Abstract
1. Fifty-four neurones of the caudal part of the nucleus reticularis thalami (nuc. ret.) were recorded during different phases of sleep and wakefulness in unanaesthetized freely moving cats.
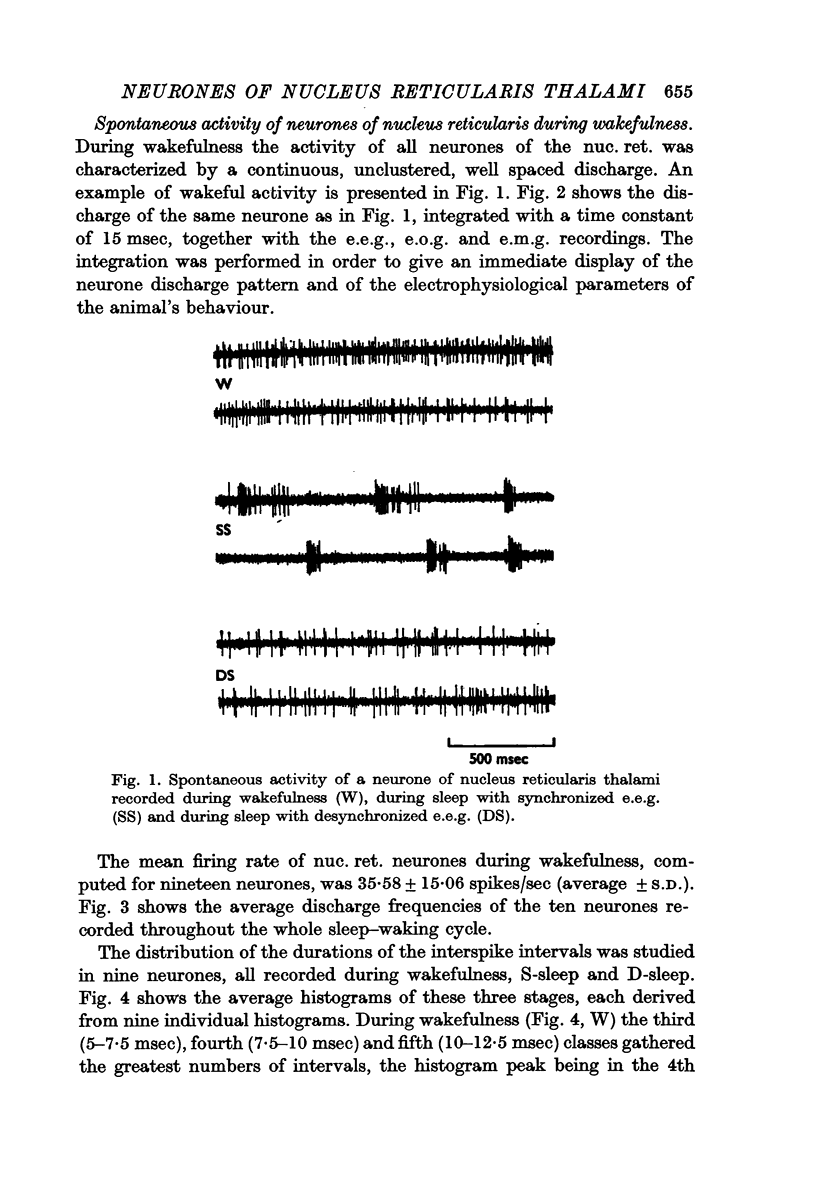
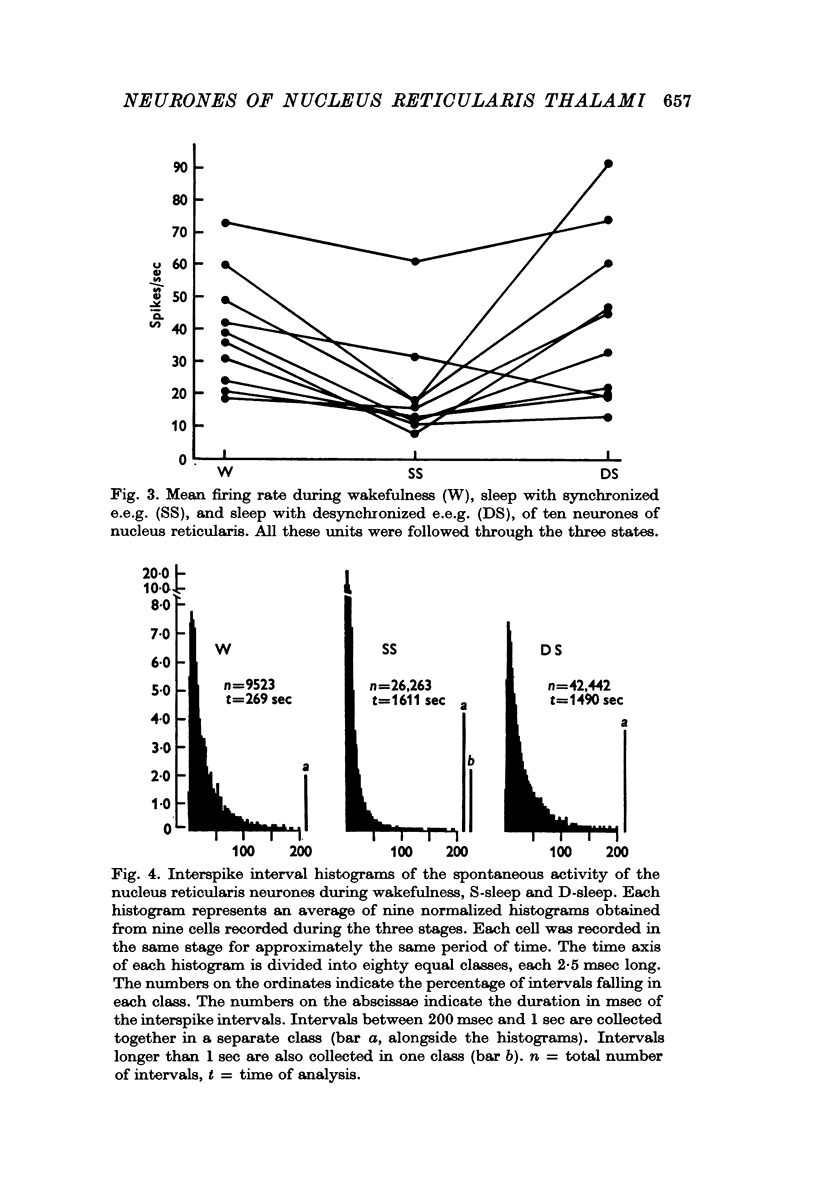
2. During wakefulness the activity of the neurones was characterized by a continuous, well-spaced discharge. The mean firing rate was 35·58 ± 15·06 spikes/sec (average ± S.D.).
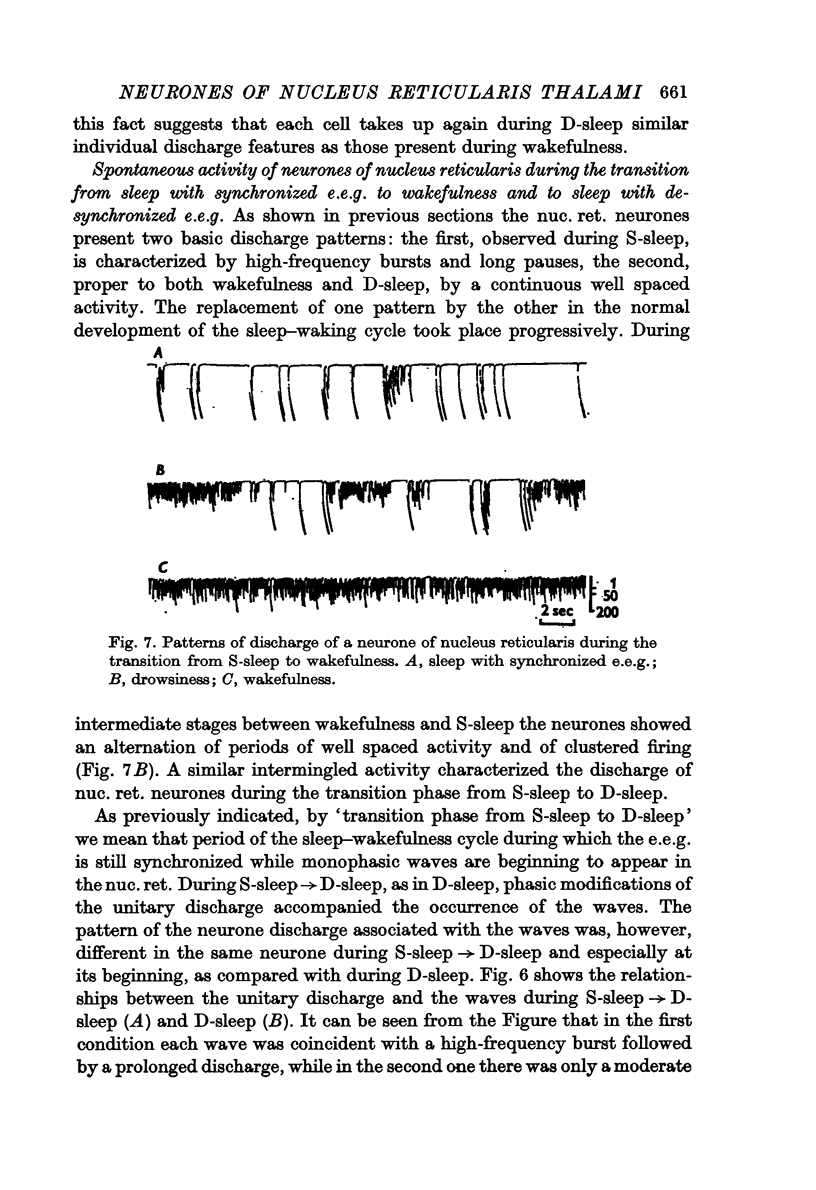
3. During sleep with synchronized e.e.g. (S-sleep) the neurones fired in high frequency bursts with long pauses in between. Each burst was formed of 10-15 spikes. Often the bursts were followed by prolonged discharges formed of spikes well spaced one from the other. Bursts followed by prolonged activity were more commonly observed at the beginning of S-sleep and during the S-sleep periods preceding sleep with desynchronized e.e.g., whereas bursts immediately followed by silence were more frequent in the S-sleep periods with e.e.g. delta waves. The long periods of silence between the bursts usually lasted over 200 msec and values greater than 1 sec were frequently found. The mean firing rate of neurones during S-sleep was 19·22 ± 10·50 spikes/sec.
4. During sleep with desynchronized e.e.g. (D-sleep) the activity of the neurones was, as during wakefulness, characterized by a continuous, well spaced, unclustered discharge. The mean firing rate was 40·00 ± 18·74 spikes/sec. During the rapid eye movements of this phase most units increased the frequency of their discharge, which, nevertheless, maintained the unclustered feature proper to the desynchronized phase of sleep.
5. Interspike interval distribution was similar during wakefulness and sleep with desynchronized e.e.g., whereas that for sleep with synchronized e.e.g. was markedly different from those for both the other stages.
6. The implications of the striking similarity between the activity of reticularis neurones during wakefulness and sleep with desynchronized e.e.g. are discussed.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O. PROPERTIES OF AFFERENT SYNAPSES AND SENSORY NEURONS IN THE LATERAL GENICULATE NUCLEUS. Int Rev Neurobiol. 1964;6:191–255. doi: 10.1016/s0074-7742(08)60770-9. [DOI] [PubMed] [Google Scholar]
- BROOKS D. C., BIZZI E. BRAIN STEM ELECTRICAL ACTIVITY DURING DEEP SLEEP. Arch Ital Biol. 1963 Oct 5;101:648–665. [PubMed] [Google Scholar]
- Berger R. J. Oculomotor control: a possible function of rem sleep. Psychol Rev. 1969 Mar;76(2):144–164. doi: 10.1037/h0027235. [DOI] [PubMed] [Google Scholar]
- Berlucchi G., Munson J. B., Rizzolatti G. Surgical immobilization of the eye and pupil, permitting stable photic stimulation of freely moving cats. Electroencephalogr Clin Neurophysiol. 1966 Nov;21(5):504–505. doi: 10.1016/0013-4694(66)90202-1. [DOI] [PubMed] [Google Scholar]
- Bizzi E. Discharge patterns of single geniculate neurons during the rapid eye movements of sleep. J Neurophysiol. 1966 Nov;29(6):1087–1095. doi: 10.1152/jn.1966.29.6.1087. [DOI] [PubMed] [Google Scholar]
- Brooks D. C. Localization of the lateral geniculate nucleus monophasic waves associated with paradoxical sleep in the cat. Electroencephalogr Clin Neurophysiol. 1967 Aug;23(2):123–133. doi: 10.1016/0013-4694(67)90102-2. [DOI] [PubMed] [Google Scholar]
- Brooks D. C. Waves associated with eye movement in the awake and sleeping cat. Electroencephalogr Clin Neurophysiol. 1968 Jun;24(6):532–541. doi: 10.1016/0013-4694(68)90042-4. [DOI] [PubMed] [Google Scholar]
- Calvet J., Calvet M. C., Langlois J. M. Diffuse cortical activation waves during so-called desynchronized EEG patterns. J Neurophysiol. 1965 Sep;28(5):893–907. doi: 10.1152/jn.1965.28.5.893. [DOI] [PubMed] [Google Scholar]
- EVARTS E. V. Effects of sleep and waking on spontaneous and evoked discharge of single units in visual cortex. Fed Proc. 1960 Dec;19:828–837. [PubMed] [Google Scholar]
- EVARTS E. V. TEMPORAL PATTERNS OF DISCHARGE OF PYRAMIDAL TRACT NEURONS DURING SLEEP AND WAKING IN THE MONKEY. J Neurophysiol. 1964 Mar;27:152–171. doi: 10.1152/jn.1964.27.2.152. [DOI] [PubMed] [Google Scholar]
- Ephron H. S., Carrington P. Rapid eye movement sleep and cortical homeostasis. Psychol Rev. 1966 Nov;73(6):500–526. doi: 10.1037/h0023888. [DOI] [PubMed] [Google Scholar]
- Feldman M., Cohen B. Electrical activity in the lateral geniculate body of the alert monkey associated with eye movements. J Neurophysiol. 1968 May;31(3):455–466. doi: 10.1152/jn.1968.31.3.455. [DOI] [PubMed] [Google Scholar]
- Findlay A. L., Hayward J. N. Spontaneous activity of single neurones in the hypothalamus of rabbits during sleep and waking. J Physiol. 1969 Mar;201(1):237–258. doi: 10.1113/jphysiol.1969.sp008753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H. Single unit activity in lateral geniculate body and optic tract of unrestrained cats. J Physiol. 1960 Jan;150:91–104. doi: 10.1113/jphysiol.1960.sp006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouvet M. Neurophysiology of the states of sleep. Physiol Rev. 1967 Apr;47(2):117–177. doi: 10.1152/physrev.1967.47.2.117. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T. Spontaneous unitary discharges of the mesencephalic reticular formation during sleep and wakefulness in normal and chronically blinded cats. Brain Res. 1969 Jul;14(2):506–509. doi: 10.1016/0006-8993(69)90125-5. [DOI] [PubMed] [Google Scholar]
- Maffei L., Moruzzi G., Rizzolatti G. Influence of sleep and wakefulness on the response of lateral geniculate units to sinewave photic stimulation. Arch Ital Biol. 1965 Dec 10;103(4):596–608. [PubMed] [Google Scholar]
- Maffei L., Rizzolatti G. Effect of synchronized sleep on the response of lateral geniculate units to flashes of light. Arch Ital Biol. 1965 Dec 10;103(4):609–622. [PubMed] [Google Scholar]
- Mukhametov L. M., Rizzolatti G., Seitun A. An analysis of the spontaneous activity of lateral geniculate neurons and of optic tract fibers in free moving cats. Arch Ital Biol. 1970 Apr;108(2):325–347. [PubMed] [Google Scholar]
- Mukhametov L. M., Rizzolatti G. Unit responses of the lateral geniculate body to light flashes in free moving unrestrained cats. Experientia. 1968 Sep 15;24(9):911–911. doi: 10.1007/BF02138648. [DOI] [PubMed] [Google Scholar]
- Mukhametov L., Rizzolatti G. Effect of sleep and waking on flash evoked discharges of lateral geniculate units in unrestrained cats. Brain Res. 1969 Apr;13(2):404–406. doi: 10.1016/0006-8993(69)90300-x. [DOI] [PubMed] [Google Scholar]
- Roffwarg H. P., Muzio J. N., Dement W. C. Ontogenetic development of the human sleep-dream cycle. Science. 1966 Apr 29;152(3722):604–619. doi: 10.1126/science.152.3722.604. [DOI] [PubMed] [Google Scholar]
- Sakakura H., Iwama K. Effects of bilateral eye enucleation upon single unit activity of the lateral geniculate body in free behaving cats. Brain Res. 1967 Dec;6(4):667–678. doi: 10.1016/0006-8993(67)90124-2. [DOI] [PubMed] [Google Scholar]
- Sakakura H. Spontaneous and evoked unitary activities of cat lateral geniculate neurons in sleep and wakefulness. Jpn J Physiol. 1968 Feb 15;18(1):23–42. doi: 10.2170/jjphysiol.18.23. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. Structural organization of nonspecific thalamic nuclei and their projection toward cortex. Brain Res. 1967 Sep;6(1):60–94. doi: 10.1016/0006-8993(67)90183-7. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Scheibel A. B. The organization of the nucleus reticularis thalami: a Golgi study. Brain Res. 1966 Jan;1(1):43–62. doi: 10.1016/0006-8993(66)90104-1. [DOI] [PubMed] [Google Scholar]


