Abstract
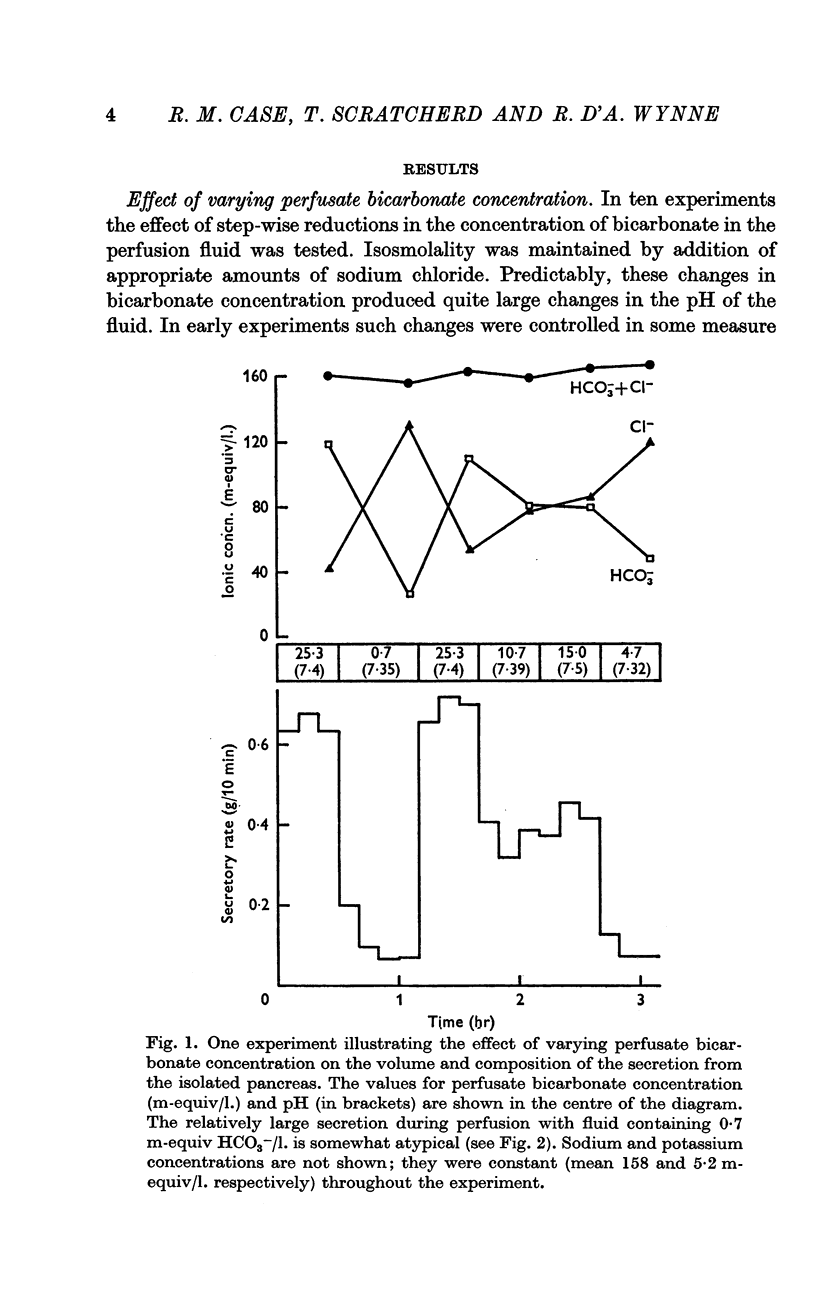
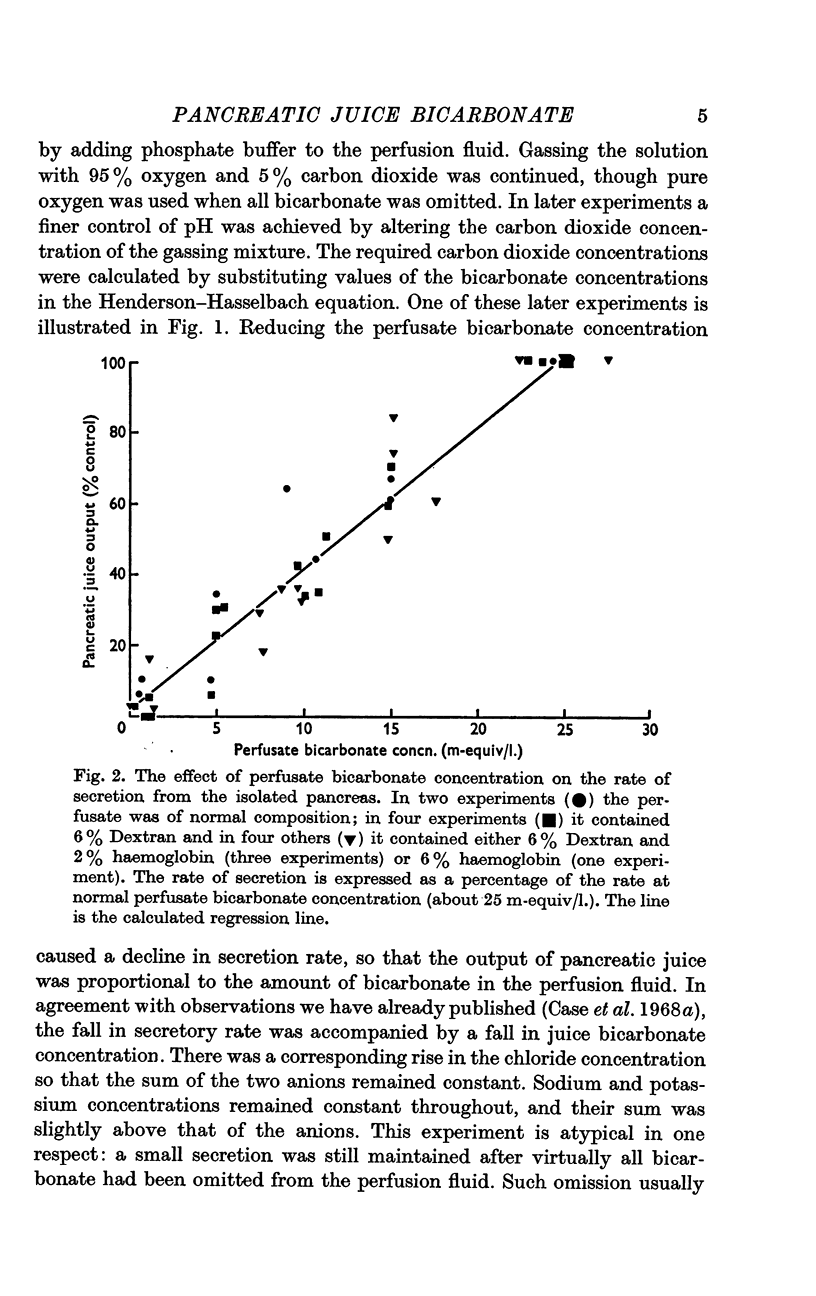
1. The rate of secretion from a saline-perfused preparation of the cat's pancreas is directly proportional to the perfusate bicarbonate concentration. When all bicarbonate is omitted, secretion completely or almost completely ceases.
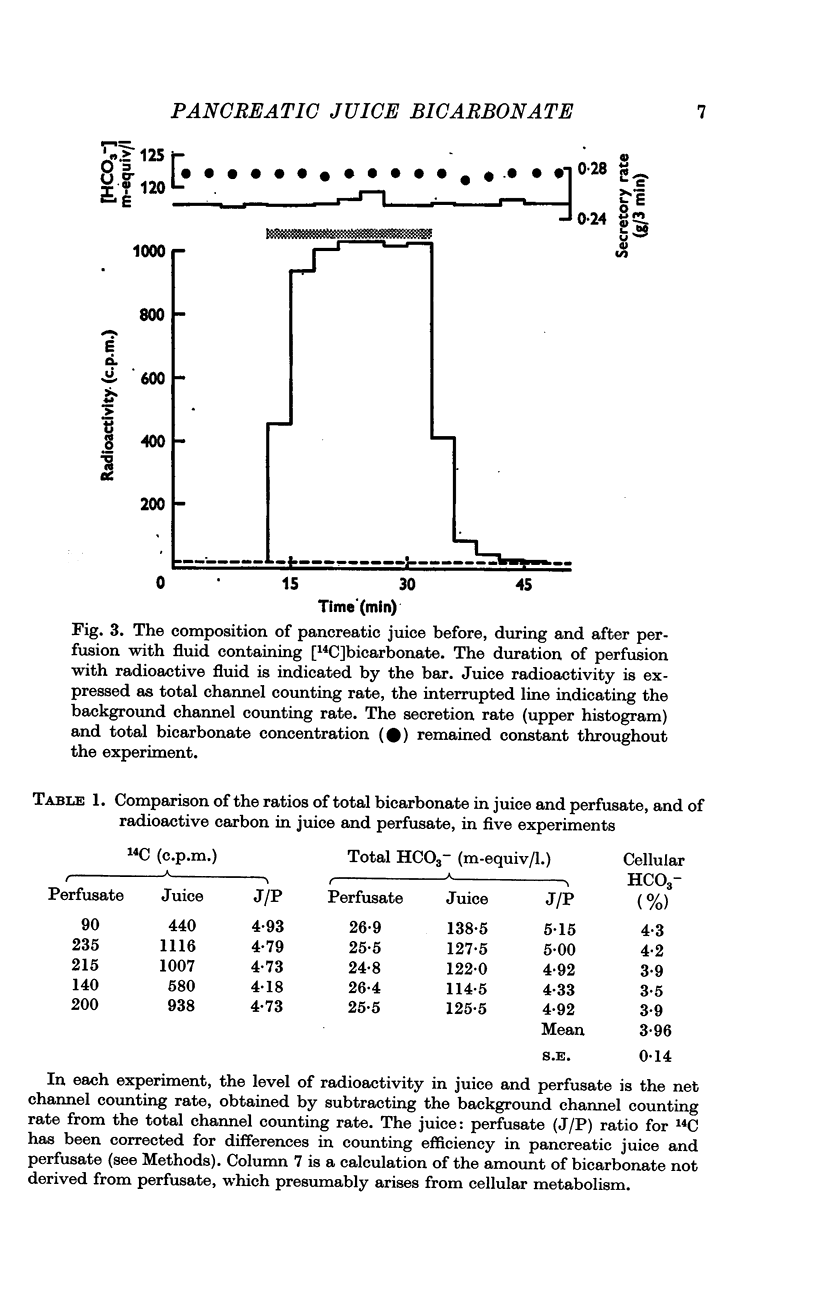
2. Incorporation of [14C]bicarbonate into the perfusion fluid results in its prompt appearance in the juice. The radioactive label is concentrated four to five times in the juice just as the total juice bicarbonate is four to five times greater than perfusate bicarbonate.
3. These two observations suggest that about 95% of pancreatic juice bicarbonate is derived from perfusate (plasma) bicarbonate.
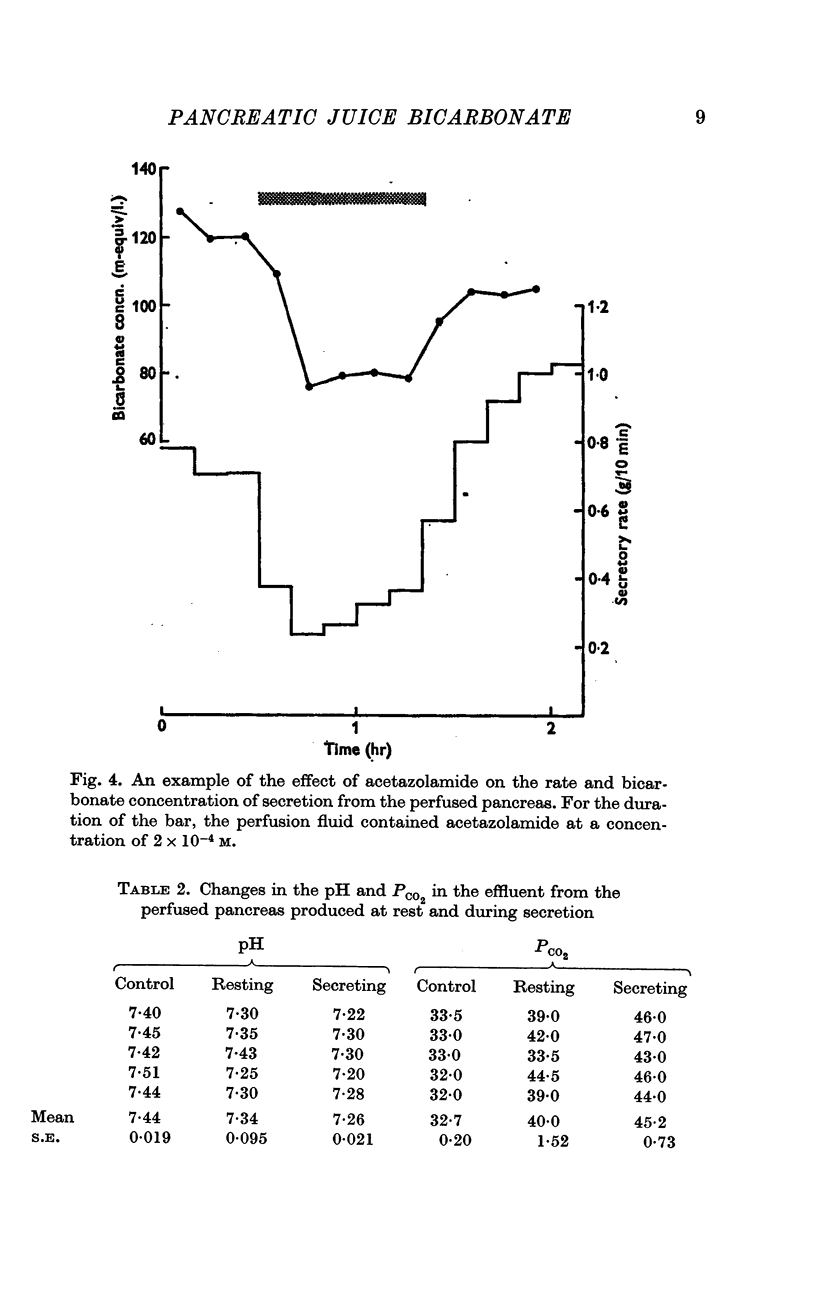
4. The inhibition of pancreatic secretion from the perfused gland by acetazolamide is similar to that observed in the intact animal.
5. There is a fall in pH and rise in PCO2 in the perfusion fluid leaving the gland which is greater during secretion than at rest.
6. It is therefore suggested that during secretion, hydrogen ions pass from the gland into the perfusate (plasma), thus increasing the production of carbon dioxide from circulating bicarbonate. This carbon dioxide diffuses into the cell, is rehydrated (partly under the influence of carbonic anhydrase) and finally is secreted, thus establishing the necessary gradient for the continued diffusion of carbon dioxide into the cell.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRNBAUM D., HOLLANDER F. Inhibition of pancreatic secretion by the carbonic anhydrase inhibitor 2-acetylamino-1,3,4-thiadiazole-5-sulfonamide, diamox (#6063). Am J Physiol. 1953 Aug;174(2):191–195. doi: 10.1152/ajplegacy.1953.174.2.191. [DOI] [PubMed] [Google Scholar]
- Brodsky W. A., Schilb T. P. Mechanism of acidification in turtle bladder. Fed Proc. 1967 Sep;26(5):1314–1321. [PubMed] [Google Scholar]
- CRICK J., HARPER A. A., RAPER H. S. On the preparation of secretin and pancreozymin. J Physiol. 1949 Dec;110(3-4):367–376. doi: 10.1113/jphysiol.1949.sp004445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Harper A. A., Scratcherd T. The secretion of electrolytes and enzymes by the pancreas of the anaesthetized cat. J Physiol. 1969 Apr;201(2):335–348. doi: 10.1113/jphysiol.1969.sp008759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Harper A. A., Scratcherd T. Water and electrolyte secretion by the perfused pancreas of the cat. J Physiol. 1968 May;196(1):133–149. doi: 10.1113/jphysiol.1968.sp008499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'SILVA J. L., NEIL M. W. The potassium water and glycogen contents of the perfused rat liver. J Physiol. 1954 Jun 28;124(3):515–527. doi: 10.1113/jphysiol.1954.sp005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKMAN S. R., MORRILL G. A. Stimulation of respiration and secretion of mouse pancreas in vitro. Am J Physiol. 1957 Sep;190(3):403–407. doi: 10.1152/ajplegacy.1957.190.3.403. [DOI] [PubMed] [Google Scholar]
- DREILING D. A., JANOWITZ H. D., HALPERN M. The effect of a carbonic anhydrase inhibitor, diamox, on human pancreatic secretion. Gastroenterology. 1955 Aug;29(2):262–279. [PubMed] [Google Scholar]
- Francis G. E., Hawkins J. D. Liquid scintillation counting of aqueous H3-and C14-protein solutions at room temperature. Int J Appl Radiat Isot. 1967 Apr;18(4):223–230. doi: 10.1016/0020-708x(67)90085-3. [DOI] [PubMed] [Google Scholar]
- KINNEY V. R., CODE C. F. CANINE ILEAL CHLORIDE ABSORPTION: EFFECT OF CARBONIC ANHYDRASE INHIBITOR ON TRANSPORT. Am J Physiol. 1964 Nov;207:998–1004. doi: 10.1152/ajplegacy.1964.207.5.998. [DOI] [PubMed] [Google Scholar]
- Kitahara S., Fox K. R., Hogben C. A. Depression of chloride transport by carbonic anhydrase inhibitors in the absence of carbonic anhydrase. Nature. 1967 May 20;214(5090):836–837. doi: 10.1038/214836a0. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Pak B. H., Hong S. S., Pak H. K., Hong S. K. Effects of acetazolamide and acid-base changes on biliary and pancreatic secretion. Am J Physiol. 1966 Mar;210(3):624–628. doi: 10.1152/ajplegacy.1966.210.3.624. [DOI] [PubMed] [Google Scholar]
- RAWLS J. A., Jr, WISTRAND P. J., MAREN T. H. EFFECTS OF ACID-BASE CHANGES AND CARBONIC ANHYDRASE INHIBITION ON PANCREATIC SECRETION. Am J Physiol. 1963 Oct;205:651–657. doi: 10.1152/ajplegacy.1963.205.4.651. [DOI] [PubMed] [Google Scholar]
- ROTHMAN S. S., BROOKS F. P. ELECTROLYTE SECRETION FROM RABBIT PANCREAS IN VITRO. Am J Physiol. 1965 Jun;208:1171–1176. doi: 10.1152/ajplegacy.1965.208.6.1171. [DOI] [PubMed] [Google Scholar]
- Rothman S. S., Brooks F. P. Pancreatic secretion in vitro in "Cl-free," "Co-2-free," and low-Na+environment. Am J Physiol. 1965 Oct;209(4):790–796. doi: 10.1152/ajplegacy.1965.209.4.790. [DOI] [PubMed] [Google Scholar]
- SANDERSON P. H. Potentiometric determination of chloride in biological fluids. Biochem J. 1952 Nov;52(3):502–505. doi: 10.1042/bj0520502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz I., Yamagata A., Weske M. Micropuncture studies on the pancreas of the rabbit. Pflugers Arch. 1969;308(3):277–290. doi: 10.1007/BF00586559. [DOI] [PubMed] [Google Scholar]
- Slegers J. F., Moons W. M. Effect of acetazolamide on the chloride shift and the sodium pump in secretory cells. Nature. 1968 Oct 12;220(5163):181–182. doi: 10.1038/220181a0. [DOI] [PubMed] [Google Scholar]


