Abstract
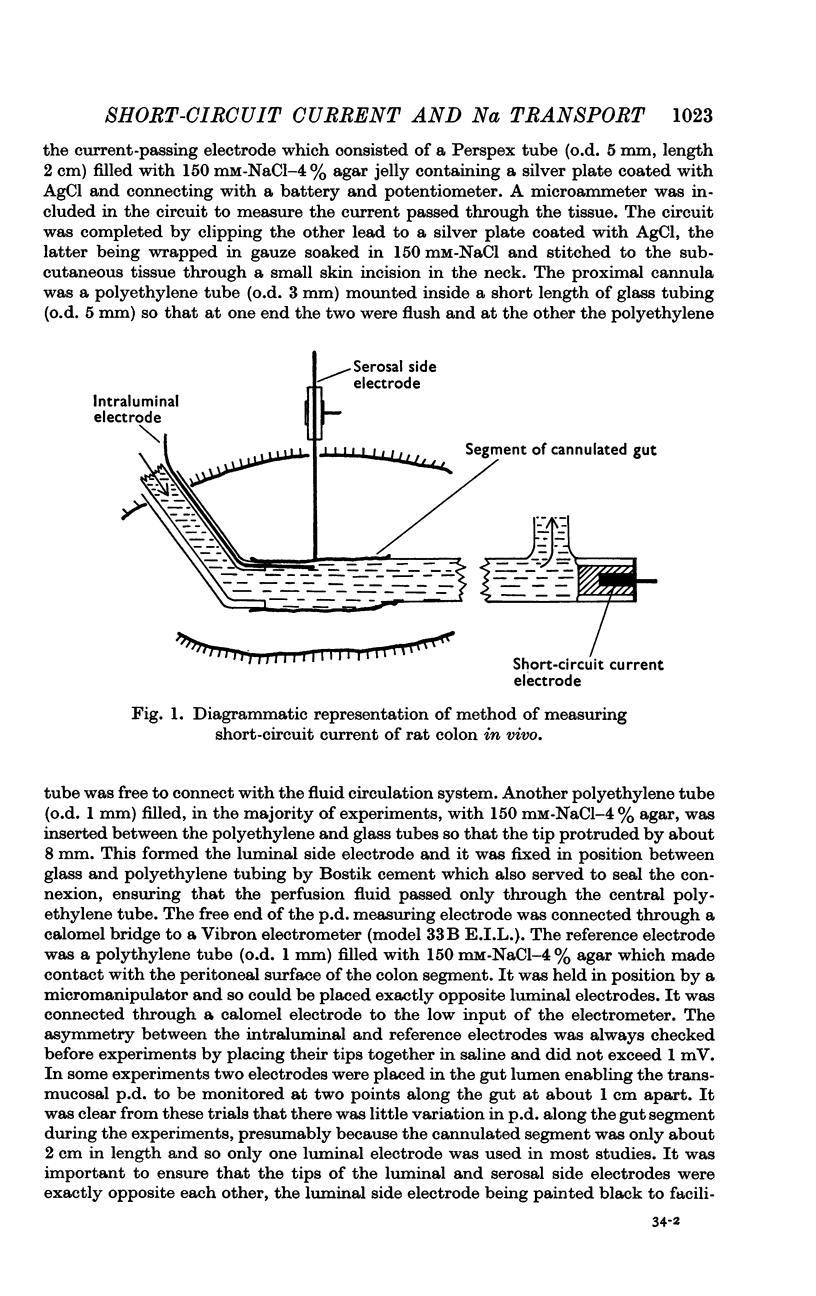
1. A method for measurement of short-circuit current and for applying a voltage clamp to segments of rat colon in vivo is described.
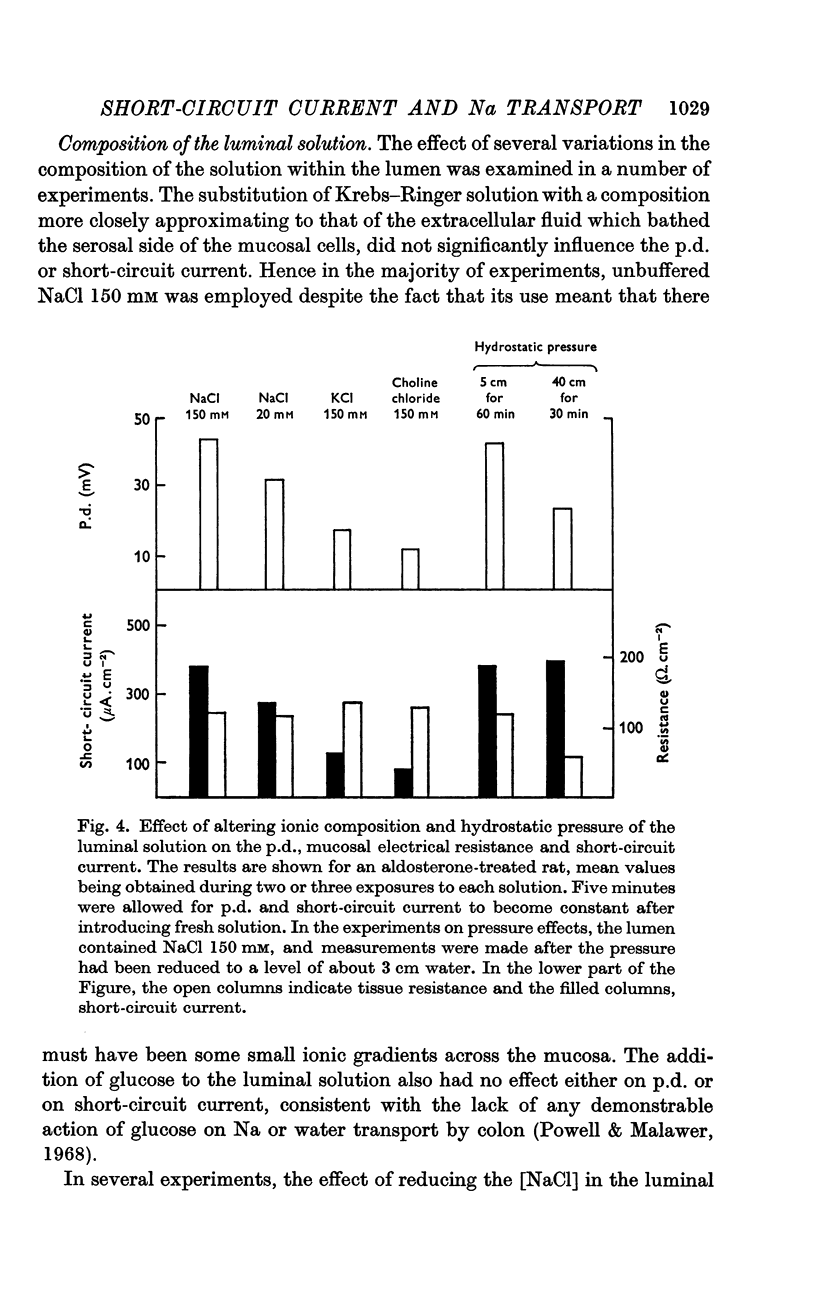
2. The mucosa behaved as an ohmic resistor of average resistance 154 Ω/cm2 although brief transient effects were frequently observed. Tissue resistance was independent of considerable changes in ionic strength and composition of the luminal solution.
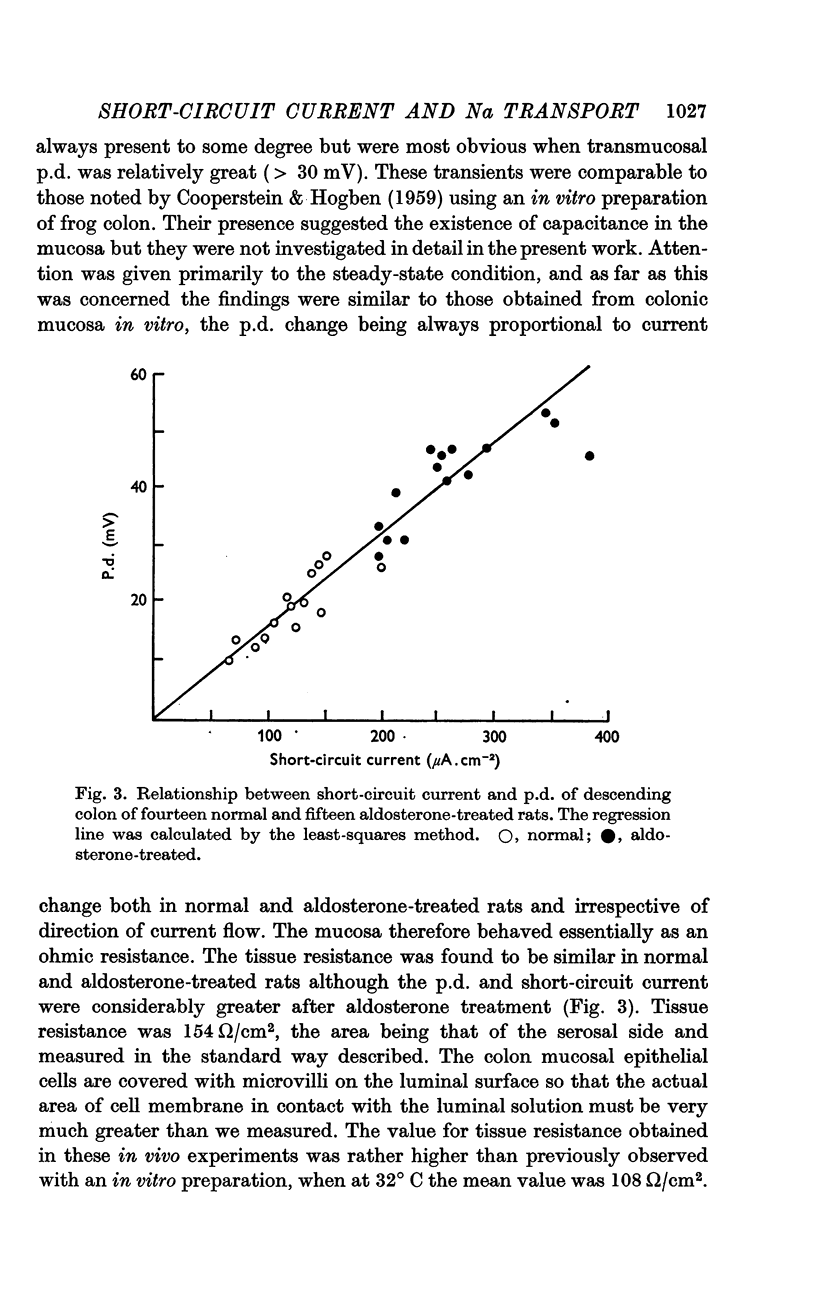
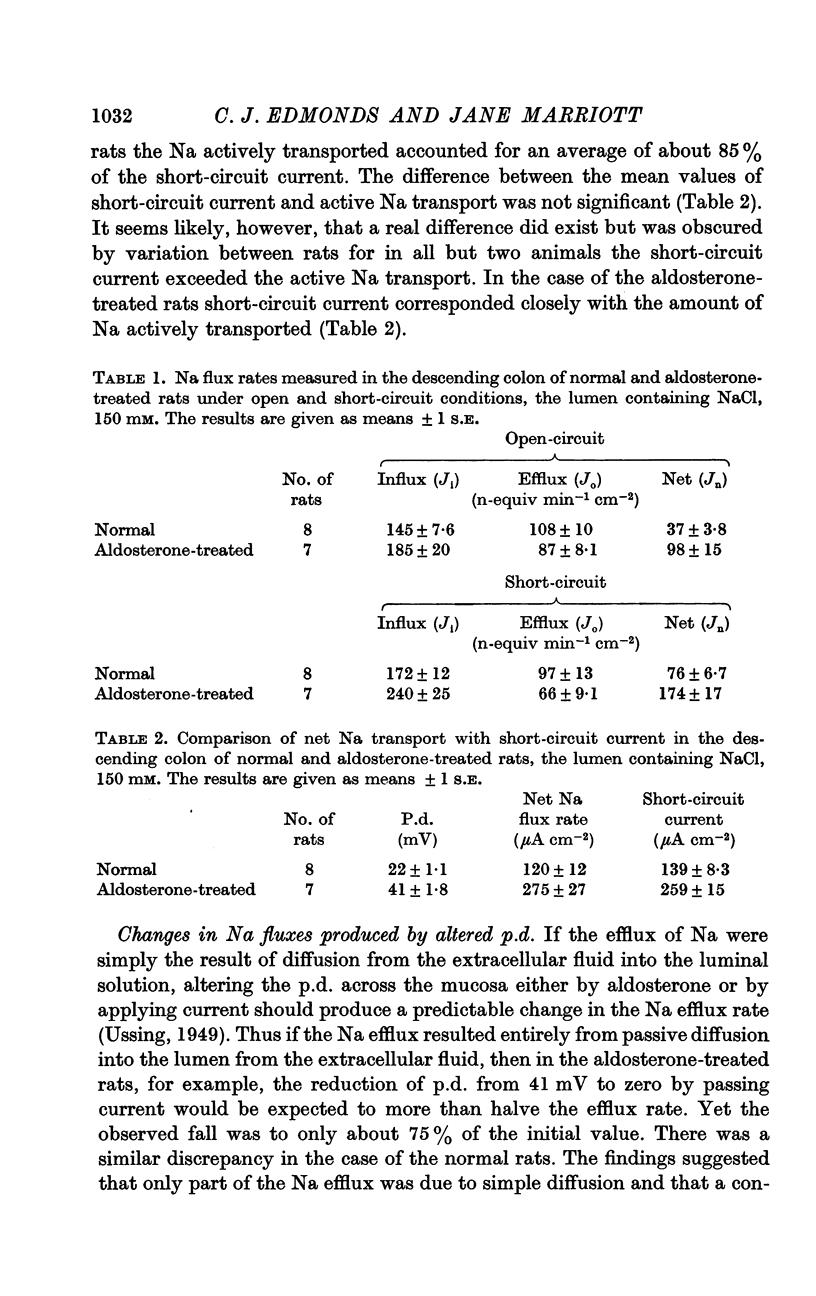
3. The short-circuit current averaged 120 μA/cm2 in normal rats. Aldosterone intravenously raised the p.d., short-circuit current rising proportionately and tissue resistance being unchanged. The effects of various modifications of the intraluminal solution in respect to composition, hydrostatic pressure and pH were examined. An increase in the osmolality of the luminal solution sufficient to abolish water absorption did not affect p.d. or short-circuit current.
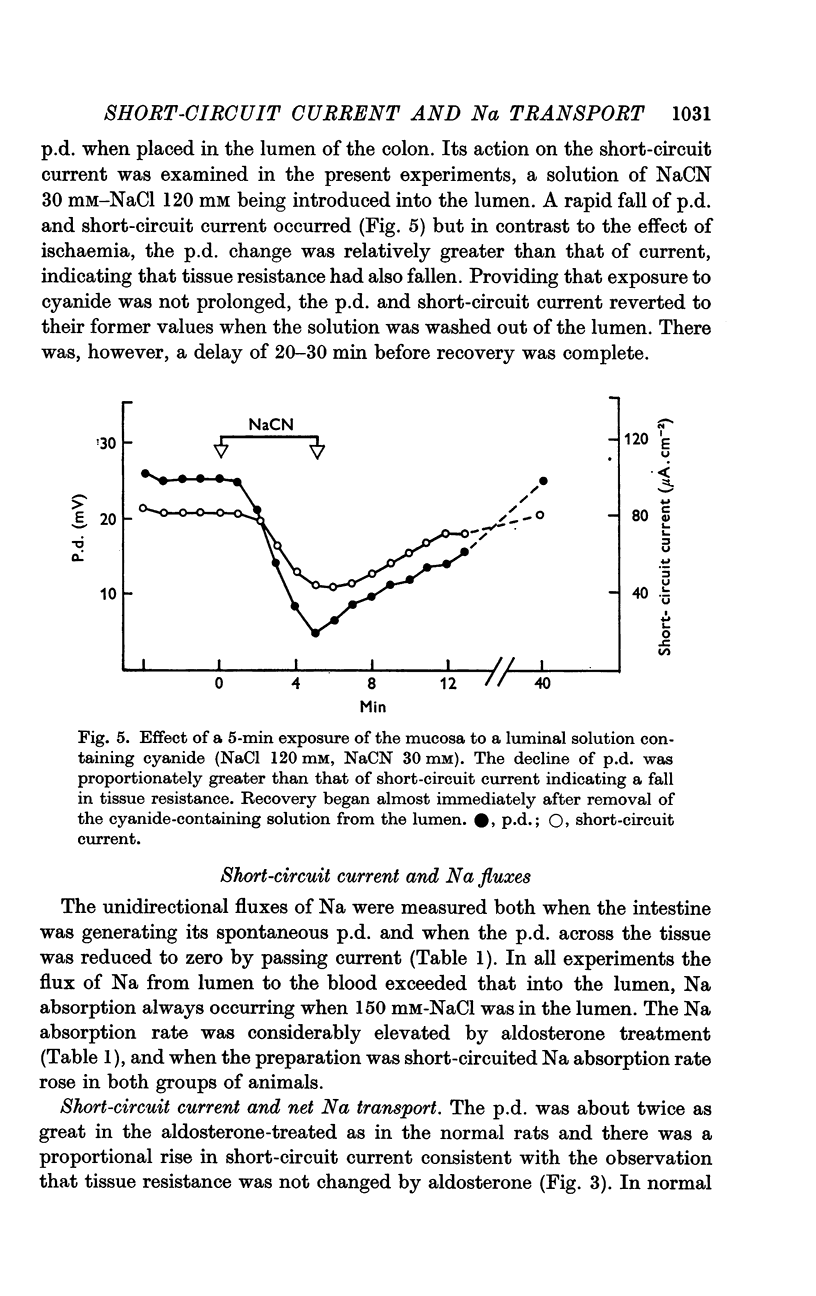
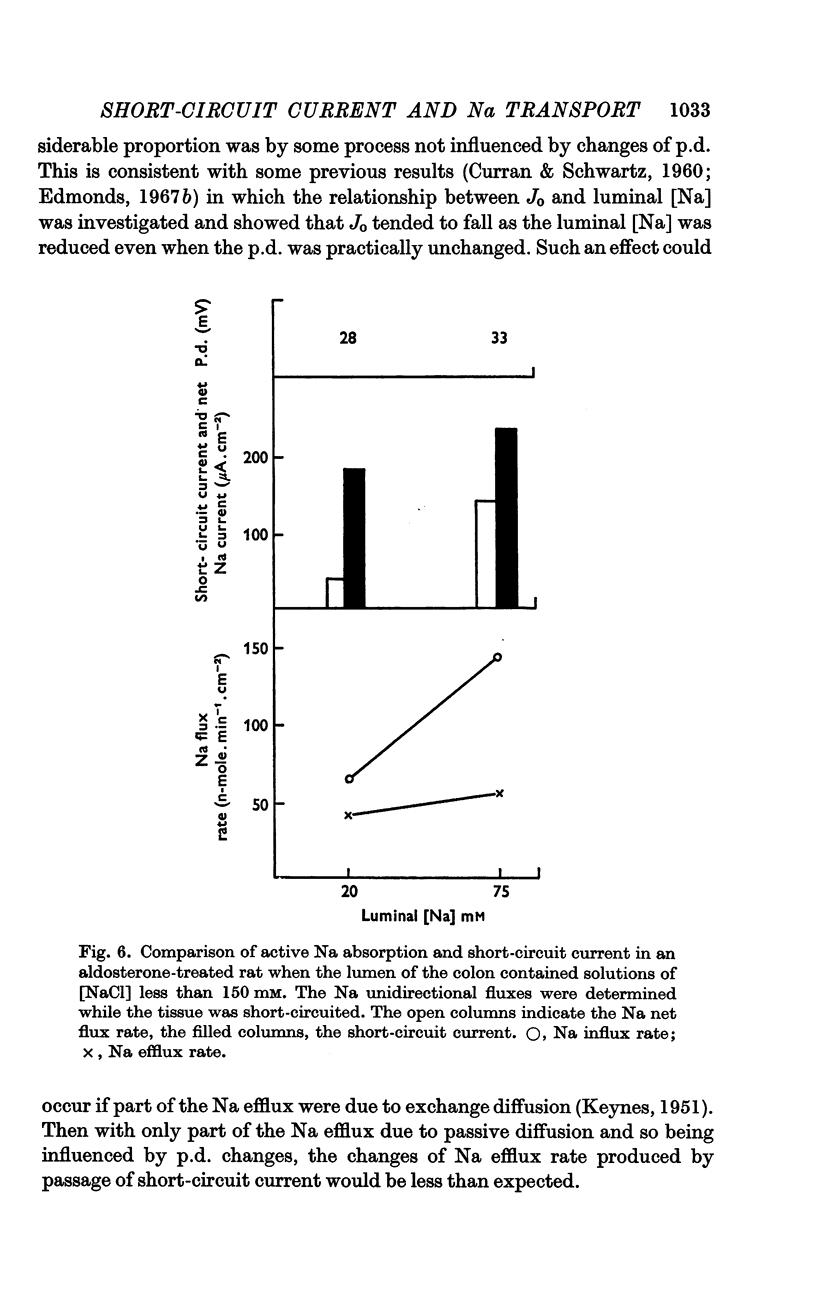
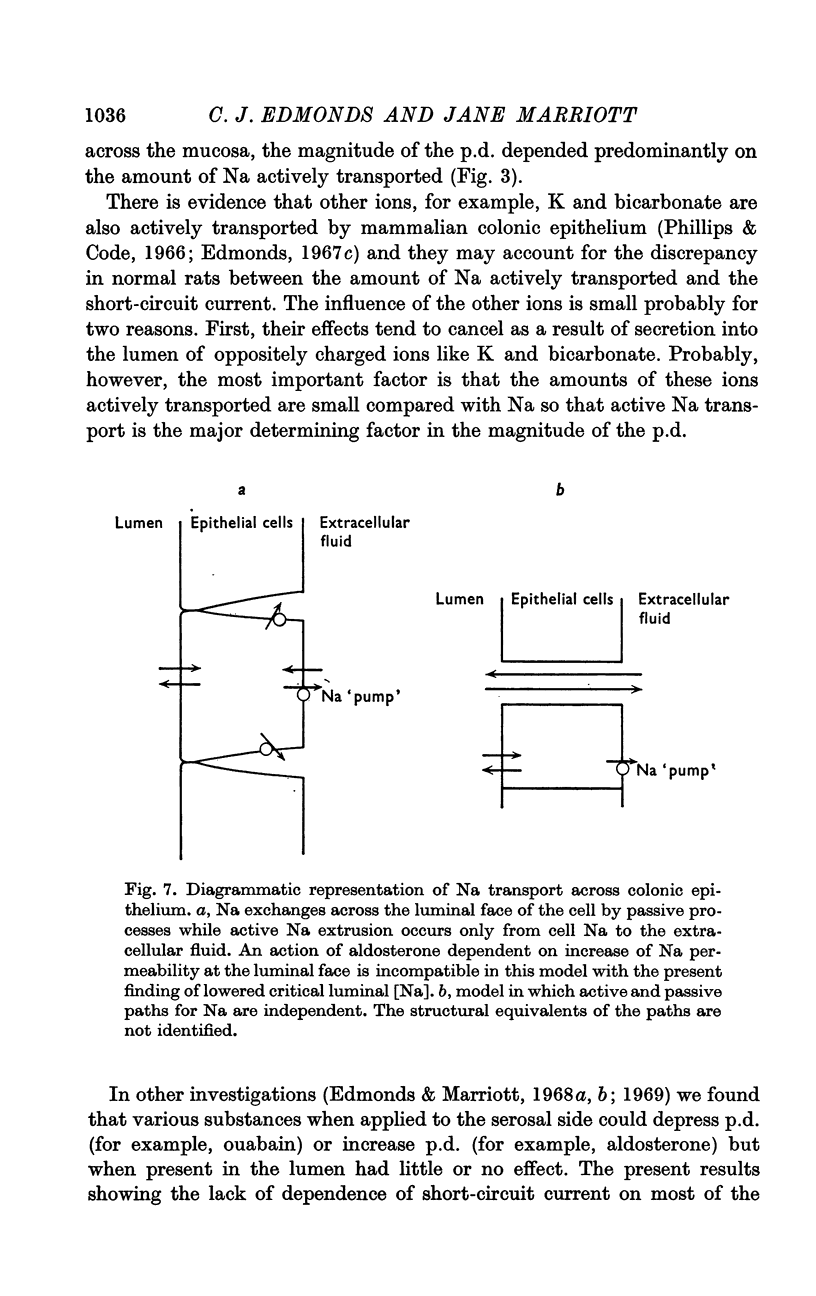
4. The short-circuit current measured with 150 mM-NaCl in the lumen was almost completely accounted for by active Na absorption both in normal and aldosterone-treated rats. The changes in Na efflux rate produced by voltage clamping suggested that only part of Na efflux was due to simple diffusion. With lower [NaCl] in the lumen, the short-circuit current exceeded that atributable to active Na absorption, the discrepancy increasing with reduction of [NaCl].
5. The luminal [Na] at which Na efflux and influx rates were equal was reduced by aldosterone, an effect which is probably responsible for the low stool [Na] of aldosterone treated animals. The significance of this finding in terms of the mode of action of aldosterone is discussed.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASANO T. METABOLIC DISTURBANCES AND SHORT-CIRCUIT CURRENT ACROSS INTESTINAL WALL OF RAT. Am J Physiol. 1964 Aug;207:415–422. doi: 10.1152/ajplegacy.1964.207.2.415. [DOI] [PubMed] [Google Scholar]
- Barnaby C. F., Edmonds C. J. Use of a miniature GM counter and a whole body counter in the study of potassium transport by the colon of normal, sodium-depleted and adrenalectomized rats in vivo. J Physiol. 1969 Dec;205(3):647–665. doi: 10.1113/jphysiol.1969.sp008988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R. J., Smyth D. H., Wright E. M. Short-circuit current and solute transfer by rat jejunum. J Physiol. 1965 Nov;181(2):410–431. doi: 10.1113/jphysiol.1965.sp007770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPERSTEIN I. L., HOGBEN C. A. Ionic transfer across the isolated frog large intestine. J Gen Physiol. 1959 Jan 20;42(3):461–473. doi: 10.1085/jgp.42.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRABBE J., DEWEER P. ACTION OF ALDOSTERONE ON THE BLADDER AND SKIN OF THE TOAD. Nature. 1964 Apr 18;202:298–299. doi: 10.1038/202298a0. [DOI] [PubMed] [Google Scholar]
- CURRAN P. F., SCHWARTZ G. F. Na, Cl, and water transport by rat colon. J Gen Physiol. 1960 Jan;43:555–571. doi: 10.1085/jgp.43.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRAN P. F., SOLOMON A. K. Ion and water fluxes in the ileum of rats. J Gen Physiol. 1957 Sep 20;41(1):143–168. doi: 10.1085/jgp.41.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron R. C., Leme C. E., Wilson D. R., Ing T. S., Wrong O. M. The effect of adrenal steroids on stool composition, as revealed by in vivo dialysis of faeces. Clin Sci. 1969 Aug;37(1):151–167. [PubMed] [Google Scholar]
- Cofré G., Crabbé J. Active sodium transport by the colon of Bufo marinus: stimulation by aldosterone and antidiuretic hormone. J Physiol. 1967 Jan;188(2):177–190. doi: 10.1113/jphysiol.1967.sp008132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman I. S., Fimognari G. M. On the biochemical mechanism of action of aldosterone. Recent Prog Horm Res. 1968;24:1–44. doi: 10.1016/b978-1-4831-9827-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J., Marriott J. C. The effect of aldosterone and adrenalectomy on the electrical potential difference of rat colon and on the transport of sodium, potassium, chloride and bicarbonate. J Endocrinol. 1967 Dec;39(4):517–531. doi: 10.1677/joe.0.0390517. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J., Marriott J. C. The effect of aldosterone on the electrical activity of rat colon. J Endocrinol. 1969 Jul;44(3):363–377. doi: 10.1677/joe.0.0440363. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J., Marriott J. Electrical potential and short circuit current of an in vitro preparation of rat colon mucosa. J Physiol. 1968 Feb;194(2):479–494. doi: 10.1113/jphysiol.1968.sp008419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J., Marriott J. Factors influencing the electrical potential across the mucosa of rat colon. J Physiol. 1968 Feb;194(2):457–478. doi: 10.1113/jphysiol.1968.sp008418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J. The gradient of electrical potential difference and of sodium and potassium of the gut contents along the caecum and colon of normal and sodium-depleted rats. J Physiol. 1967 Dec;193(3):571–588. doi: 10.1113/jphysiol.1967.sp008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J. Transport of potassium by the colon of normal and sodium-depleted rats. J Physiol. 1967 Dec;193(3):603–617. doi: 10.1113/jphysiol.1967.sp008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds C. J. Transport of sodium and secretion of potassium and bicarbonate by the colon of normal and sodium-depleted rats. J Physiol. 1967 Dec;193(3):589–602. doi: 10.1113/jphysiol.1967.sp008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles-Baillien M., Schoffeniels E. Fluxes of inorganic ions across the isolated intestinal epithelium of the greek tortoise. Arch Int Physiol Biochim. 1967 Dec;75(5):754–762. doi: 10.3109/13813456709084932. [DOI] [PubMed] [Google Scholar]
- KEYNES R. D. The ionic movements during nervous activity. J Physiol. 1951 Jun;114(1-2):119–150. doi: 10.1113/jphysiol.1951.sp004608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAF A., ANDERSON J., PAGE L. B. Active sodium transport by the isolated toad bladder. J Gen Physiol. 1958 Mar 20;41(4):657–668. doi: 10.1085/jgp.41.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILNE M. D., MUEHRCKE R. C., AIRD I. Primary aldosteronism. Q J Med. 1957 Jul;26(103):317–333. [PubMed] [Google Scholar]
- Nutbourne D. M. The effect of small hydrostatic pressure gradients on the rate of active sodium transport across isolated living frog-skin membranes. J Physiol. 1968 Mar;195(1):1–18. doi: 10.1113/jphysiol.1968.sp008442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips S. F., Code C. F. Sorption of potassium in the small and the large intestine. Am J Physiol. 1966 Sep;211(3):607–613. doi: 10.1152/ajplegacy.1966.211.3.607. [DOI] [PubMed] [Google Scholar]
- Powell D. W., Malawer S. J. Relationship between water and solute transport from isosmotic solutions by rat intestine in vivo. Am J Physiol. 1968 Jul;215(1):49–55. doi: 10.1152/ajplegacy.1968.215.1.49. [DOI] [PubMed] [Google Scholar]
- Richards P. Clinical investigation of the effects of adrenal corticosteroid excess on the colon. Lancet. 1969 Mar 1;1(7592):437–442. doi: 10.1016/s0140-6736(69)91480-9. [DOI] [PubMed] [Google Scholar]
- Sharp G. W., Leaf A. Mechanism of action of aldosterone. Physiol Rev. 1966 Oct;46(4):593–633. doi: 10.1152/physrev.1966.46.4.593. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]


