Abstract
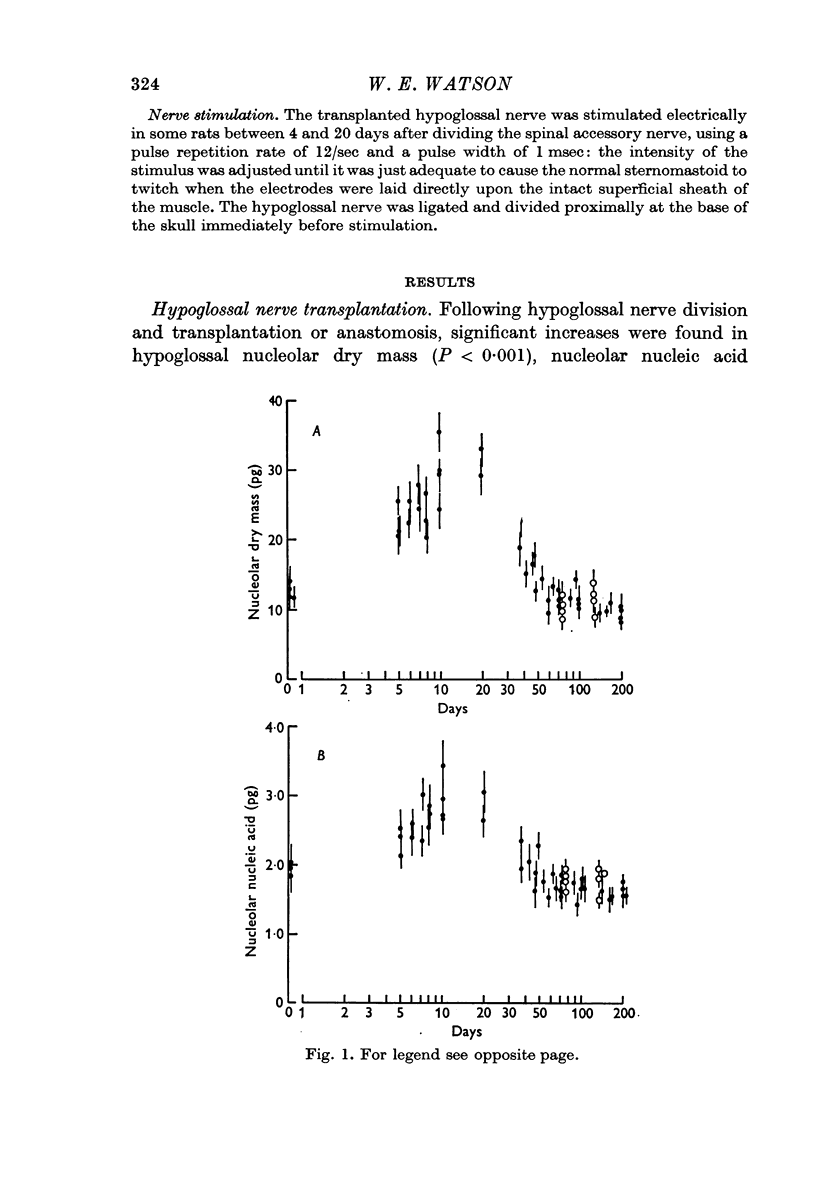
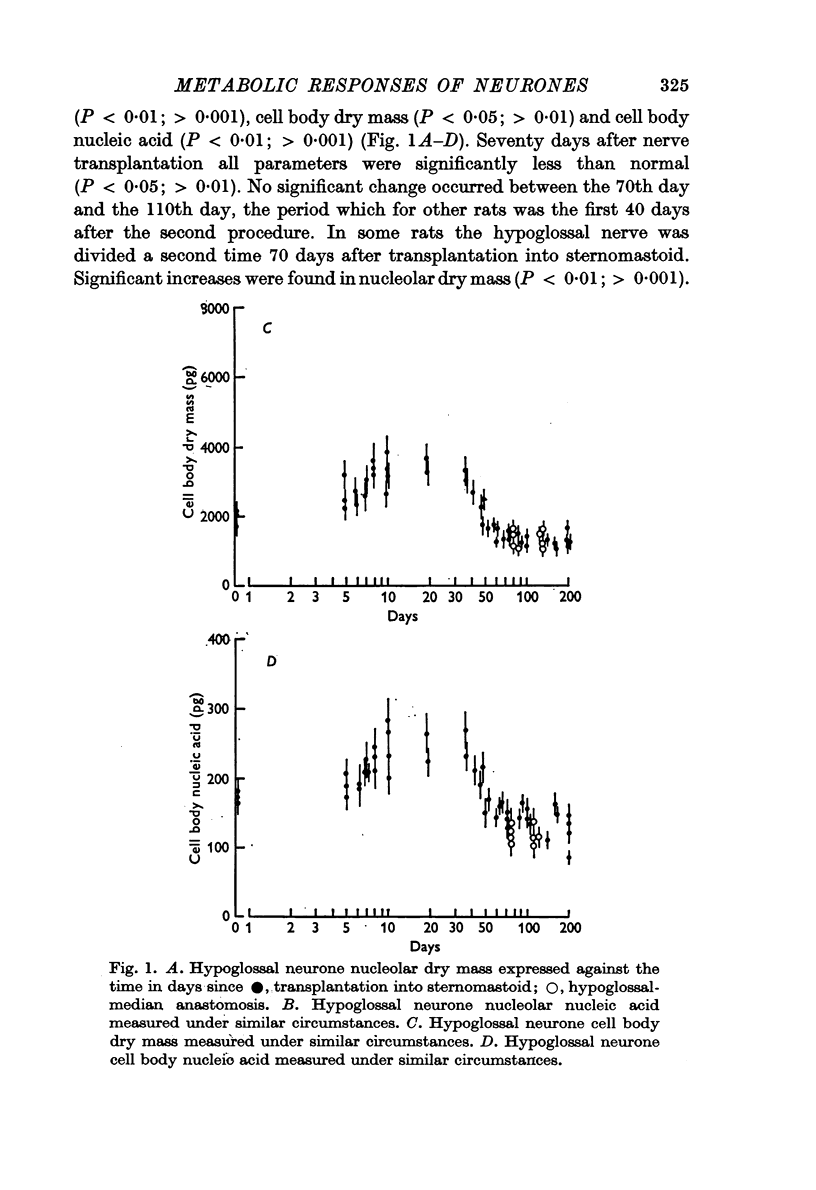
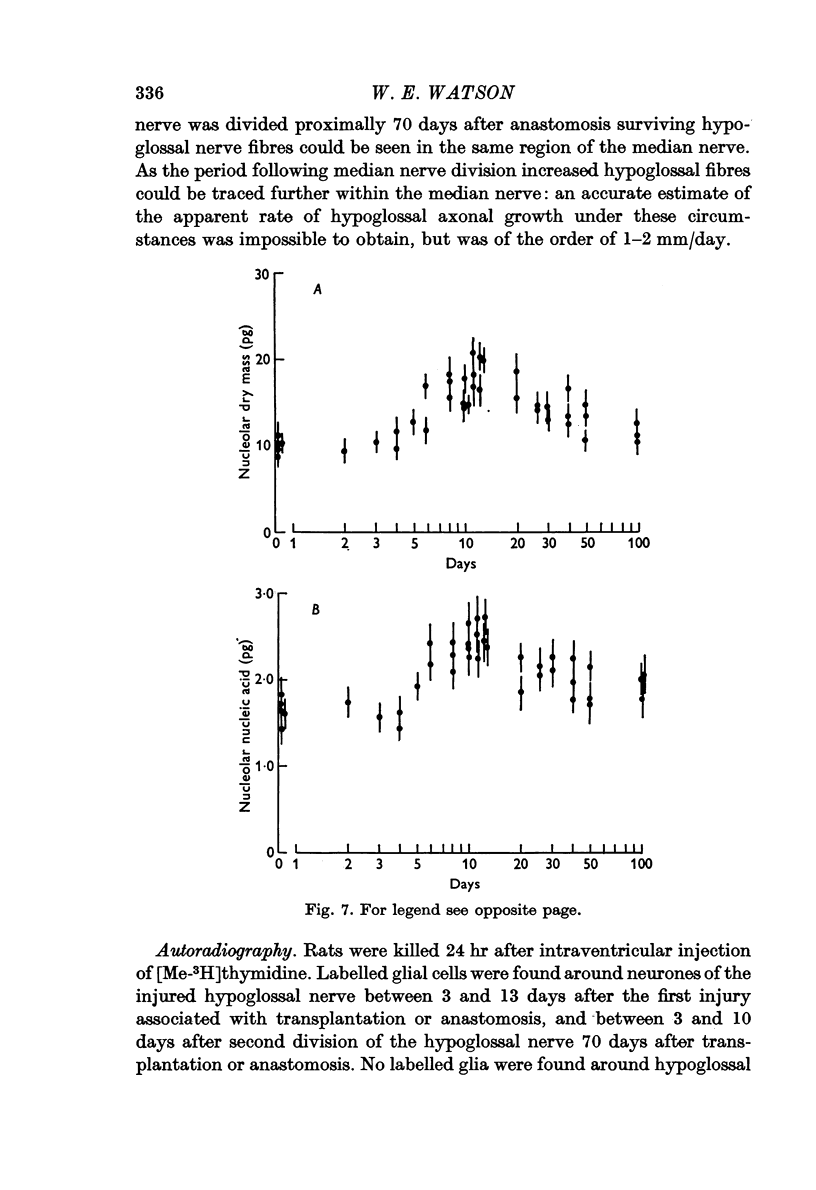
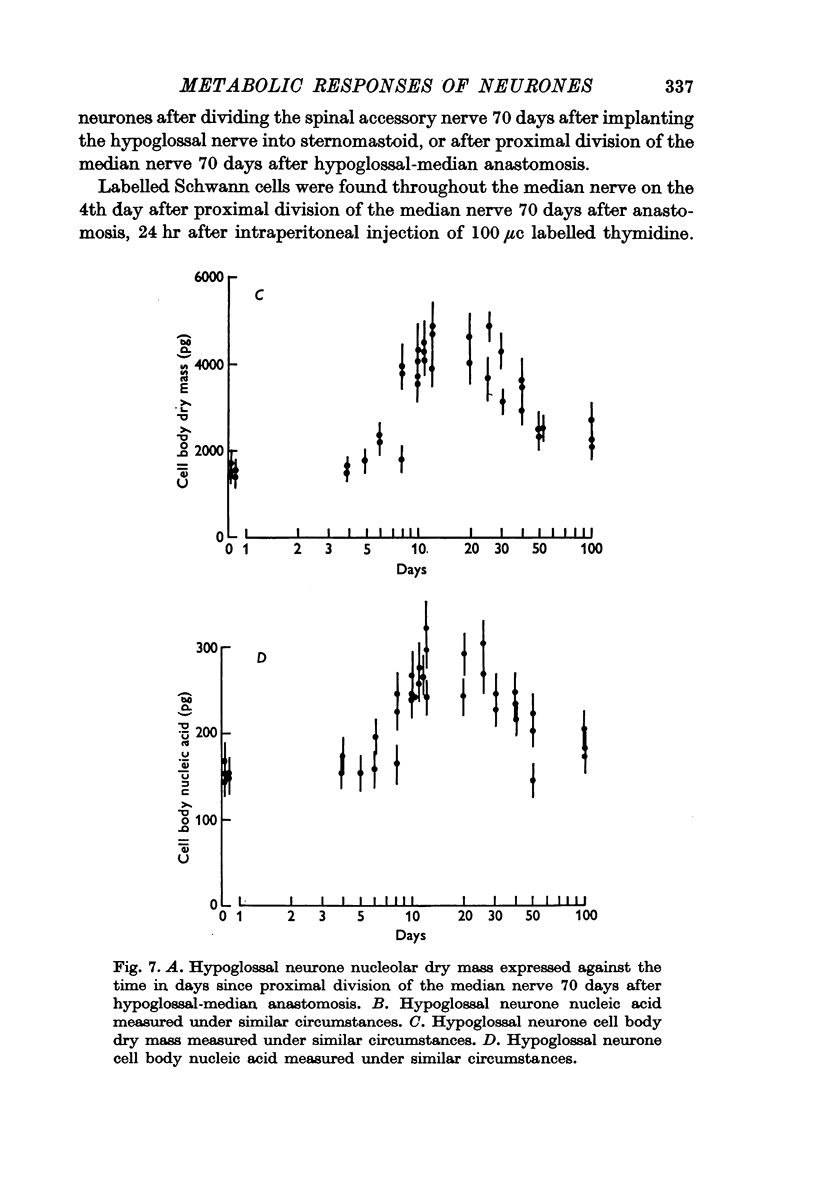
1. The nucleolar and cell body dry mass and nucleic acid content of hypoglossal neurones were measured in adult rats using interference microscopy and ultra-violet absorption microspectrography.
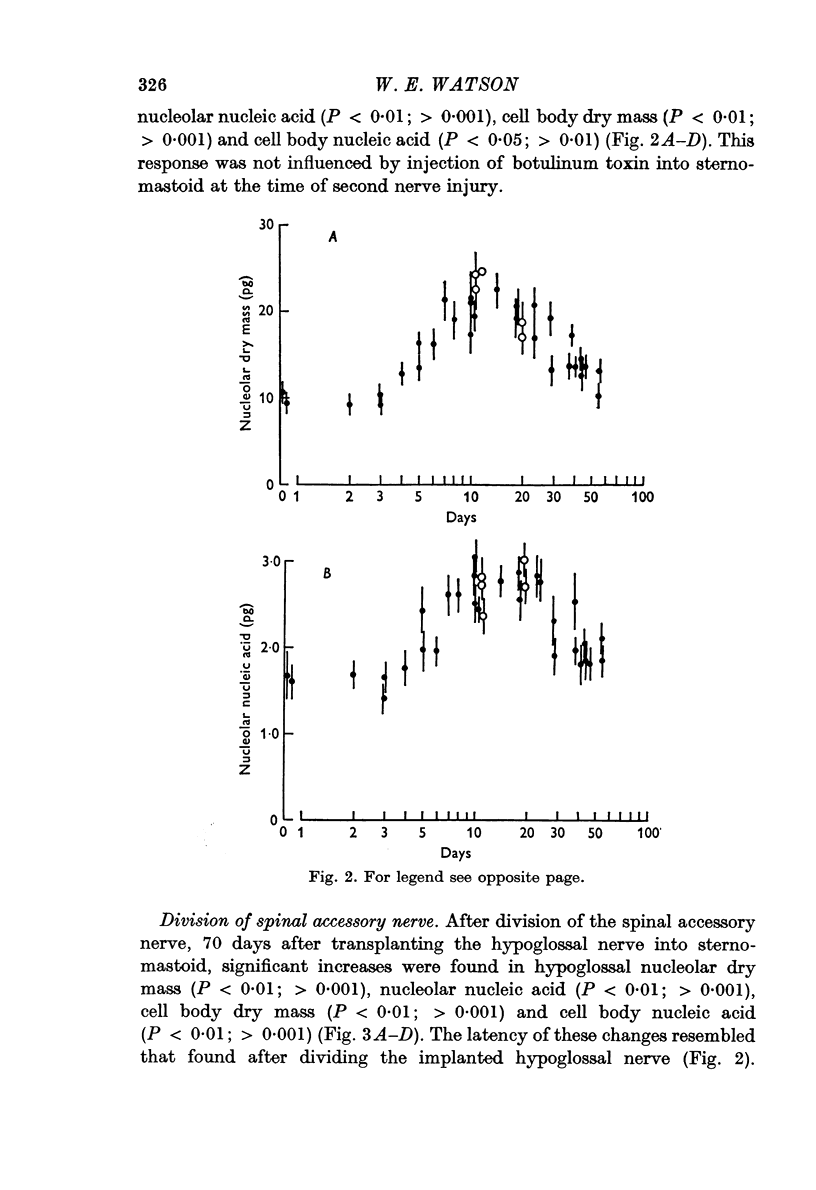
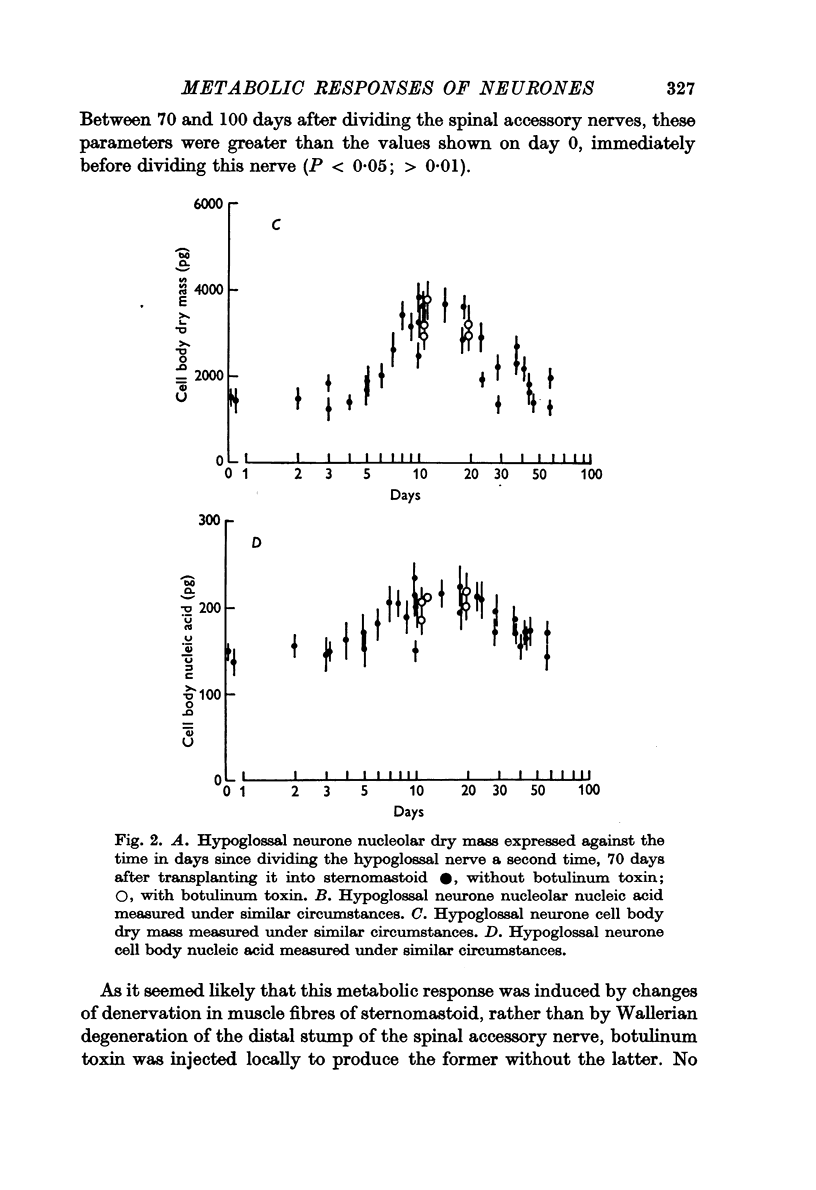
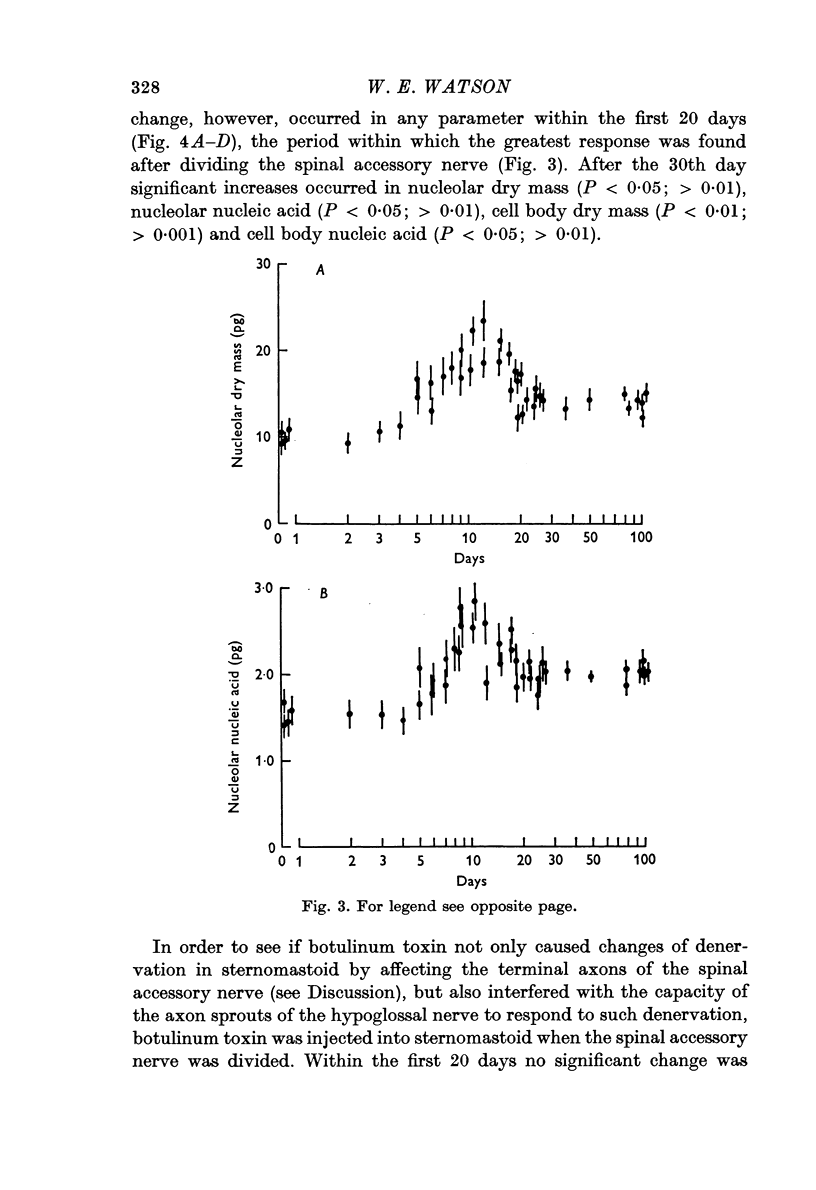
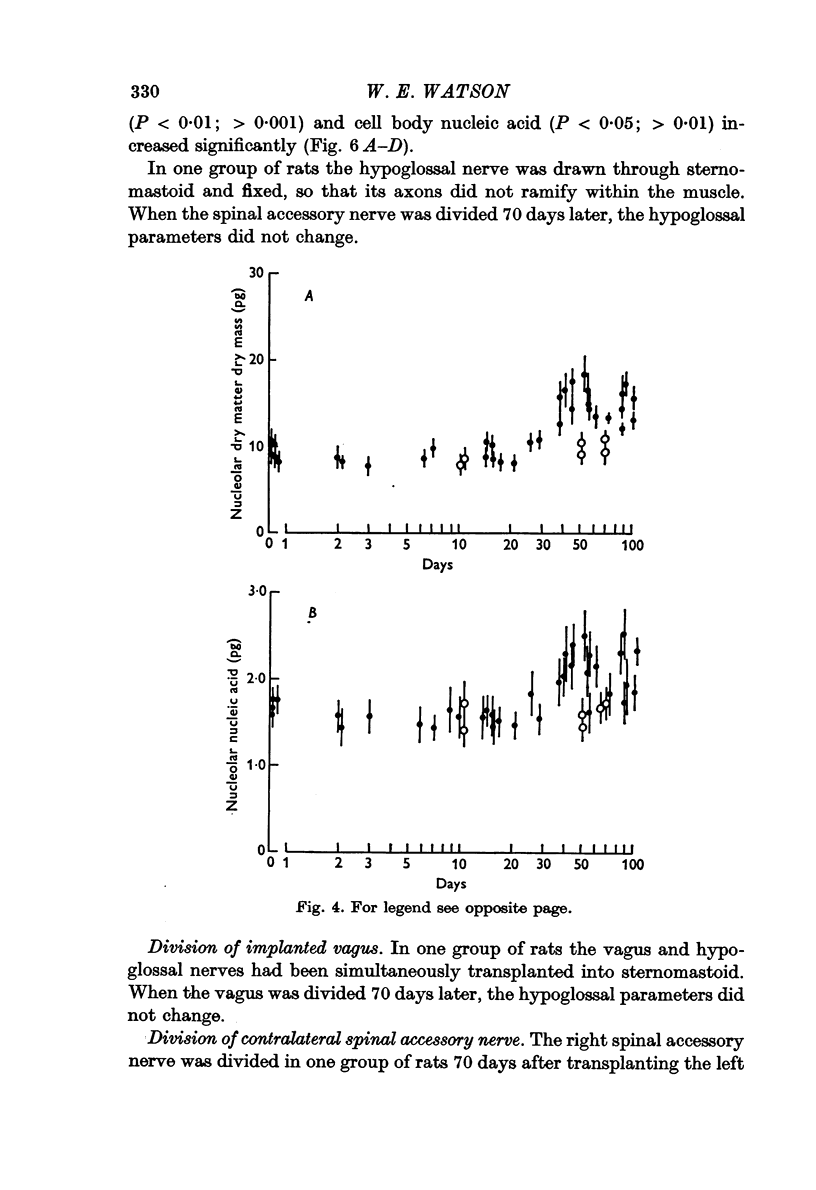
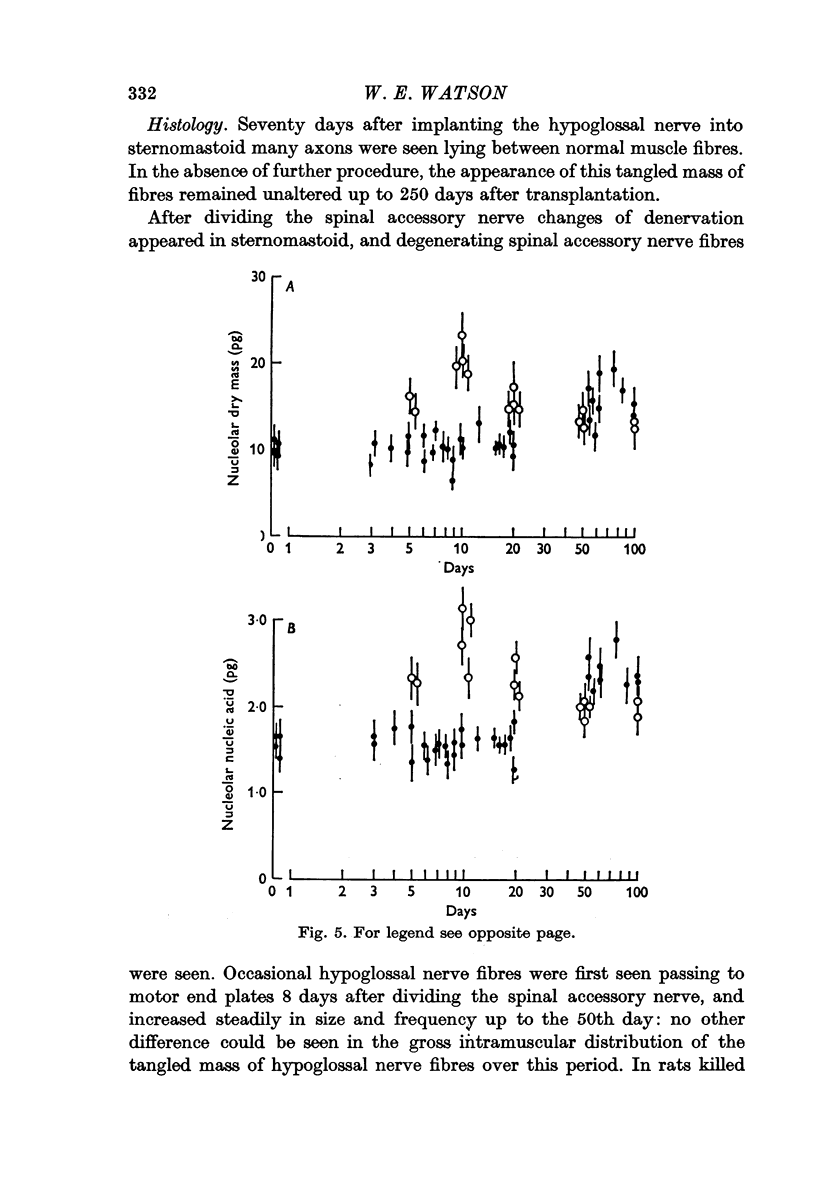
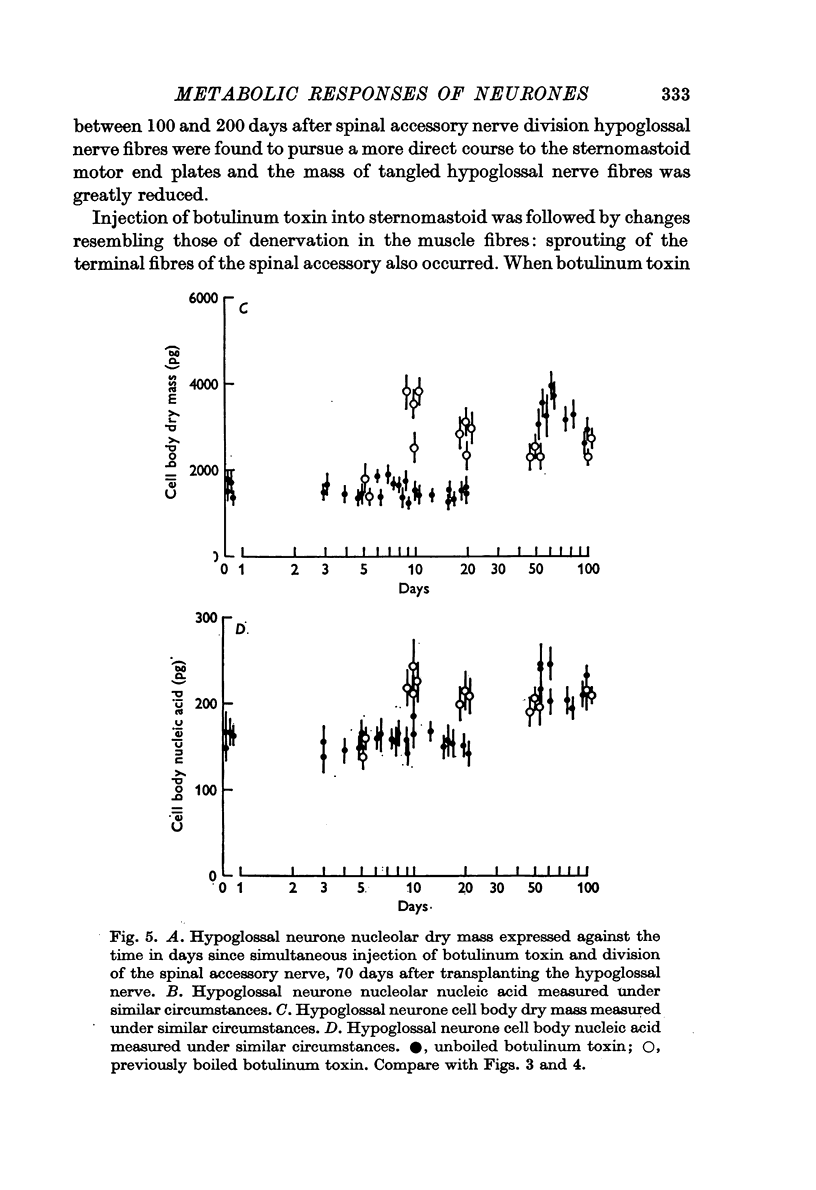
2. The left hypoglossal nerve was transplanted into the ipsilateral sternomastoid. Seventy days later the sternomastoid was denervated by dividing the ipsilateral spinal accessory nerve. This was followed by metabolic changes in hypoglossal nerve cells.
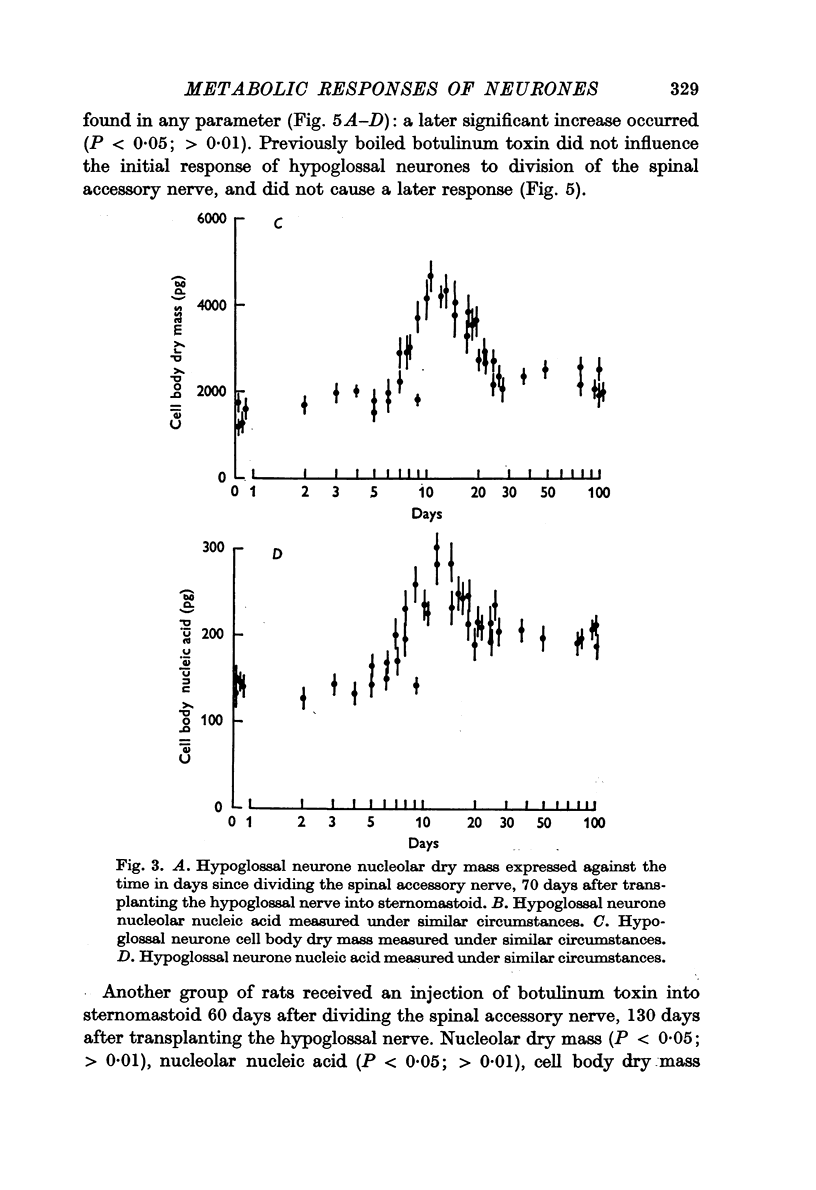
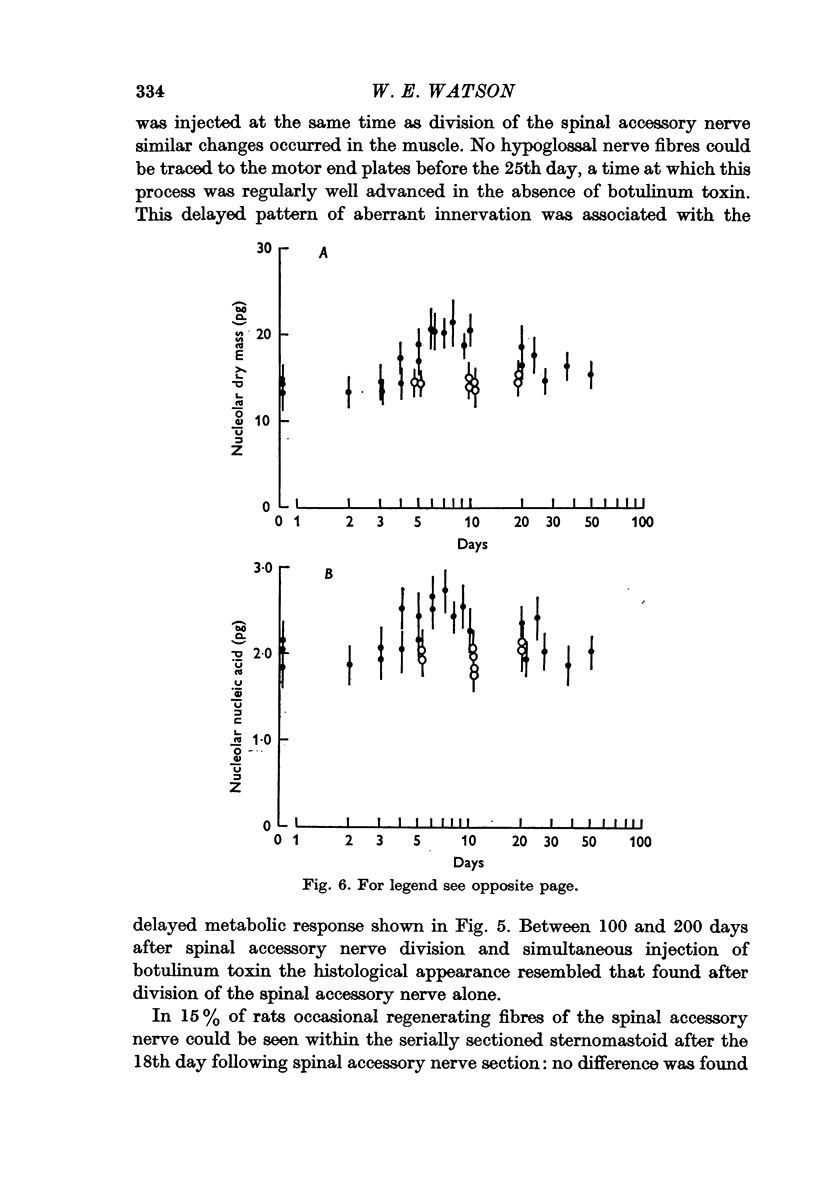
3. The changes induced in hypoglossal neurones by division of the ipsilateral accessory nerve did not occur if botulinum toxin was injected locally at the same time.
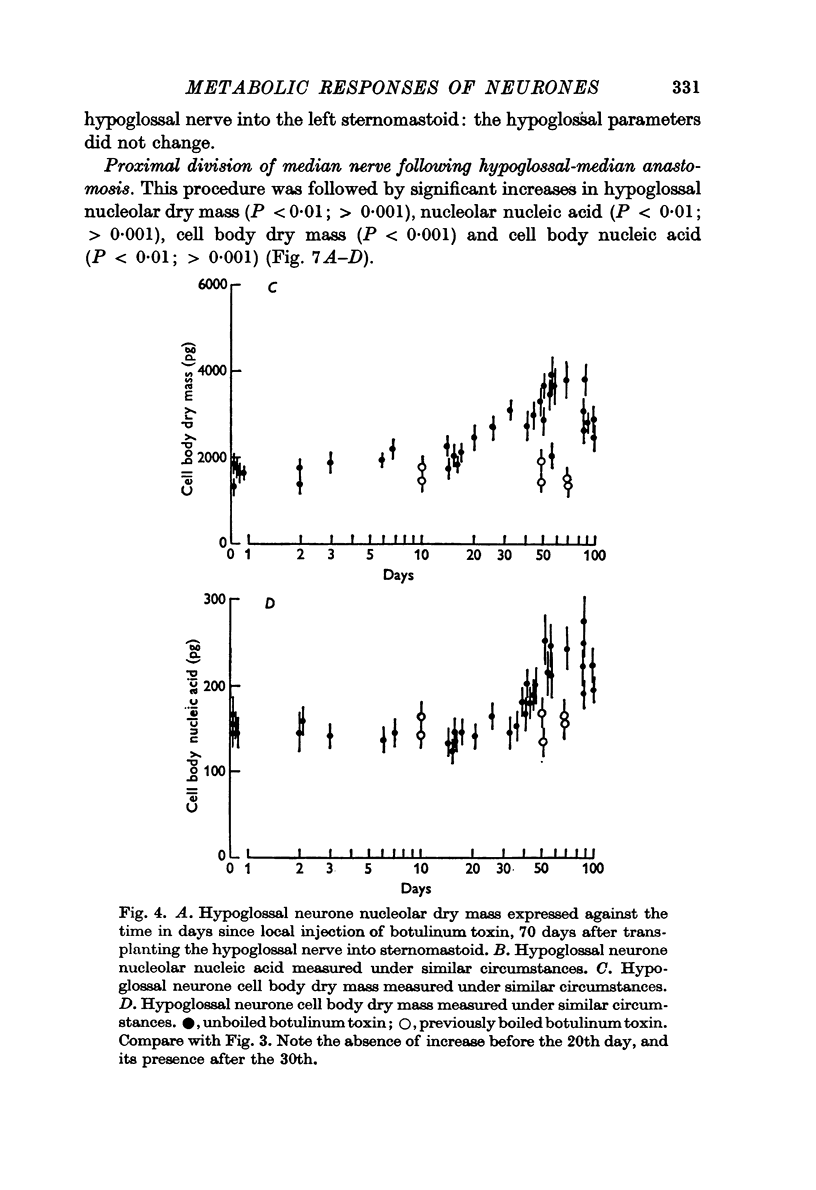
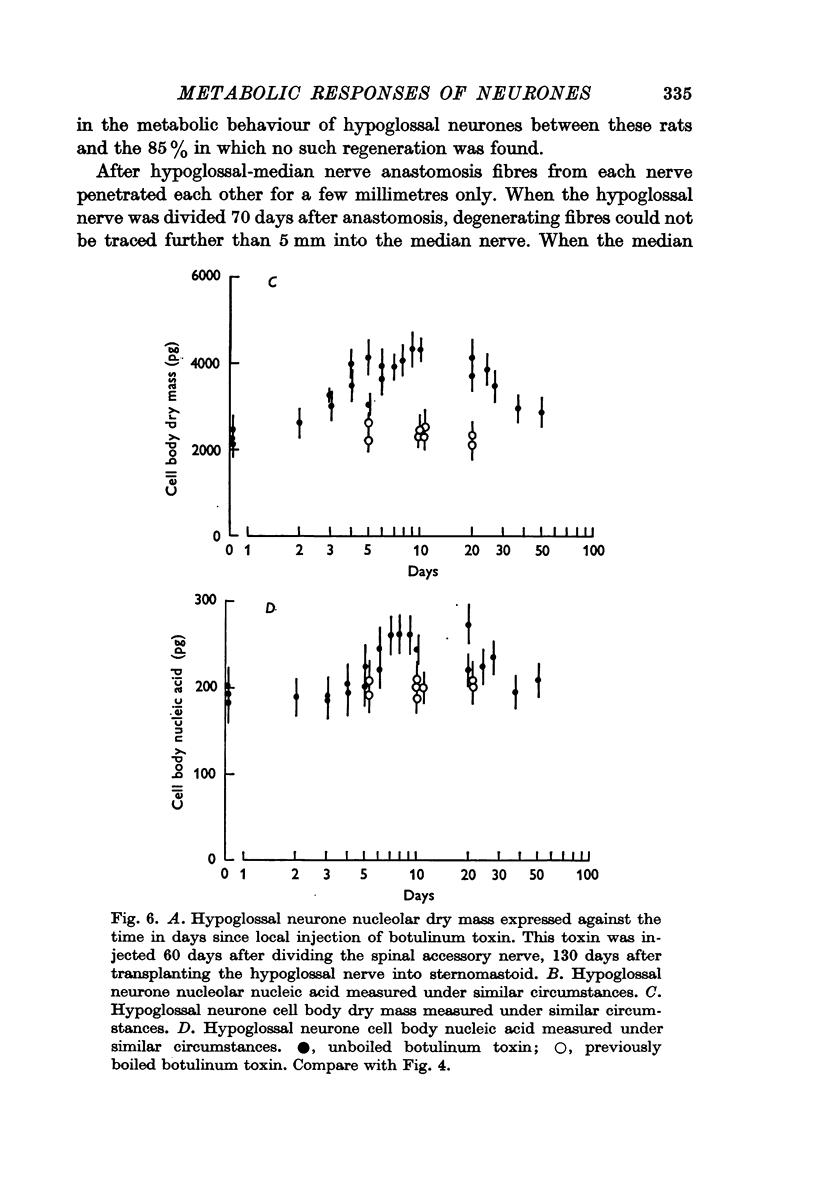
4. In other rats the left hypoglossal nerve was anastomosed to the proximal stump of the ipsilateral median nerve simultaneously divided at the level of the wrist. Seventy days later this median nerve was divided in the axilla. This was followed by metabolic changes in hypoglossal nerve cells.
5. These results are discussed in relation to the possible roles of reacting Schwann cells, degenerating axoplasm and denervated muscle in maintaining aspects of the metabolic response of nerve cells to injury.
6. It is suggested that the synthesis of acetylcholine by an axonal ending, or its release, is dependent upon the presence of an adjacent membrane which can respond to it, and that the metabolic changes measured in the nerve cell body are secondary to this response of the axon terminal.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBACHE N. A further survey of the action of Clostridium botulinum toxin upon different types of autonomic nerve fibre. J Physiol. 1951 Mar;113(1):1–17. doi: 10.1113/jphysiol.1951.sp004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AXELSSON J., THESLEFF S. A study of supersensitivity in denervated mammalian skeletal muscle. J Physiol. 1959 Jun 23;147(1):178–193. doi: 10.1113/jphysiol.1959.sp006233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken J. T. Growth of nerve implants in voluntary muscle. J Anat. 1950 Jan;84(Pt 1):38–49. [PMC free article] [PubMed] [Google Scholar]
- Aitken J. T., Sharman M., Young J. Z. Maturation of regenerating nerve fibres with various peripheral connexions. J Anat. 1947 Jan;81(Pt 1):1–22.2. [PMC free article] [PubMed] [Google Scholar]
- Ambache N. The peripheral action of Cl. botulinum toxin. J Physiol. 1949 Mar 15;108(2):127–141. [PMC free article] [PubMed] [Google Scholar]
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRATTGARD S. O., EDSTROM J. E., HYDEN H. The chemical changes in regenerating neurons. J Neurochem. 1957;1(4):316–325. doi: 10.1111/j.1471-4159.1957.tb12088.x. [DOI] [PubMed] [Google Scholar]
- BROOKS V. B. An intracellular study of the action of repetitive nerve volleys and of botulinum toxin on miniature end-plate potentials. J Physiol. 1956 Nov 28;134(2):264–277. doi: 10.1113/jphysiol.1956.sp005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B. The action of botulinum toxin on motor-nerve filaments. J Physiol. 1954 Mar 29;123(3):501–515. doi: 10.1113/jphysiol.1954.sp005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A. S. V., DICKENS F., ZATMAN L. J. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949 Aug;109(1-2):10–24. doi: 10.1113/jphysiol.1949.sp004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D., Ip M. C. Sprouting and degeneration of mammalian motor axons in normal and de-afferentated skeletal muscle. Proc R Soc Lond B Biol Sci. 1966 Jan 18;163(993):538–554. doi: 10.1098/rspb.1966.0008. [DOI] [PubMed] [Google Scholar]
- Cammermeyer J. Endothelial and intramural karyokinesis during retrograde reaction in the facial nucleus of rabbits of varying age. Ergeb Anat Entwicklungsgesch. 1965;38:23–45. [PubMed] [Google Scholar]
- DESMEDT J. E. The physio-pathology of neuromuscular transmission and the trophic influence of motor innervation. Am J Phys Med. 1959 Dec;38:248–261. [PubMed] [Google Scholar]
- DRAHOTA Z., HUDLICKA O. Effect of denervation on potassium-42 uptake by the rat muscle in vitro. Nature. 1960 Apr 30;186:396–397. doi: 10.1038/186396a0. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Strich S. J. The effects of botulinum toxin on the pattern of innervation of skeletal muscle in the mouse. Q J Exp Physiol Cogn Med Sci. 1968 Jan;53(1):84–89. doi: 10.1113/expphysiol.1968.sp001948. [DOI] [PubMed] [Google Scholar]
- Elsberg C. A. EXPERIMENTS ON MOTOR NERVE REGENERATION AND THE DIRECT NEUROTIZATION OF PARALYZED MUSCLES BY THEIR OWN AND BY FOREIGN NERVES. Science. 1917 Mar 30;45(1161):318–320. doi: 10.1126/science.45.1161.318. [DOI] [PubMed] [Google Scholar]
- Fex S., Sonesson B., Thesleff S., Zelená J. Nerve implants in botulinum poisoned mammalian muscle. J Physiol. 1966 Jun;184(4):872–882. doi: 10.1113/jphysiol.1966.sp007953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAMBLE H. J. COMPARATIVE ELECTRON-MICROSCOPIC OBSERVATIONS ON THE CONNECTIVE TISSUES OF A PERIPHERAL NERVE AND A SPINAL NERVE ROOT IN THE RAT. J Anat. 1964 Jan;98:17–26. [PMC free article] [PubMed] [Google Scholar]
- GUTH L., ZALEWSKI A. A. Disposition of cholinesterase following implantation of nerve into innervated and denervated muscle. Exp Neurol. 1963 Apr;7:316–326. doi: 10.1016/0014-4886(63)90078-5. [DOI] [PubMed] [Google Scholar]
- GWYN D. G., AITKEN J. T. NEW MOTOR END-PLATES AND THEIR RELATIONSHIP TO MUSCLE FIBRE INJURY. Nature. 1964 Aug 8;203:651–652. doi: 10.1038/203651a0. [DOI] [PubMed] [Google Scholar]
- Guth L. "Trophic" influences of nerve on muscle. Physiol Rev. 1968 Oct;48(4):645–687. doi: 10.1152/physrev.1968.48.4.645. [DOI] [PubMed] [Google Scholar]
- Gutmann E., Young J. Z. The re-innervation of muscle after various periods of atrophy. J Anat. 1944 Jan;78(Pt 1-2):15–43. [PMC free article] [PubMed] [Google Scholar]
- Gwyn D. G., Aitken J. T. The formation of new motor endplates in mammalian skeletal muscle. J Anat. 1966 Jan;100(Pt 1):111–126. [PMC free article] [PubMed] [Google Scholar]
- HARRIS E. J., NICHOLLS J. G. The effect of denervation on the rate of entry of potassium into frog muscle. J Physiol. 1956 Feb 28;131(2):473–476. doi: 10.1113/jphysiol.1956.sp005476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE DEVELOPMENT OF ACETYLCHOLINE SENSITIVITY IN NERVE-FREE SEGMENTS OF SKELETAL MUSCLE. J Physiol. 1964 Mar;170:389–396. doi: 10.1113/jphysiol.1964.sp007339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILEDI R. Induced innervation of end-plate free muscle segments. Nature. 1962 Jan 20;193:281–282. doi: 10.1038/193281a0. [DOI] [PubMed] [Google Scholar]
- MILEDI R. Properties of regenerating neuromuscular synapses in the frog. J Physiol. 1960 Nov;154:190–205. doi: 10.1113/jphysiol.1960.sp006573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLLS J. G. The electrical properties of denervated skeletal muscle. J Physiol. 1956 Jan 27;131(1):1–12. doi: 10.1113/jphysiol.1956.sp005440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter R. Synaptic potentials in hypoglossal motoneurones. J Physiol. 1965 Sep;180(1):209–224. [PMC free article] [PubMed] [Google Scholar]
- SHANTHAVEERAPPA T. R., BOURNE G. H. The 'perineural epithelium', a metabolically active, continuous, protoplasmic cell barrier surrounding peripheral nerve fasciculi. J Anat. 1962 Oct;96:527–537. [PMC free article] [PubMed] [Google Scholar]
- Sjöstrand J. Proliferative changes in glial cells during nerve regeneration. Z Zellforsch Mikrosk Anat. 1965 Nov 15;68(4):481–493. doi: 10.1007/BF00347712. [DOI] [PubMed] [Google Scholar]
- Sunderland S. The connective tissues of peripheral nerves. Brain. 1965 Nov;88(4):841–854. doi: 10.1093/brain/88.4.841. [DOI] [PubMed] [Google Scholar]
- THESLEFF S. Supersensitivity of skeletal muscle produced by botulinum toxin. J Physiol. 1960 Jun;151:598–607. doi: 10.1113/jphysiol.1960.sp006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. An autoradiographic study of the incorporation of nucleic-acid precursors by neurones and glia during nerve regeneration. J Physiol. 1965 Oct;180(4):741–753. doi: 10.1113/jphysiol.1965.sp007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. Observations on the nucleolar and total cell body nucleic acid of injured nerve cells. J Physiol. 1968 Jun;196(3):655–676. doi: 10.1113/jphysiol.1968.sp008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson W. E. The response of motor neurones to intramuscular injection of botulinum toxin. J Physiol. 1969 Jun;202(3):611–630. doi: 10.1113/jphysiol.1969.sp008830. [DOI] [PMC free article] [PubMed] [Google Scholar]


