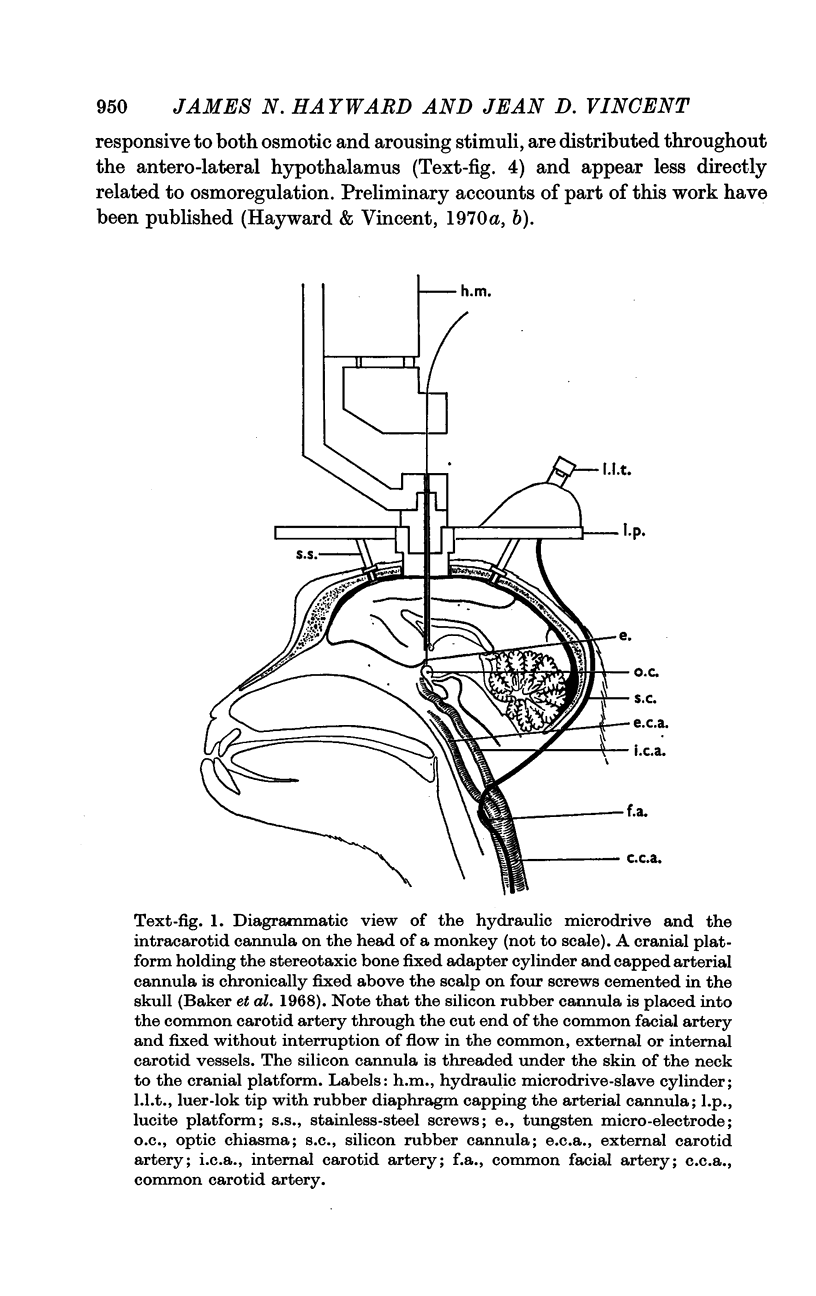
Abstract
1. We recorded with tungsten micro-electrodes the activity of single neurones in the supraoptic nucleus (NSO) and adjacent regions of the hypothalamus while repeatedly injecting solutions of varying tonicity into the common carotid artery of trained, unanaesthetized monkeys who accepted the experimental restraints without anxiety.
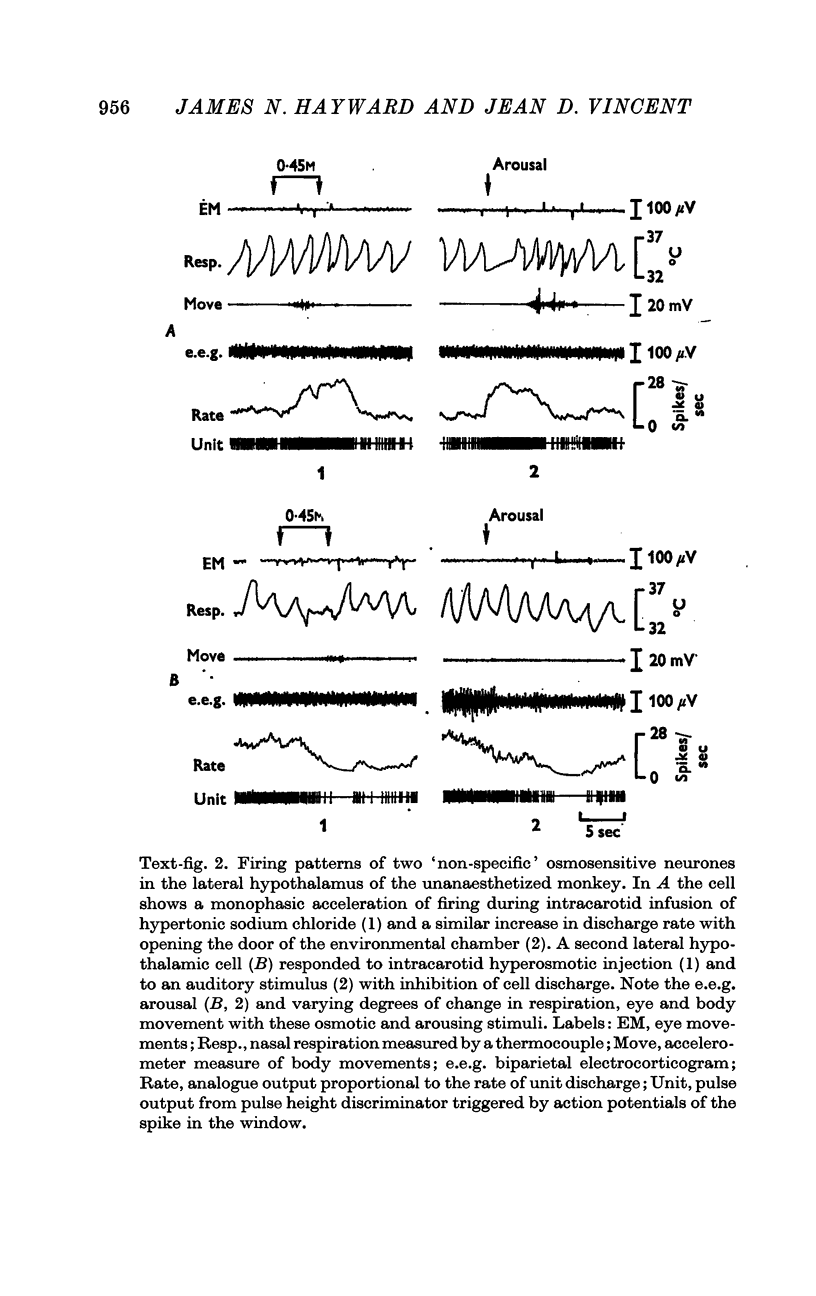
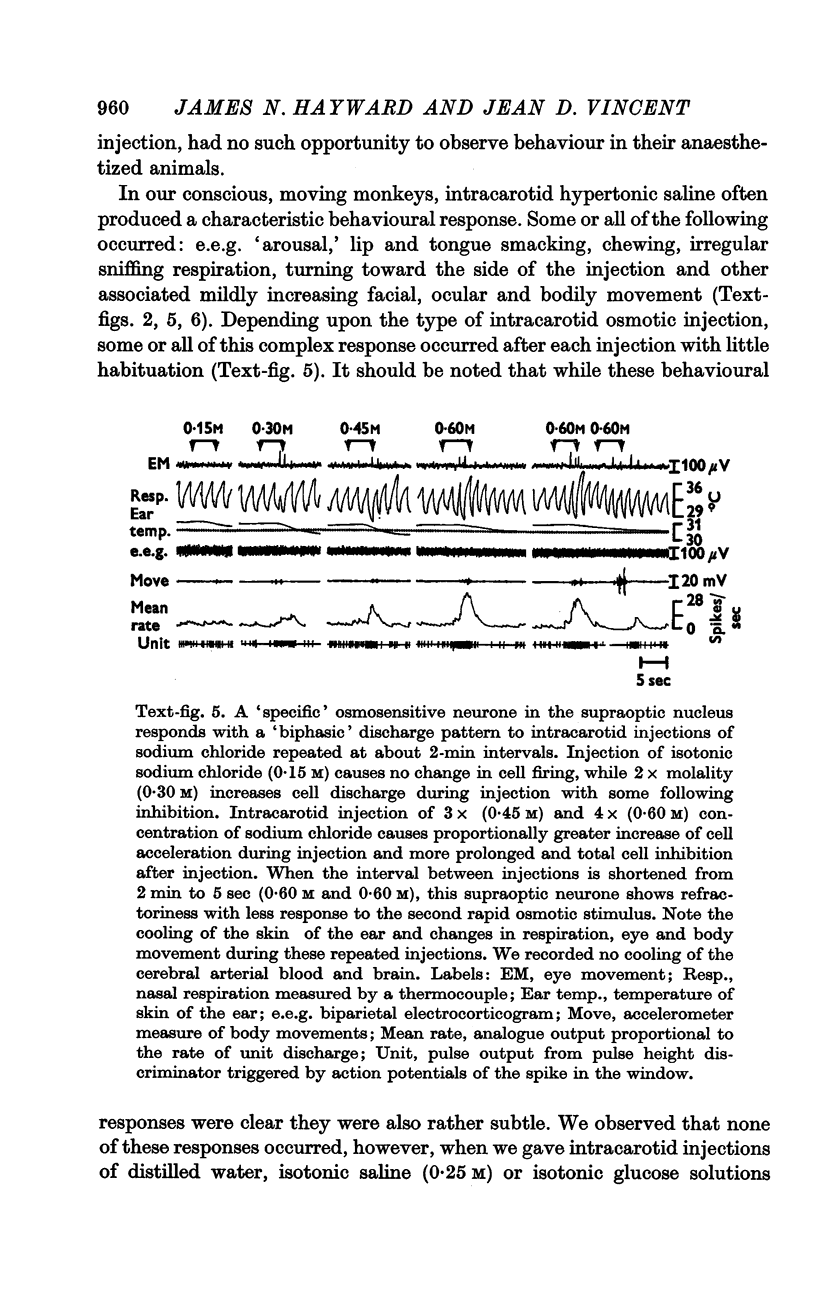
2. Intracarotid injections of mildly hypertonic solutions of sodium chloride produced a characteristic behavioural response during and immediately after injection: e.e.g. `arousal,' lip and tongue smacking, chewing, irregular sniffing respiration and associated mildly increased movement of face, eyes and body.
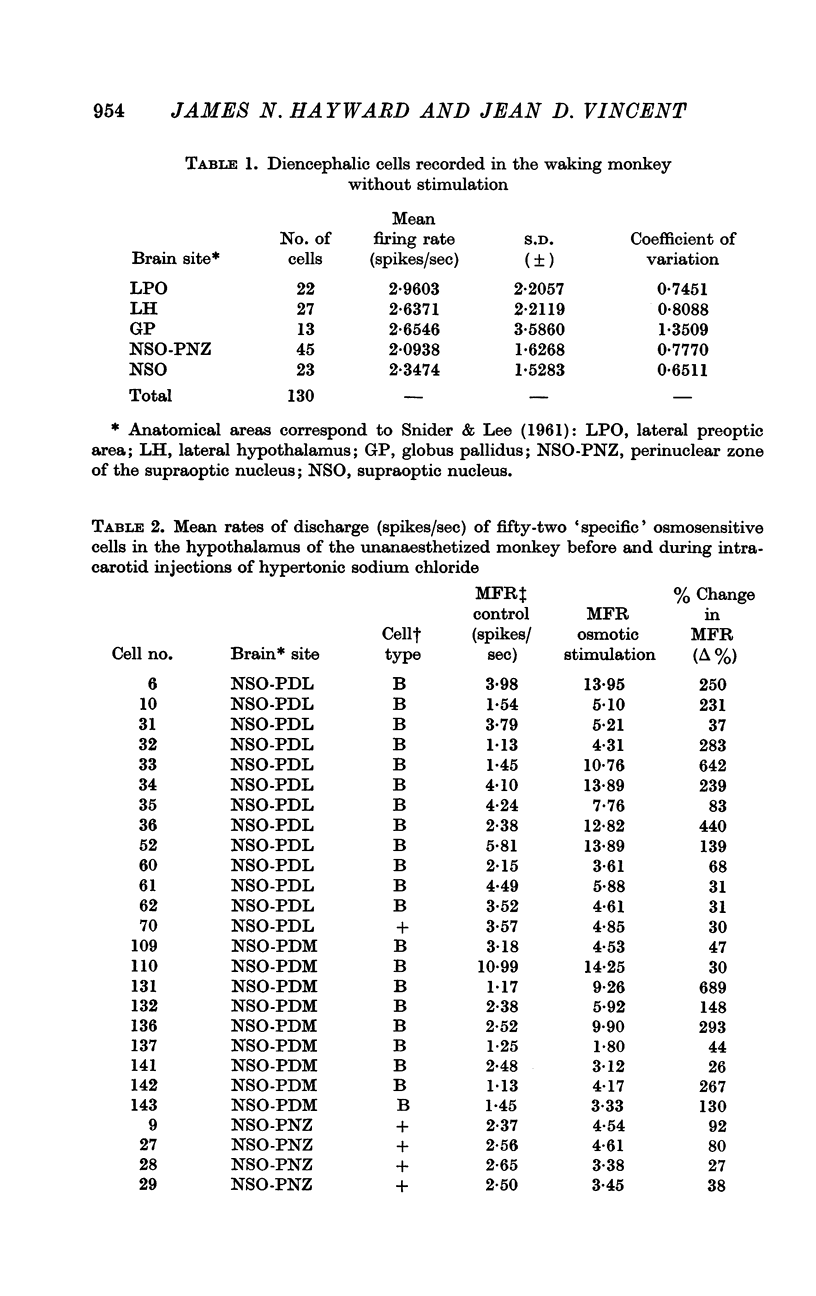
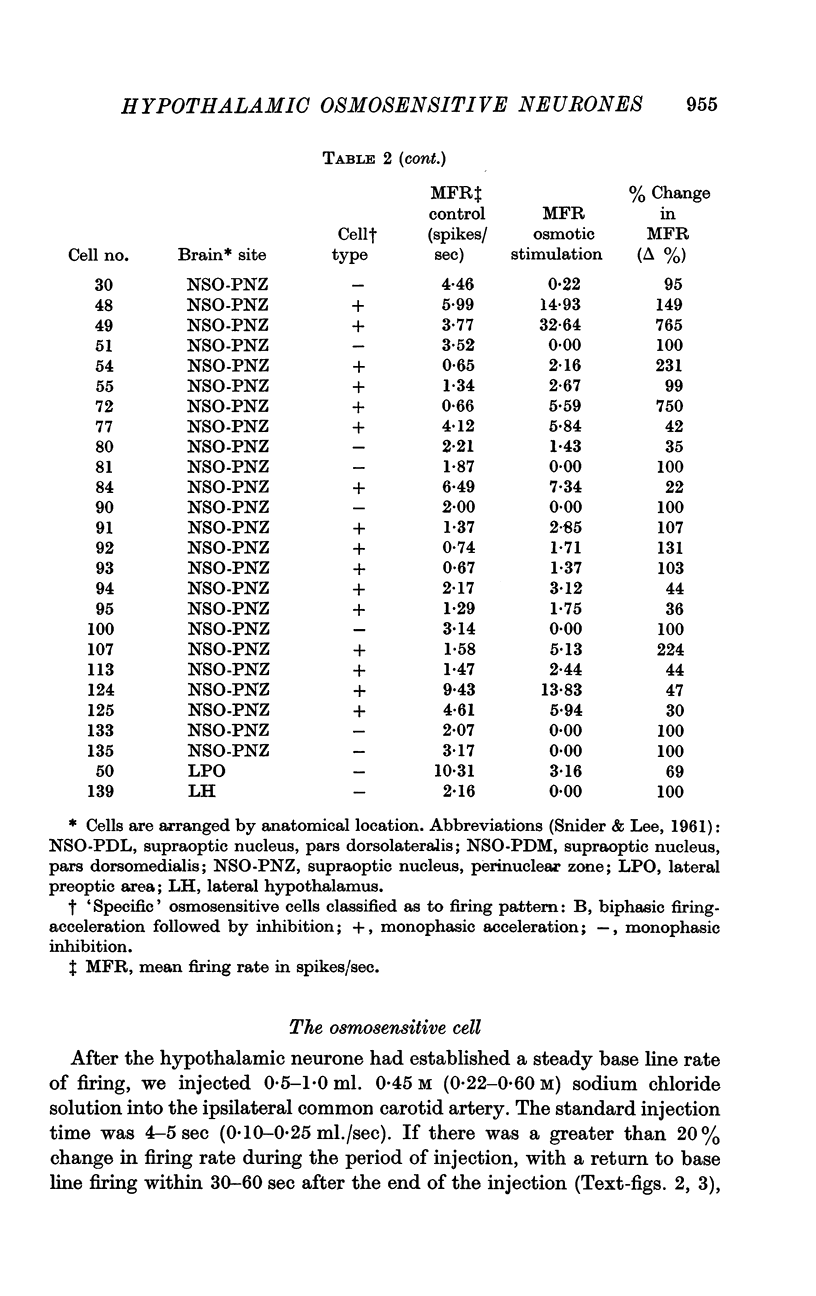
3. Of the 130 cells analysed during hypertonic intracarotid injections, 105 (81%) were osmosensitive. Twenty-five (19%) of the cells studied during similar injections were non-osmosensitive. On the basis of the anatomical location of the cells, the pattern of discharge to intracarotid osmotic stimuli and the response to arousing sensory stimuli, we divided the osmosensitive cells into two major groups, `specific' and `non-specific' osmosensitive cells.
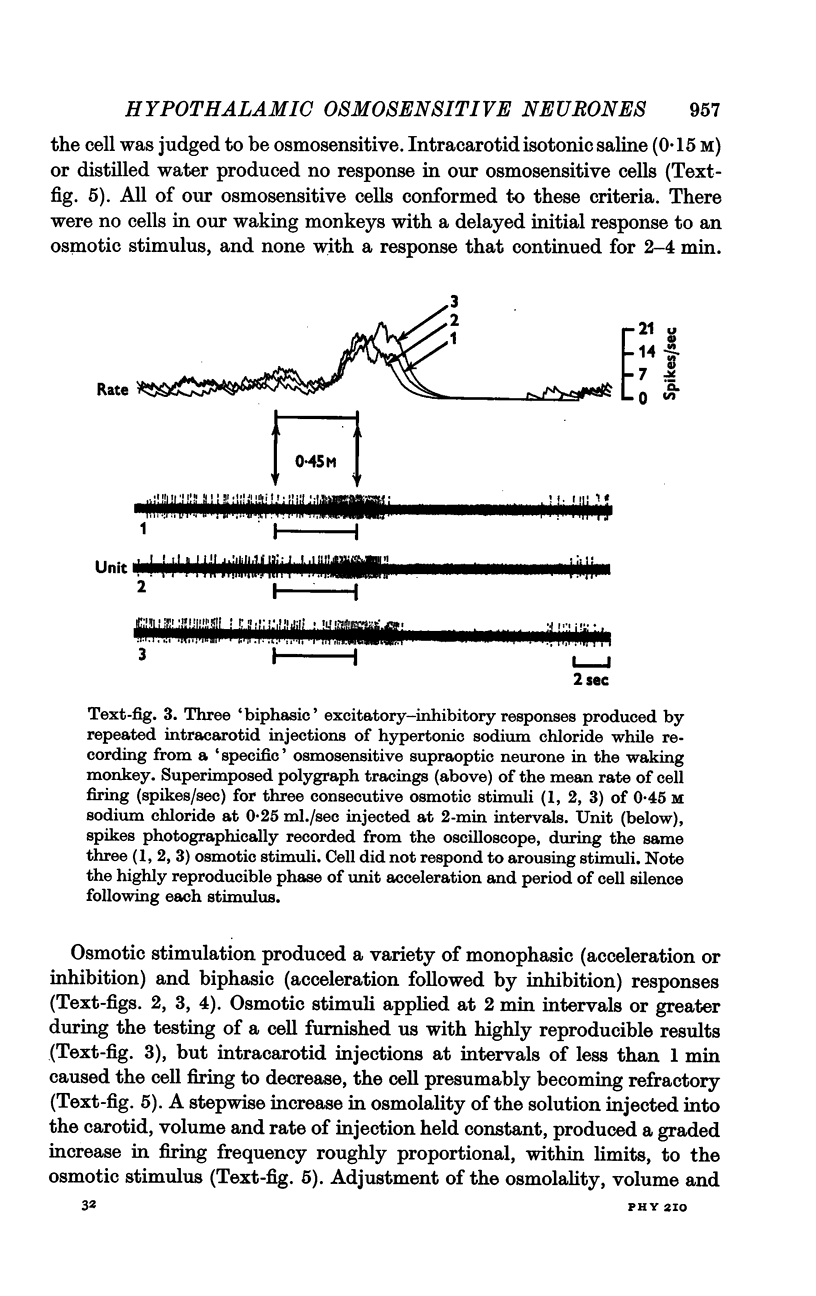
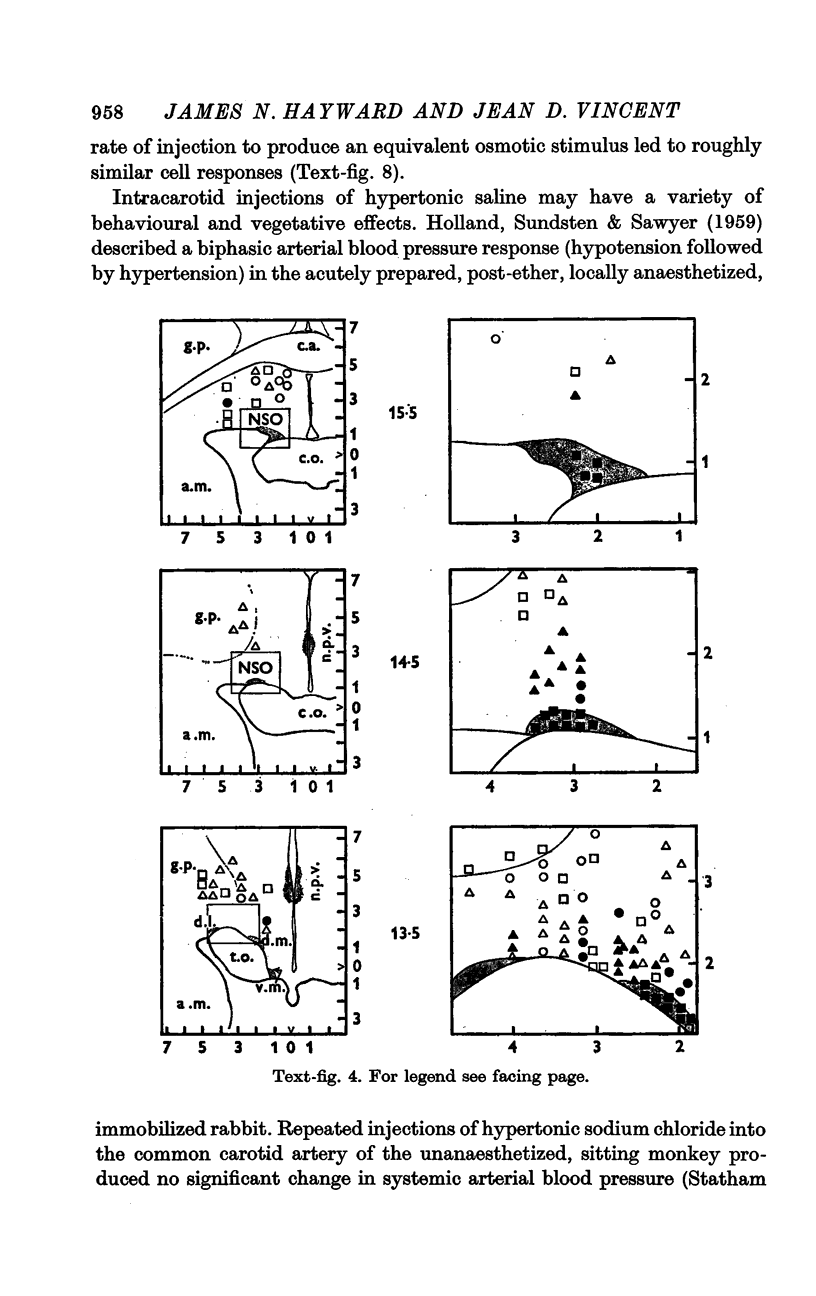
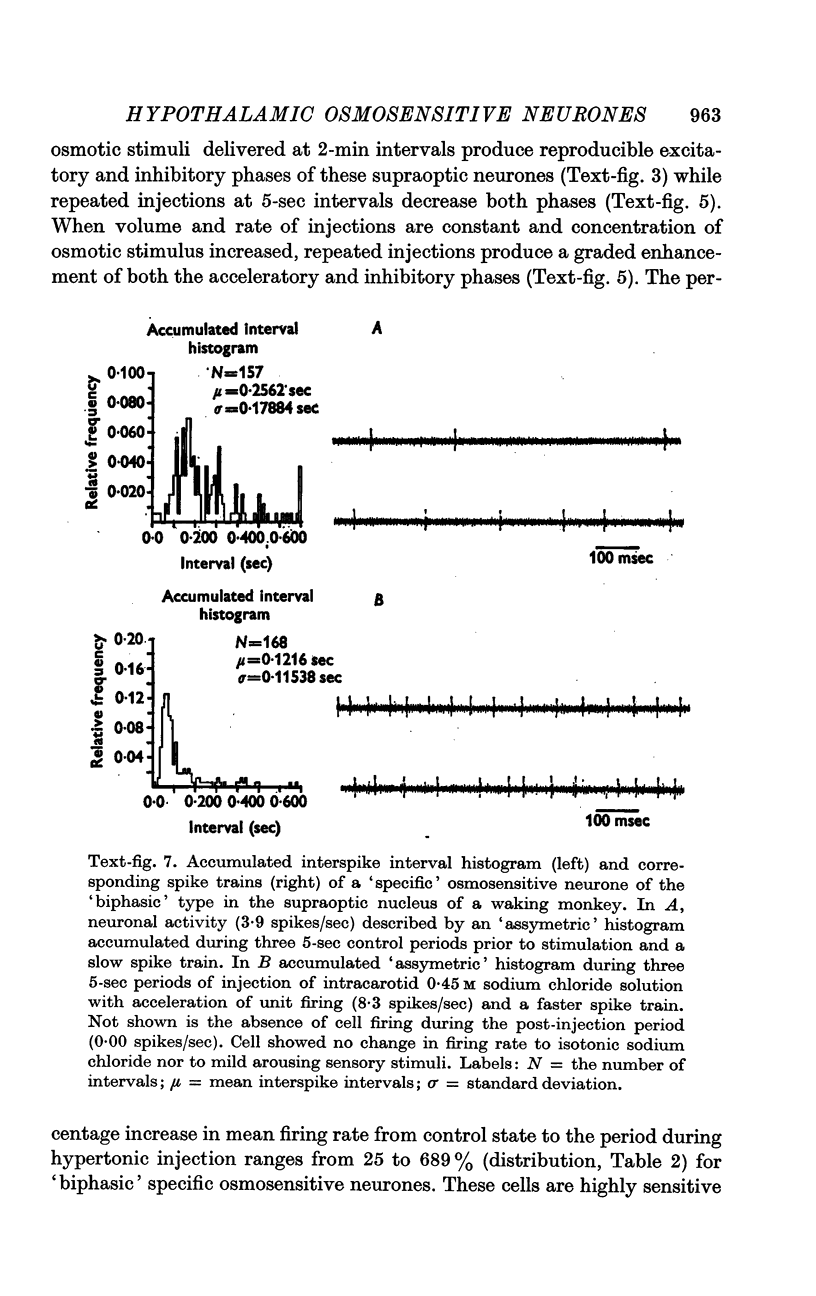
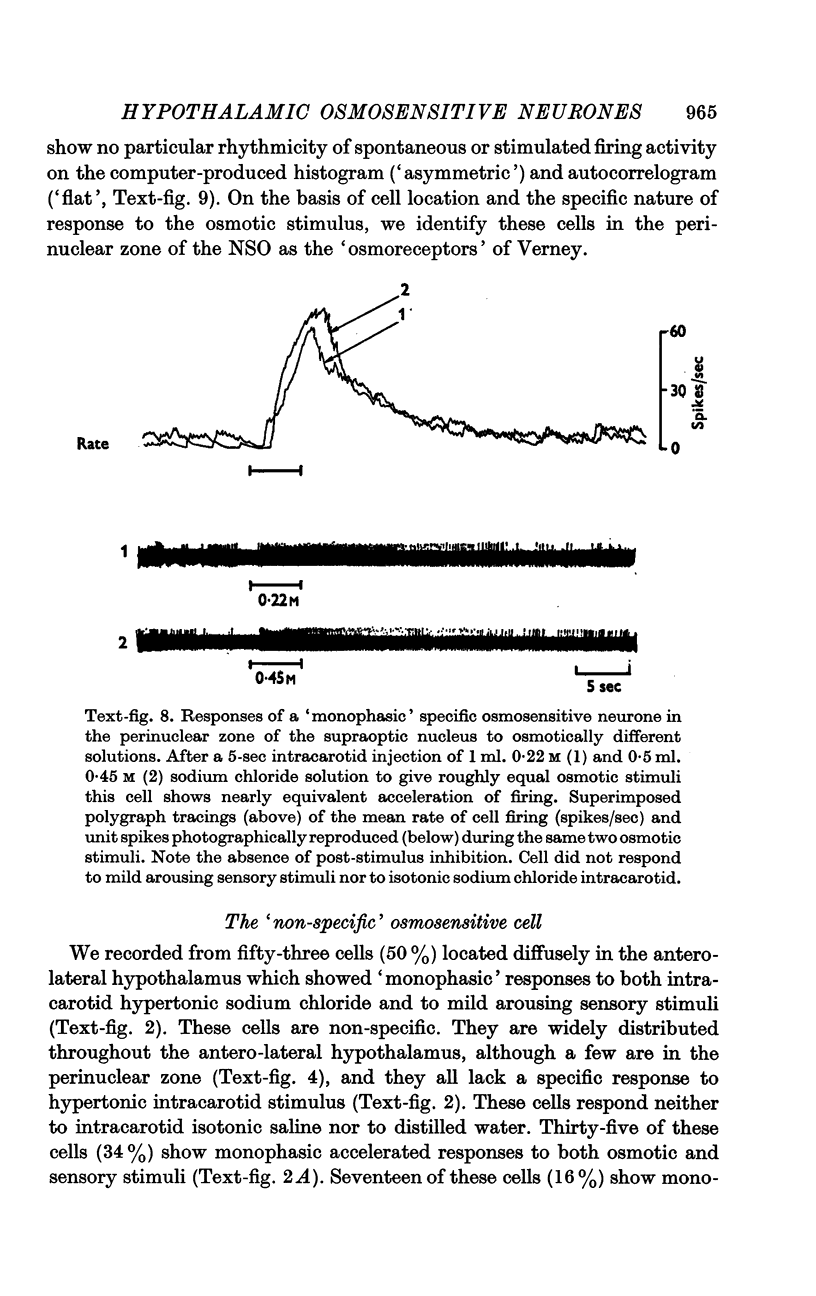
4. Fifty-two (50%) of the osmosensitive cells we labelled `specific' because they responded to an intracarotid injection of hypertonic sodium chloride, generally did not respond to non-noxious arousing sensory stimuli and were located in or near the supraoptic nucleus. We found two subtypes of these `specific' osmosensitive cells: (a) twenty-one (20%) NSO cells with `biphasic' responses, that is, acceleration followed by inhibition; (b) thirty-one (30%) cells in the immediate perinuclear zone of the NSO with `monophasic' responses, subdivided into twenty-one (20%) cells that accelerated and ten (10%) that were inhibited.
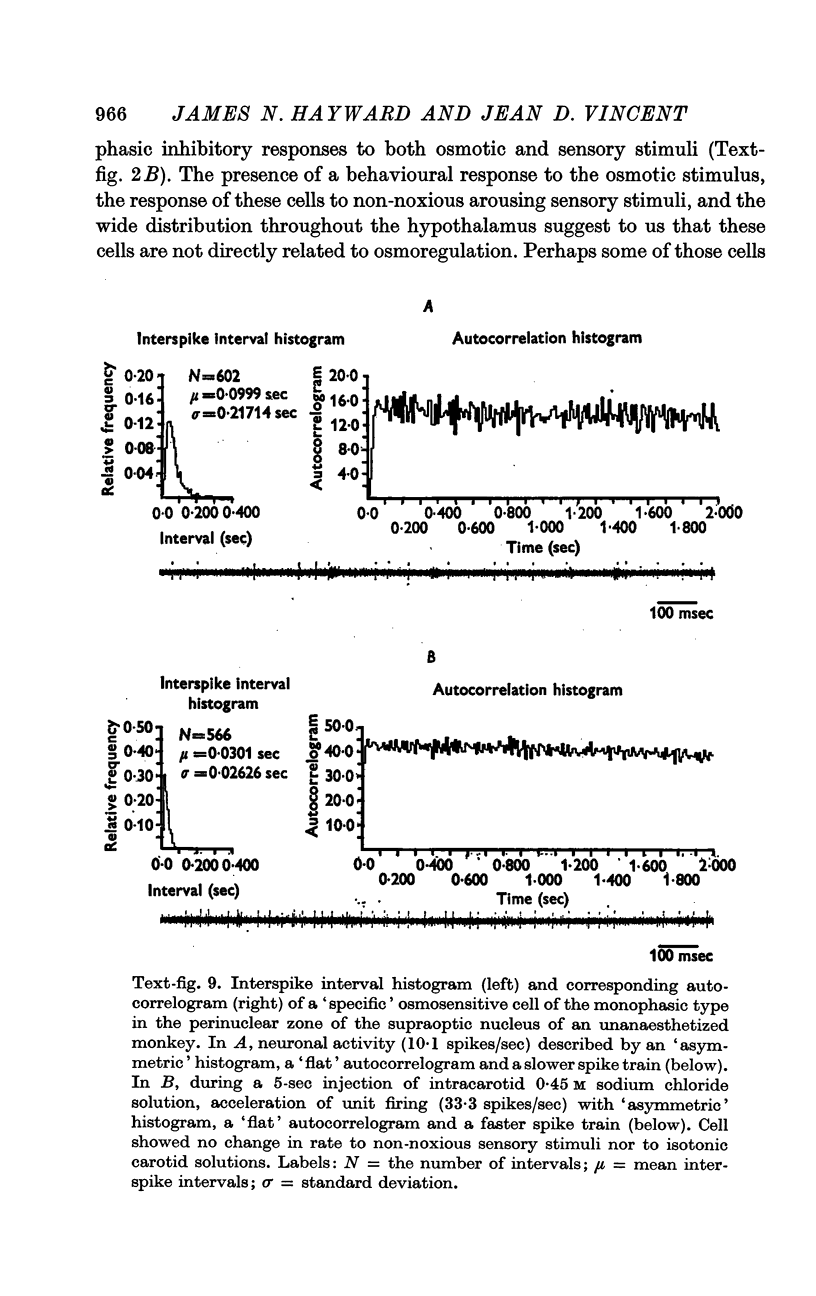
5. Fifty-three osmosensitive cells (50%), located diffusely in the anterolateral hypothalamus, were `non-specific', responding both to intracarotid injections of hypertonic sodium chloride and also to sensory stimuli that were mildly arousing. Two groups of `non-specific' osmosensitive cells showed monophasic responses; thirty-five (34%) cells accelerated and seventeen (16%) of them were inhibited.
6. The `monophasic' specific osmosensitive neurones lying in the immediate perinuclear zone of the supraoptic nucleus in the primate could conceivably be the `osmoreceptors' of Verney. The `biphasic' specific osmosensitive neurones in the NSO may well represent the secretory cells of this system. From our data, the `non-specific' osmosensitive neurones, scattered diffusely in the anterolateral hypothalamus, have little to do with osmoregulation. Some of these cells located in the perinuclear zone of the NSO could act as interneurones, however, conveying afferent input to the osmoreceptor-secretory complex of the supraoptic nucleus.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROOKS C. M., USHIYAMA J., LANGE G. Reactions of neurons in or near the supraoptic nuclei. Am J Physiol. 1962 Mar;202:487–490. doi: 10.1152/ajplegacy.1962.202.3.487. [DOI] [PubMed] [Google Scholar]
- Bak A. F. Testing metal micro-electrodes. Electroencephalogr Clin Neurophysiol. 1967 Feb;22(2):186–187. doi: 10.1016/0013-4694(67)90162-9. [DOI] [PubMed] [Google Scholar]
- Baker M. A., Burrell E., Penkhus J., Hayward J. N. Capping and stabilizing chronic intravascular cannulae. J Appl Physiol. 1968 Apr;24(4):577–579. doi: 10.1152/jappl.1968.24.4.577. [DOI] [PubMed] [Google Scholar]
- Baker M. A., Hayward J. N. Carotid rete and brain temperature of cat. Nature. 1967 Oct 14;216(5111):139–141. doi: 10.1038/216139a0. [DOI] [PubMed] [Google Scholar]
- Brooks C. M., Ishikawa T., Koizumi K., Lu H. H. Activity of neurones in the paraventricular nucleus of the hypothalamus and its control. J Physiol. 1966 Jan;182(1):217–231. doi: 10.1113/jphysiol.1966.sp007820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEMENTE C. D., SUTIN J., SILVERSTONE J. T. Changes in electrical activity of the medulla on the intravenous injection of hypertonic solutions. Am J Physiol. 1957 Jan;188(1):193–198. doi: 10.1152/ajplegacy.1956.188.1.193. [DOI] [PubMed] [Google Scholar]
- CROSS B. A., GREEN J. D. Activity of single neurones in the hypothalamus: effect of osmotic and other stimuli. J Physiol. 1959 Oct;148:554–569. doi: 10.1113/jphysiol.1959.sp006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evarts E. V. A technique for recording activity of subcortical neurons in moving animals. Electroencephalogr Clin Neurophysiol. 1968 Jan;24(1):83–86. doi: 10.1016/0013-4694(68)90070-9. [DOI] [PubMed] [Google Scholar]
- Findlay A. L., Hayward J. N. Spontaneous activity of single neurones in the hypothalamus of rabbits during sleep and waking. J Physiol. 1969 Mar;201(1):237–258. doi: 10.1113/jphysiol.1969.sp008753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYWARD J. N., SMITH W. K. ANTIDIURETIC RESPONSE TO ELECTRICAL STIMULATION IN BRAIN STEM OF THE MONKEY. Am J Physiol. 1964 Jan;206:15–20. doi: 10.1152/ajplegacy.1964.206.1.15. [DOI] [PubMed] [Google Scholar]
- HAYWARD J. N., SMITH W. K. INFLUENCE OF LIMBIC SYSTEM ON NEUROHYPOPHYSIS. Arch Neurol. 1963 Aug;9:171–177. doi: 10.1001/archneur.1963.00460080081010. [DOI] [PubMed] [Google Scholar]
- HOLLAND R. C., SUNDSTEN J. W., SAWYER C. H. Effects of intracarotid injections of hypertonic solutions on arterial pressure in the rabbit. Circ Res. 1959 Sep;7:712–720. doi: 10.1161/01.res.7.5.712. [DOI] [PubMed] [Google Scholar]
- Hayward J. N., Baker M. A. A comparative study of the role of the cerebral arterial blood in the regulation of brain temperature in five mammals. Brain Res. 1969 Dec;16(2):417–440. doi: 10.1016/0006-8993(69)90236-4. [DOI] [PubMed] [Google Scholar]
- Hayward J. N., Baker M. A. Diuretic and thermoregulatory responses to preoptic cooling in the monkey. Am J Physiol. 1968 Apr;214(4):843–850. doi: 10.1152/ajplegacy.1968.214.4.843. [DOI] [PubMed] [Google Scholar]
- Hayward J. N., Baker M. A. Role of cerebral arterial blood in the regulation of brain temperature in the monkey. Am J Physiol. 1968 Aug;215(2):389–403. doi: 10.1152/ajplegacy.1968.215.2.389. [DOI] [PubMed] [Google Scholar]
- Hayward J. N. Brain temperature and thermosensitivity of nerve cells in monkey. Trans Am Neurol Assoc. 1969;94:157–159. [PubMed] [Google Scholar]
- Hayward J. N., Fairchild M. D., Stuart D. G. Hypothalamic and cortical D-C potential changes induced by stimulation of the midbrain reticular formation. Exp Brain Res. 1966;1(3):205–219. doi: 10.1007/BF00234342. [DOI] [PubMed] [Google Scholar]
- Hubel D. H. Tungsten Microelectrode for Recording from Single Units. Science. 1957 Mar 22;125(3247):549–550. doi: 10.1126/science.125.3247.549. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Koizumi K., Brooks C. M. Activity of supraoptic nucleus neurons of the hypothalamus. Neurology. 1966 Jan;16(1):101–106. doi: 10.1212/wnl.16.1.101. [DOI] [PubMed] [Google Scholar]
- JOYNT R. J. FUNCTIONAL SIGNIFICANCE OF OSMOSENSITIVE UNITS IN THE ANTERIOR HYPOTHALAMUS. Neurology. 1964 Jun;14:584–590. doi: 10.1212/wnl.14.6.584. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R. ELECTRICAL PROPERTIES OF HYPOTHALAMIC NEUROENDOCRINE CELLS. J Gen Physiol. 1964 Mar;47:691–717. doi: 10.1085/jgp.47.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOIZUMI K., ISHIKAWA T., BROOKS C. M. CONTROL OF ACTIVITY OF NEURONS IN THE SUPRAOPTIC NUCLEUS. J Neurophysiol. 1964 Sep;27:878–892. doi: 10.1152/jn.1964.27.5.878. [DOI] [PubMed] [Google Scholar]
- Komisaruk B. R., McDonald P. G., Whitmoyer D. I., Sawyer C. H. Effects of progesterone and sensory stimulation on EEG and neuronal activity in the rat. Exp Neurol. 1967 Dec;19(4):494–507. doi: 10.1016/0014-4886(67)90169-0. [DOI] [PubMed] [Google Scholar]
- Libet B., Tosaka T. Slow inhibitory and excitatory postsynaptic responses in single cells of mammalian sympathetic ganglia. J Neurophysiol. 1969 Jan;32(1):43–50. doi: 10.1152/jn.1969.32.1.43. [DOI] [PubMed] [Google Scholar]
- Martin A. R. A peak amplitude selector for electrophysiological data analysis. IEEE Trans Biomed Eng. 1969 Apr;16(2):152–159. doi: 10.1109/tbme.1969.4502629. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Gerstein G. L., Moore G. P. Neuronal spike trains and stochastic point processes. I. The single spike train. Biophys J. 1967 Jul;7(4):391–418. doi: 10.1016/S0006-3495(67)86596-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAWYER C. H., GERNANDT B. E. Effects of intracarotid and intraventricular injections of hypertonic solutions on electrical activity of the rabbit brain. Am J Physiol. 1956 Apr;185(1):209–216. doi: 10.1152/ajplegacy.1956.185.1.209. [DOI] [PubMed] [Google Scholar]
- SUDA I., KOIZUMI K. STUDY OF UNITARY ACTIVITY IN THE SUPRAOPTIC NUCLEUS OF THE HYPOTHALAMUS. Jpn J Physiol. 1963 Aug 15;13:374–385. doi: 10.2170/jjphysiol.13.374. [DOI] [PubMed] [Google Scholar]
- SUNDSTEN J. W., SAWYER C. H. Osmotic activation of neurohypophysial hormone release in rabbits with hypothalamic islands. Exp Neurol. 1961 Dec;4:548–561. doi: 10.1016/0014-4886(61)90051-6. [DOI] [PubMed] [Google Scholar]
- SUNSTEN J. W., SAWYER C. H. Electroencephalographic evidence of osmosensitive elements in olfactory bulb of dog brain. Proc Soc Exp Biol Med. 1959 Jul;101(3):524–527. doi: 10.3181/00379727-101-25004. [DOI] [PubMed] [Google Scholar]
- VON EULER C. A preliminary note on slow hypothalamic osmo-potentials. Acta Physiol Scand. 1953 Jun 26;29(1):133–136. doi: 10.1111/j.1748-1716.1953.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Vincent J. D., Benoit O., Scherrer J., Faure J. M. Activités élémentaires recueillies dans l'hypothalamus au cours de la veille et du sommeil chez le lapin chronique. J Physiol (Paris) 1967;59(1 Suppl):527–527. [PubMed] [Google Scholar]
- Woods J. W., Bard P., Bleier R. Functional capacity of the deafferented hypothalamus: water balance and responses to osmotic stimuli in the decerebrate cat and rat. J Neurophysiol. 1966 Jul;29(4):751–767. doi: 10.1152/jn.1966.29.4.751. [DOI] [PubMed] [Google Scholar]