the strips were subsequently placed into a solution of 6 M urea, 0.375 M Tris-HCl (pH 8.8), 2% SDS, 20% glycerol, and 2.5% (wt/vol) iodoacetamide for 10 min. Then, the strips were placed onto Bio-Rad Criterion precast 8 to 16% gradient gels overlaid by Bio-Rad ReadyPrep overlay agarose, and SDS-polyacrylamide gel electrophoresis (PAGE) was subsequently performed. The 2D gels were silver stained using a ProteoSilver Plus silver stain kit (Sigma); this procedure was followed by excision of the protein dots of interest, digestion with trypsin, and matrix-assisted laser desorption ionization-time of flight-based mass spectrometry analysis at the Center for Structural Biology at the University of Kentucky.
Pull down assay.
The pull down assay was performed as described earlier (27). Briefly, 10 g of yeast was resuspended in a total volume of 15 ml TG (50 mM Tris-HCl [pH 7.5], 10% glycerol, 15 mM MgCl2, and 10 mM KCl) buffer supplemented with 0.5 M NaCl, 0.1% Nonidet P-40, and 1% vol/vol yeast protease inhibitor cocktail (Sigma), ground in a mortar chilled by liquid nitrogen, and centrifuged for 15 min at 21,000 × g at 4°C. The supernatant was loaded onto an anti-FLAG M2-agarose affinity gel (Sigma), and the column was rotated for 2 h at 4°C and then placed at 37°C for 10 min and centrifuged for 5 min at 500 × g. The resin was washed four times with 5 ml of 50 mM Tris-HCl, pH 7.5, containing 150 mM NaCl and 0.1% Nonidet P-40. Sample elution was done in 2 ml washing buffer supplemented with 0.15 mg/ml FLAG peptide (Sigma) after rotation for 2 h. The obtained protein samples were analyzed by Western blotting as described previously (27) by use of anti-Ssa (generous gift from E. Craig) or anti-six-His (Amersham) antibodies. The detection was done with alkaline phosphatase conjugated to either anti-chicken or anti-mouse secondary immunoglobulin G.
SPR analysis.
The recombinant Ssa1p, Ssa2p, MBP, p33C (aa 151 to 296, soluble version of p33 [37]), and p33dN (aa 172 to 296) were expressed as fusions with MBP in E. coli. Purifications were done as described previously (36, 37). The surface plasmon resonance (SPR) experiments were performed by use of a BiaCore X system (Biacore, New Jersey) as described previously (36, 37). The CM-5 sensor chip and amine coupling kit were purchased from Biacore Inc. of New Jersey. Briefly, the purified fusion protein MBP-p33C was dialyzed in 10 mM sodium acetate, pH 5.0, and immobilized on the CM-5 sensor chip (Biacore, New Jersey) by use of amine coupling chemistry in flow cell 1. Purified MBP was immobilized on flow cell 2 as a control surface for nonspecific binding. The immobilization level of MBP-p33C was 8,000 resonance units (RU) for the binding studies. The test proteins were diluted to 1 μM in the running buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 3 mM EDTA, 0.05% surfactant P-20) and injected over the chip surface at a flow rate of 40 μl/min. The experimental data from individual binding experiments were overlaid and analyzed using BIAevaluation 4.1 software (Biacore, New Jersey).
Viral replicase purification, RdRp, and RNA assays.
Viral replicase was purified and tested as described previously (32). Total RNA from yeast cells was extracted, and Northern blotting was performed as described previously (32).
RESULTS
Proteomics analysis identifies four major host proteins in the purified CNV replicase.
To identify putative host proteins present in the CNV replicase complex, we expressed six-His/FLAG-tagged CNV p33 and p92 replication proteins together with the DI-72 RNA replicon from separate expression plasmids in yeast (Fig. 1A). After induction of transcription of the replicon RNA (repRNA) by galactose, robust replication takes place in yeast cells, producing plus-stranded repRNAs at ribosomal levels (28, 32). These yeast cells were then used to obtain solubilized active CNV replicase preparations via a two-step affinity purification (Fig. 1A) as described in Materials and Methods. The purified CNV replicase had in vitro RdRp activity on an added template that was indistinguishable from the activity of a CNV replicase preparation obtained from CNV-infected Nicotiana benthamiana plants (Fig. 1B) (24).
The two-step affinity-purified CNV replicase was then subjected to 2D gel electrophoresis to separate the proteins present in the complex (Fig. 1C). The 2D gel analysis revealed the presence of 5 to 15 proteins that were absent in control 2D gels that contained comparable preparations from yeast cells expressing singly six-His-tagged p33 and p92 proteins (Fig. 1D). We excised the unique dots, which were reproducible in five independent experiments and represented the most abundant proteins in the six-His/FLAG-tagged CNV replicase preparations, from the silver-stained 2D gels. Mass spectrometry analysis of the isolated proteins led to the identification of four proteins, namely, the CNV p33 replication protein, the Ssa1/2p molecular chaperones (Hsp70 homologues in yeast), Tdh2/3p (glyceraldehyde-3-phosphate dehydrogenase), and Pdc1p (pyruvate decarboxylase) (Fig. 1C). The above analyses could not differentiate between either (i) Ssa1p and Ssa2p or (ii) Tdh2p and Tdh3p, because of the near-perfect identity of these proteins (see Fig. S1 in the supplemental material). The fifth protein (∼35 kDa) could not be identified despite multiple attempts (not shown). The sixth protein present in the CNV replicase preparation was the p92 replication protein, which was detected by Western blotting (not shown). The additional 5 to 10 less-abundant host proteins (Fig. 1C), which were also present less reproducibly in our preparations, will be analyzed in the future.
Overall, the proteomics analysis of a highly purified functional CNV replicase revealed that both viral and host proteins are present in the complex. The identified proteins included two viral and three host proteins, whereas a characteristic acidic protein has not yet been identified.
Ssa1p and Ssa2p interact with CNV p33 in vivo and in vitro.
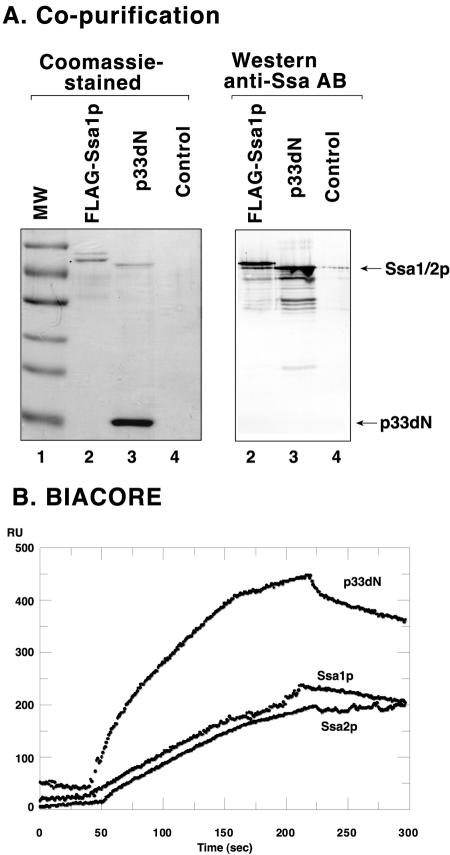
To validate the proteomics results presented above, we chose to further study the Hsp70 homologues, named Ssa1p and Ssa2p, which are almost identical (see Fig. S1 in the supplemental material); on the basis of this near identity, it is likely that both of them are present in our purified CNV replicase preparations. Initially, we performed copurification experiments from yeast extracts. To this end, we expressed FLAG-tagged p33dN (a soluble version of p33 protein [37]) in yeast, which was followed by FLAG-based affinity chromatography, SDS-PAGE, and Western blotting using anti-Ssa antibody (Fig. 2). These experiments demonstrated that Ssa1p/Ssa2p was copurified with p33dN (Fig. 2A, lanes 3, and B). The control experiment with wt yeast lacking p33dN, which was based on the same FLAG affinity purification approach, resulted in only a trace amount of copurified Ssa1p/Ssa2p (Fig. 2A, lanes 4, and B), excluding the possibility that Ssa1p/Ssa2p can bind efficiently to the affinity column.
FIG. 2.
Binding of p33 to Ssa in vivo and in vitro. (A) Copurification experiments using anti-FLAG resin were performed with yeast expressing FLAG-p33dN (lane 3) and followed by staining the SDS-PAGE gels with Coomassie blue (left panel) or by Western blotting with anti-Ssa antibody (right panel). Lanes 2 represent a FLAG-tagged affinity-purified positive control sample derived from yeast expressing FLAG-Ssa1p (note that the presence of the N-terminal FLAG tag slows down the migration of Ssa1p slightly). Lanes 4 represent a FLAG-tagged affinity-purified negative control from the wt yeast. The positions of p33dN and Ssa1/2p are indicated on the side. Molecular mass markers were (from top to bottom) 100, 70, 55, 45, 35, and 25 kDa. (B) SPR analysis of interaction between purified TBSV p33C (aa 151 to 296), fixed onto a CM-5 sensor chip at 8,000 RU, and 1 μM purified recombinant Ssa1p, Ssa2p, and p33dN (aa 172 to 296), which were separately injected in the running buffer over the chip. These proteins were expressed as MBP fusion proteins in E. coli. The interaction data shown were subtracted from the data from the control surface to account for the bulk effects of running buffer and the nonspecific binding of proteins to the chip surface.
To obtain further evidence for specific interaction between p33 and Ssa1p/Ssa2p, we performed SPR measurements with a BiaCore X system (36, 37). For the SPR analysis, we expressed these proteins in E. coli and followed this with affinity purification. The purified recombinant p33 was fixed to the surface of the chip, whereas the purified recombinant Ssa1p, Ssa2p, and p33dN (as a positive control) and MBP (negative control, not shown), respectively, were passed over the surface of the chip. The SPR analysis demonstrated interaction between p33 and Ssa1p and Ssa2p (Fig. 2B). The positive control p33dN also interacted with p33, whereas MBP did not significantly interact with p33 (not shown) (37). Note that the SPR measurements were done on high-density chips (8,000 RU; see Materials and Methods), so the data are suitable only for the identification of interactions (37).
The above-described experiments demonstrated that the p33 replication protein and Ssa1p/Ssa2p could interact both in vivo and in vitro. These findings supported the model that p33 and Ssa1p/Ssa2p are likely bound together within the CNV replicase, as suggested by their copurification in the functional replicase complex (Fig. 1C) (see Discussion).
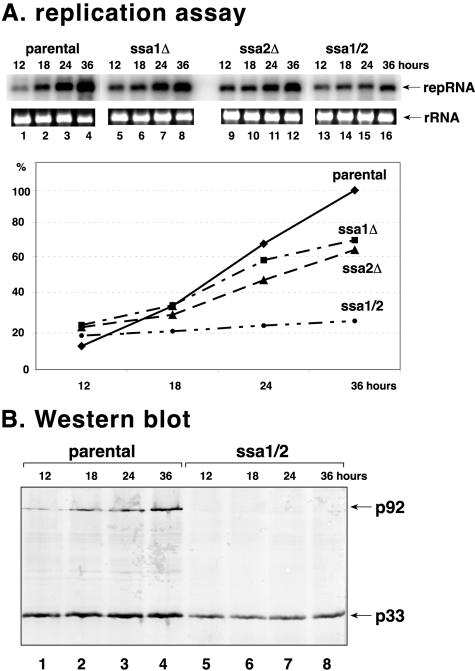
Reduced tombusvirus replication in ssa1 ssa2 double mutant yeast.
To obtain evidence about the functional relevance of Ssa1p and/or Ssa2p in tombusvirus replication, we launched repRNA replication in ssa1Δ, ssa2Δ, and double mutant (ssa1 ssa2) yeast (Fig. 3A). A time course analysis of plus-stranded repRNA accumulation done by use of Northern blotting demonstrated that single deletions reduced repRNA accumulation only slightly (30 to 35%) (Fig. 3A, lanes 5 to 12) compared to what was seen with the parental yeast (lanes 1 to 4). In contrast, the double mutant strain (ssa1 ssa2) lacking functional SSA1 and SSA2 genes showed ∼75% reduction in repRNA accumulation (Fig. 3A, lanes 13 to 16). These data suggest that Ssa1p and Ssa2p are important for tombusvirus replication and that they can functionally complement each other.
FIG. 3.
Effect of Ssa1/2p on viral RNA accumulation and replicase protein levels. (A) Northern blot analysis of total RNA extracts for DI-72 RNA accumulation at 12 (lanes 1, 5, 9, and 13), 18 (lanes 2, 6, 10, and 14), 24 (lanes 3, 7, 11, and 15), and 36 (lanes 4, 8, 12, and 16) h after induction using the 3′ end of DI-72 as a probe. The ethidium bromide-stained agarose gel shows the rRNA bands as loading controls. Each strain was transformed with three plasmids (see Fig. 1A). The accumulation level of DI-72 RNA in the parental yeast strain 36 h after induction was chosen as the 100% value. (B) Western analysis of p33 and p92 replicase proteins in total protein samples using anti-His antibody. The samples were collected 12 (lanes 1 and 5), 18 (lanes 2 and 6), 24 (lanes 3 and 7), and 36 (lanes 4 and 8) h after the induction of DI-72 RNA replication. Note that comparable amounts of cells were used for protein extraction. Both p33 and p92 are expressed from the constitutive ADH1 promoter; thus, their concentrations changed only slightly during incubation.
Western blot analysis of p33 and p92 proteins in the wt and double mutant yeast strains revealed that levels of both p33 and p92 dropped markedly in double mutant yeast strains (Fig. 3B, lanes 5 to 8). These data suggest that Ssa1p and Ssa2p might be important for stabilizing p33 and, especially, the p92 protein.
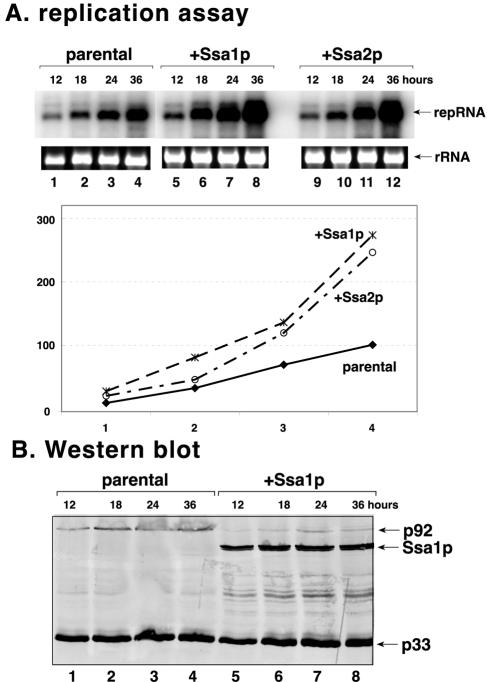
Overexpression of Ssa1p or Ssa2p stimulates tombusvirus replication in yeast.
To further test the effect of Ssa1p and Ssa2p on tombusvirus replication, we overexpressed them separately in the wt yeast from plasmids via the inducible GAL1 promoter. A time course analysis of the accumulation of plus-stranded repRNA done with Northern blotting demonstrated that the overexpression of Ssa1p and Ssa2p stimulated repRNA accumulation, which increased by 2.5- to 3-fold (Fig. 4A, lanes 5 to 12) compared to the that for wt yeast (lanes 1 to 4). Therefore, these data further support the notion that Ssa1p and Ssa2p play complementary roles in tombusvirus replication.
FIG. 4.
Increased accumulation of viral RNA in Ssa overexpression strains. (A) Northern blot analysis of total RNA extracts for DI-72 RNA accumulation at 12 (lanes 1, 5, and 9), 18 (lanes 2, 6, and 10), 24 (lanes 3, 7, and 11), and 36 (lanes 4, 8, and 12) h after induction using the 3′ end of DI-72 as a probe. See further details in the legend to Fig. 3. (B) Western analysis of p33 and p92 replicase proteins in total protein samples using anti-His antibody. Further details are as for Fig. 3. Note that the overexpressed Ssa1p carried a six-His tag, which was detected by the anti-His antibody, whereas the wt Ssa1p was not detected (lanes 1 to 4).
To test if the overexpression of Ssa1p affects the amounts of viral replication proteins, we performed Western blot analysis on total protein extracts obtained from the above-described yeast strains. This experiment demonstrated that Ssa1p was indeed overexpressed in the selected strain (Fig. 4B, lanes 5 to 8). Moreover, the amounts of p33 were comparable in the parental and Ssa1p overexpression yeast strains (Fig. 4B), whereas the amount of p92 was decreased by approximately fivefold in the Ssa1p overexpression strain (lanes 5 to 8). Thus, in spite of the small amount of p92, the replication of repRNA was stimulated, suggesting that the reduced amount of p92 available is more active in the Ssa1p overexpression strain than in wt yeast.
Overexpression of Ssa1p or Ssa2p in yeast results in more-active tombusvirus replicase.
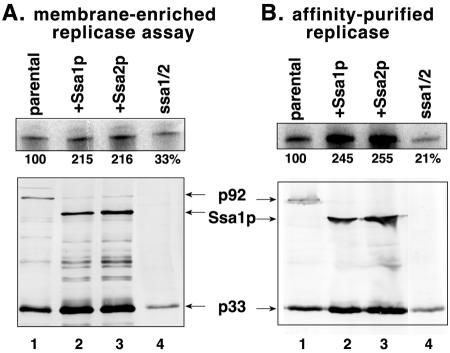
To further decipher the role for Ssa1/2p proteins in tombusviral replication, we isolated membrane-enriched fractions from comparable amounts of yeast cells for each strain and performed replicase assays on the copurified (endogenous) RNA templates (Fig. 5A) as described previously (31, 32). We also performed an additional assay that included solubilized affinity-purified CNV replicase preparations with added RNA templates (Fig. 5B) (31, 32). These experiments showed that the overexpression of either Ssa1p or Ssa2p resulted in approximately 2- to 2.5-fold-more-active replicase preparations than those obtained from the parental yeast, whereas the replicase preparations from the ssa1 ssa2 double mutant yeast strain lacking functional SSA1 and SSA2 genes showed three- to fourfold less replicase activity on both endogenous (Fig. 4A, lane 4) and exogenous (Fig. 4B, lane 4) templates. Western blot analysis of the replicase preparations showed that the levels of p33 were approximately the same, while p92 was less abundant in the replicase from yeast overexpressing either Ssa1p or Ssa2p than in the replicase from the wt yeast (Fig. 4A and B). In contrast, the amounts of both p33 and p92 in the replicase preparations from the ssa1 ssa2 double mutant yeast strain were less than in that of the wt yeast. Altogether, these experiments suggest that Ssa1p and Ssa2p might be involved in multiple steps, including the stabilization of the replicase proteins, in the assembly and/or activity of the replicase (see Discussion).
FIG. 5.
Characterization of CNV replicase activity derived from yeast. (A) Top: PAGE analysis of the 32P-labeled in vitro replicase products derived from the endogenous templates, which are present in the enriched membrane fractions, obtained from the shown yeast strains. The relative RdRp activities of the enriched membrane fractions are shown as percentages of the RdRp activity obtained from the parental yeast strain. Bottom: Western blot analysis of p33 and p92 levels in the various replicase preparations. See the legend for Fig. 3 for further details. (B) Activity of solubilized and affinity-purified CNV replicase preparations obtained from the shown yeast strains. Each preparation was tested in the presence of exogenous RI/III(−) template containing the minus-stranded regions I and III of DI-72 (Fig. 1A) in a standard CNV replicase assay. See further details in the legend for Fig. 3A.
DISCUSSION
Earlier cell biology and biochemistry works have revealed that the tombusvirus replicase contains p33 and p92 replication proteins and the viral RNA (27, 32, 40), while the identity of putative host factors in the complex remained unknown. The proteomics approach used in this work led to the identification of three host proteins together with the viral p33 and p92 replication proteins within the highly purified replicase complex. An additional abundant acidic ∼35-kDa host protein could not be identified by using the same mass spectrometry-based proteomics approach. The identified Ssa1/2p, Tdh2/3p, and Pdc1p proteins were not present in preparations that lacked FLAG-tagged viral proteins (Fig. 1C and D), suggesting that these host proteins were stably associated with the functional replicase complex.
The cytosolic Ssa proteins belong to one of the most abundant subfamilies of the Hsp70 molecular chaperones (4, 9). The four members within the subfamily (Ssa1p to Ssa4p) show more than 80% amino acid identity, and Ssa1p and Ssa2p have almost identical sequences. At least one of the Ssa proteins must be expressed at a high level in yeast for vegetative growth. While Ssa1p and Ssa2p are expressed constitutively, the expression of Ssa3p and Ssa4p is induced by heat shock or other stresses (5, 8, 17, 54). Among the biochemical/cellular features of Ssa1p and Ssa2p which could be relevant for tombusvirus replication are participation in protein translocation, protein folding, and assembly of protein complexes (5, 8, 17, 54).
Hsp70 proteins play roles in the replication of other viruses as well. For example, the polymerase of respiratory syncytial virus has been shown to colocalize with Hsp70 to lipid raft membranes and virus-induced inclusion bodies (6). The involvement of Hsp70 and Hsp90 in the receptor entry of dengue virus has also been shown (38). Moreover, additional members of the heat shock protein family were shown to be important for the assembly of the BMV replicase (45), stimulation of the polymerase activity of influenza virus (22), the enhancement of Flock House virus replication (18), and the activation of reverse transcriptase for hepadnaviruses (16, 43) or to be involved in the assembly of closterovirus virions (3). Therefore, molecular chaperones seem to be recruited by various viruses to play important roles in virus replication and other processes.
In contrast to the Ssa proteins, the known cellular functions of Tdh2/3p as glyceraldehyde-3-phosphate dehydrogenase and Pdc1p as pyruvate decarboxylase make them unlikely candidates for participation in viral replication. However, Tdh2p was found to bind to the tombusvirus RNA in vitro (S. Serva and P. D. Nagy, unpublished data), suggesting that Tdh2/3p might be associated with the tombusvirus replicase via association with the viral RNA template. The possible relevance of Tdh2p in virus replication is further supported by binding of Gpdh2 (Tdh2p in yeast) and related Gpdh proteins to HCV, hepatitis A virus, and hepatitis delta virus RNAs (10). In addition, Tdh2p was observed to be part of large cellular complexes involved in RNA transport and RNA metabolism in yeast (Saccharomyces genome database, http://www.yeastgenome.org/). The possible function of Pdc1p, if any, in the tombusvirus replicase is currently unknown.
Follow-up experiments with Ssa1/2p demonstrated that Ssa1p could bind to p33 in vitro and in vivo (Fig. 2), explaining its presence in the CNV replicase. Single deletion of SSA1 or SSA2 genes inhibited TBSV repRNA replication only by 30%, suggesting that Ssa1/2p could complement each other in tombusvirus replication. This relatively small reduction in repRNA accumulation in single-deletion strains also explains why we did not identify SSA genes in the genome-wide screen, which included the complete yeast single-gene knock-out library (29). However, dual deletion of SSA1 and SSA2 genes did reduce repRNA accumulation by 75%, whereas their overexpression increased repRNA replication by approximately threefold, suggesting that these proteins play significant roles in tombusvirus replication. The above data cannot exclude the possibility that the function of Ssa proteins might be essential for tombusvirus replication, because the SSA3 and SSA4 genes were still present and they might have partially complemented (up to 25%) the nonfunctional Ssa1/2p in the dual mutant strain. Yeast is not viable when all four SSA genes are missing (9, 51), which prevents analysis of tombusvirus replication in the absence of all Ssa proteins.
Interestingly, the amounts of p33 and p92 were decreased in the dual mutant strain, suggesting that Ssa1/2p might affect the stability of the replication proteins. Surprisingly, however, we found that the overexpression of Ssa1p or Ssa2p also reduced the amount of p92 but not that of p33. We also found that the overexpression of several other yeast proteins, which are not known to affect TBSV replication directly, can reduce p92 levels (not shown; M. Jonczyk and Nagy, unpublished), suggesting that p92 expression could be affected nonspecifically by a high level of plasmid-based expression of other proteins in yeast. Interestingly, the smaller amount of p92 was over twofold more active in the purified replicase from the Ssa1p overexpression strain than the larger amount of p92 present in the replicase purified from the wt yeast. Altogether, these findings are consistent with the model that Ssa1/2p are possibly involved in stabilizing p33 and p92 proteins and improving their biochemical activities as well.
We propose that Ssa1/2p might play a role in the assembly of the viral replicase. Indeed, Ssa1/2p copurified with the replicase (Fig. 1C) and interacted with p33 in vitro and in vivo (Fig. 2). Furthermore, based on the molecular chaperone function of Ssa1/2p, we speculate that the refolding of p33 and/or p92 after their association with the peroxisomal membrane could be a mechanism leading to the activation of the tombusvirus replicase complex. The viral RNA template could also affect this step by providing an assembly platform (31, 32). The replicase assembly/activation functions of Ssa1/2p, however, do not exclude the possibility that Ssa1/2p could also affect the intracellular transport of the replicase proteins. It is unlikely that the only role of Ssa1/2p in TBSV replication is to promote correct folding of p33/p92 in the cytoplasm, where Ssa1/2p is known to perform its protein folding function. This is because Ssa1/2p is a cytosolic protein; however, we copurified it with the replicase complex from the membrane fraction. Based on these observations, we propose that Ssa1/2p might contribute in multiple ways to TBSV replicase functions.
The tombusvirus replicase complex is bound to the peroxisomal membranes (27) in cells. Thus, the purification of the replicase complex required solubilization of membranes with detergents, which could have resulted in the partial disassembly of the complex and/or the loss of some of the host factors associated weakly with the replicase complex. In addition, the purification method used favored the identification of host proteins, which are associated permanently or stably with the replicase, while those factors associated temporally with the replicase or present in low abundance might not be identified with our approach. Therefore, the number of host factors associated with the replicase complex is likely higher than that presented in this paper. More dynamic and sophisticated approaches will be needed to identify those host factors in the future.
Supplementary Material
Acknowledgments
We thank Judit Pogany and K. S. Rajendran for their critical comments and helpful suggestions.
This work was supported by NIH and by the Kentucky Tobacco Research and Development Center at the University of Kentucky.
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Ahlquist, P. 2002. RNA-dependent RNA polymerases, viruses, and RNA silencing. Science 296:1270-1273. [DOI] [PubMed] [Google Scholar]
- 2.Ahlquist, P., A. O. Noueiry, W. M. Lee, D. B. Kushner, and B. T. Dye. 2003. Host factors in positive-strand RNA virus genome replication. J. Virol. 77:8181-8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzhanova, D. V., A. J. Napuli, R. Creamer, and V. V. Dolja. 2001. Cell-to-cell movement and assembly of a plant closterovirus: roles for the capsid proteins and Hsp70 homolog. EMBO J. 20:6997-7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baxter, B. K., and E. A. Craig. 1998. Suppression of an Hsp70 mutant phenotype in Saccharomyces cerevisiae through loss of function of the chromatin component Sin1p/Spt2p. J. Bacteriol. 180:6484-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker, J., W. Walter, W. Yan, and E. A. Craig. 1996. Functional interaction of cytosolic hsp70 and a DnaJ-related protein, Ydj1p, in protein translocation in vivo. Mol. Cell. Biol. 16:4378-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, G., H. W. Rixon, J. Steel, T. P. McDonald, A. R. Pitt, S. Graham, and R. J. Sugrue. 2005. Evidence for an association between heat shock protein 70 and the respiratory syncytial virus polymerase complex within lipid-raft membranes during virus infection. Virology 338:69-80. [DOI] [PubMed] [Google Scholar]
- 7.Buck, K. W. 1996. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Virus Res. 47:159-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukau, B., E. Deuerling, C. Pfund, and E. A. Craig. 2000. Getting newly synthesized proteins into shape. Cell 101:119-122. [DOI] [PubMed] [Google Scholar]
- 9.Cristofari, G., and J. L. Darlix. 2002. The ubiquitous nature of RNA chaperone proteins. Prog. Nucleic Acid Res. Mol. Biol. 72:223-268. [DOI] [PubMed] [Google Scholar]
- 10.Dollenmaier, G., and M. Weitz. 2003. Interaction of glyceraldehyde-3-phosphate dehydrogenase with secondary and tertiary RNA structural elements of the hepatitis A virus 3′ translated and non-translated regions. J. Gen. Virol. 84:403-414. [DOI] [PubMed] [Google Scholar]
- 11.Dubuisson, J., F. Penin, and D. Moradpour. 2002. Interaction of hepatitis C virus proteins with host cell membranes and lipids. Trends Cell Biol. 12:517-523. [DOI] [PubMed] [Google Scholar]
- 12.Evans, M. J., C. M. Rice, and S. P. Goff. 2004. Phosphorylation of hepatitis C virus nonstructural protein 5A modulates its protein interactions and viral RNA replication. Proc. Natl. Acad. Sci. USA 101:13038-13043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gietz, R. D., and R. A. Woods. 2002. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350:87-96. [DOI] [PubMed] [Google Scholar]
- 14.Herold, J., and R. Andino. 2001. Poliovirus RNA replication requires genome circularization through a protein-protein bridge. Mol. Cell 7:581-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano, M., S. Kaneko, T. Yamashita, H. Luo, W. Qin, Y. Shirota, T. Nomura, K. Kobayashi, and S. Murakami. 2002. Direct interaction between nucleolin and hepatitis C virus NS5B. J. Biol. Chem. [DOI] [PubMed]
- 16.Hu, J., D. Flores, D. Toft, X. Wang, and D. Nguyen. 2004. Requirement of heat shock protein 90 for human hepatitis B virus reverse transcriptase function. J. Virol. 78:13122-13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, J. L., and E. A. Craig. 1997. Protein folding in vivo: unraveling complex pathways. Cell 90:201-204. [DOI] [PubMed] [Google Scholar]
- 18.Kampmueller, K. M., and D. J. Miller. 2005. The cellular chaperone heat shock protein 90 facilitates Flock House virus RNA replication in Drosophila cells. J. Virol. 79:6827-6837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kao, C. C., P. Singh, and D. J. Ecker. 2001. De novo initiation of viral RNA-dependent RNA synthesis. Virology 287:251-260. [DOI] [PubMed] [Google Scholar]
- 20.Kyono, K., M. Miyashiro, and I. Taguchi. 2002. Human eukaryotic initiation factor 4AII associates with hepatitis C virus NS5B protein in vitro. Biochem. Biophys. Res. Commun. 292:659-666. [DOI] [PubMed] [Google Scholar]
- 21.McBride, A. E., A. Schlegel, and K. Kirkegaard. 1996. Human protein Sam68 relocalization and interaction with poliovirus RNA polymerase in infected cells. Proc. Natl. Acad. Sci. USA 93:2296-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Momose, F., T. Naito, K. Yano, S. Sugimoto, Y. Morikawa, and K. Nagata. 2002. Identification of Hsp90 as a stimulatory host factor involved in influenza virus RNA synthesis. J. Biol. Chem. 277:45306-45314. [DOI] [PubMed] [Google Scholar]
- 23.Monkewich, S., H. X. Lin, M. R. Fabian, W. Xu, H. Na, D. Ray, O. A. Chernysheva, P. D. Nagy, and K. A. White. 2005. The p92 polymerase coding region contains an internal RNA element required at an early step in tombusvirus genome replication. J. Virol. 79:4848-4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagy, P. D., and J. Pogany. 2000. Partial purification and characterization of Cucumber necrosis virus and Tomato bushy stunt virus RNA-dependent RNA polymerases: similarities and differences in template usage between tombusvirus and carmovirus RNA-dependent RNA polymerases. Virology 276:279-288. [DOI] [PubMed] [Google Scholar]
- 25.Osman, T. A., and K. W. Buck. 1997. The tobacco mosaic virus RNA polymerase complex contains a plant protein related to the RNA-binding subunit of yeast eIF-3. J. Virol. 71:6075-6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oster, S. K., B. Wu, and K. A. White. 1998. Uncoupled expression of p33 and p92 permits amplification of tomato bushy stunt virus RNAs. J. Virol. 72:5845-5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panavas, T., C. M. Hawkins, Z. Panaviene, and P. D. Nagy. 2005. The role of the p33:p33/p92 interaction domain in RNA replication and intracellular localization of p33 and p92 proteins of Cucumber necrosis tombusvirus. Virology 338:81-95. [DOI] [PubMed] [Google Scholar]
- 28.Panavas, T., and P. D. Nagy. 2003. Yeast as a model host to study replication and recombination of defective interfering RNA of Tomato bushy stunt virus. Virology 314:315-325. [DOI] [PubMed] [Google Scholar]
- 29.Panavas, T., E. Serviene, J. Brasher, and P. D. Nagy. 2005. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of RNA viruses. Proc. Natl. Acad. Sci. USA 102:7326-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panaviene, Z., J. M. Baker, and P. D. Nagy. 2003. The overlapping RNA-binding domains of p33 and p92 replicase proteins are essential for tombusvirus replication. Virology 308:191-205. [DOI] [PubMed] [Google Scholar]
- 31.Panaviene, Z., T. Panavas, and P. D. Nagy. 2005. Role of an internal and two 3′-terminal RNA elements in assembly of tombusvirus replicase. J. Virol. 79:10608-10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panaviene, Z., T. Panavas, S. Serva, and P. D. Nagy. 2004. Purification of the cucumber necrosis virus replicase from yeast cells: role of coexpressed viral RNA in stimulation of replicase activity. J. Virol. 78:8254-8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantaleo, V., L. Rubino, and M. Russo. 2003. Replication of Carnation Italian ringspot virus defective interfering RNA in Saccharomyces cerevisiae. J. Virol. 77:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pogany, J., K. A. White, and P. D. Nagy. 2005. Specific binding of tombusvirus replication protein p33 to an internal replication element in the viral RNA is essential for replication. J. Virol. 79:4859-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quadt, R., C. C. Kao, K. S. Browning, R. P. Hershberger, and P. Ahlquist. 1993. Characterization of a host protein associated with brome mosaic virus RNA-dependent RNA polymerase. Proc. Natl. Acad. Sci. USA 90:1498-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rajendran, K. S., and P. D. Nagy. 2003. Characterization of the RNA-binding domains in the replicase proteins of tomato bushy stunt virus. J. Virol. 77:9244-9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajendran, K. S., and P. D. Nagy. 2004. Interaction between the replicase proteins of Tomato Bushy Stunt virus in vitro and in vivo. Virology 326:250-261. [DOI] [PubMed] [Google Scholar]
- 38.Reyes-Del Valle, J., S. Chavez-Salinas, F. Medina, and R. M. Del Angel. 2005. Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J. Virol. 79:4557-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rust, R. C., L. Landmann, R. Gosert, B. L. Tang, W. Hong, H. P. Hauri, D. Egger, and K. Bienz. 2001. Cellular COPII proteins are involved in production of the vesicles that form the poliovirus replication complex. J. Virol. 75:9808-9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholthof, K. B., H. B. Scholthof, and A. O. Jackson. 1995. The tomato bushy stunt virus replicase proteins are coordinately expressed and membrane associated. Virology 208:365-369. [DOI] [PubMed] [Google Scholar]
- 41.Serviene, E., N. Shapka, C. P. Cheng, T. Panavas, B. Phuangrat, J. Baker, and P. D. Nagy. 2005. Genome-wide screen identifies host genes affecting viral RNA recombination. Proc. Natl. Acad. Sci. USA 102:10545-10550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen, X., and P. S. Masters. 2001. Evaluation of the role of heterogeneous nuclear ribonucleoprotein A1 as a host factor in murine coronavirus discontinuous transcription and genome replication. Proc. Natl. Acad. Sci. USA 98:2717-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavis, J. E., B. Massey, and Y. Gong. 1998. The duck hepatitis B virus polymerase is activated by its RNA packaging signal, epsilon. J. Virol. 72:5789-5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tellinghuisen, T. L., and C. M. Rice. 2002. Interaction between hepatitis C virus proteins and host cell factors. Curr. Opin. Microbiol. 5:419-427. [DOI] [PubMed] [Google Scholar]
- 45.Tomita, Y., T. Mizuno, J. Diez, S. Naito, P. Ahlquist, and M. Ishikawa. 2003. Mutation of host DnaJ homolog inhibits brome mosaic virus negative-strand RNA synthesis. J. Virol. 77:2990-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsujimoto, Y., T. Numaga, K. Ohshima, M. A. Yano, R. Ohsawa, D. B. Goto, S. Naito, and M. Ishikawa. 2003. Arabidopsis TOBAMOVIRUS MULTIPLICATION (TOM) 2 locus encodes a transmembrane protein that interacts with TOM1. EMBO J. 22:335-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tu, H., L. Gao, S. T. Shi, D. R. Taylor, T. Yang, A. K. Mircheff, Y. Wen, A. E. Gorbalenya, S. B. Hwang, and M. M. Lai. 1999. Hepatitis C virus RNA polymerase and NS5A complex with a SNARE-like protein. Virology 263:30-41. [DOI] [PubMed] [Google Scholar]
- 48.Waggoner, S., and P. Sarnow. 1998. Viral ribonucleoprotein complex formation and nucleolar-cytoplasmic relocalization of nucleolin in poliovirus-infected cells. J. Virol. 72:6699-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walter, B. L., T. B. Parsley, E. Ehrenfeld, and B. L. Semler. 2002. Distinct poly(rC) binding protein KH domain determinants for poliovirus translation initiation and viral RNA replication. J. Virol. 76:12008-12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, Y., and X. Zhang. 1999. The nucleocapsid protein of coronavirus mouse hepatitis virus interacts with the cellular heterogeneous nuclear ribonucleoprotein A1 in vitro and in vivo. Virology 265:96-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werner-Washburne, M., D. E. Stone, and E. A. Craig. 1987. Complex interactions among members of an essential subfamily of hsp70 genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 7:2568-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White, K. A., and P. D. Nagy. 2004. Advances in the molecular biology of tombusviruses: gene expression, genome replication, and recombination. Prog. Nucleic Acid Res. Mol. Biol. 78:187-226. [DOI] [PubMed] [Google Scholar]
- 53.Yamanaka, T., T. Ohta, M. Takahashi, T. Meshi, R. Schmidt, C. Dean, S. Naito, and M. Ishikawa. 2000. TOM1, an Arabidopsis gene required for efficient multiplication of a tobamovirus, encodes a putative transmembrane protein. Proc. Natl. Acad. Sci. USA 97:10107-10112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Young, J. C., J. M. Barral, and F. Ulrich Hartl. 2003. More than folding: localized functions of cytosolic chaperones. Trends Biochem. Sci. 28:541-547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.