Abstract
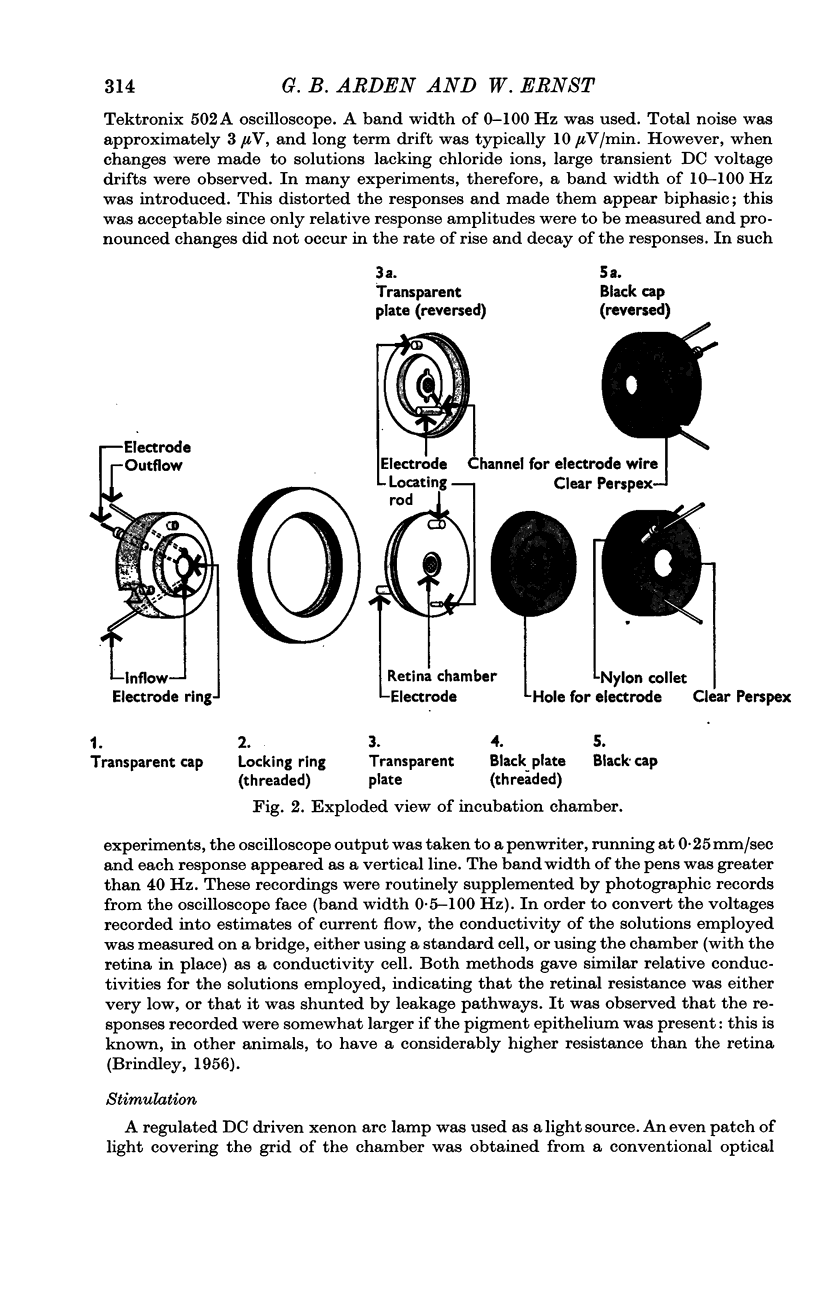
1. Pigeon cone receptor potentials have been identified in a preparation of isolated, incubated retina.
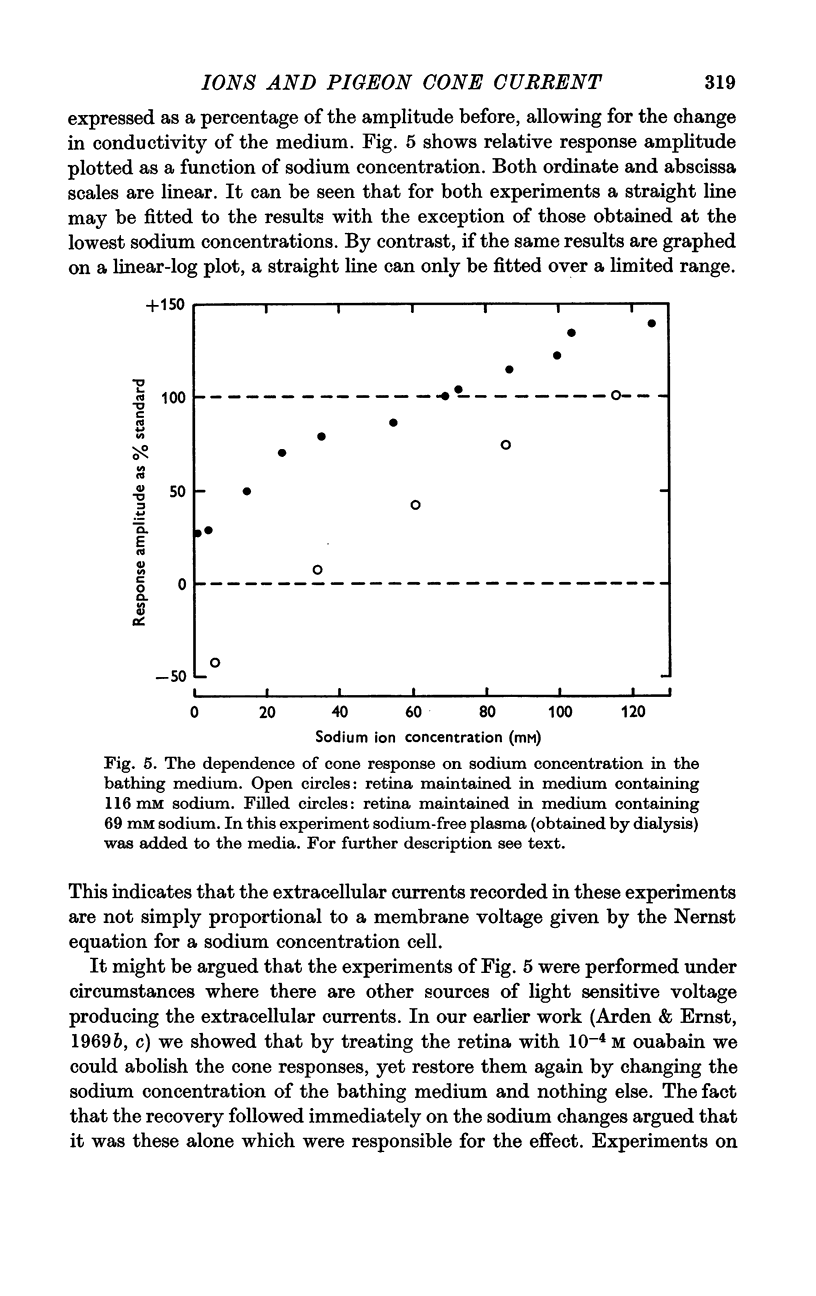
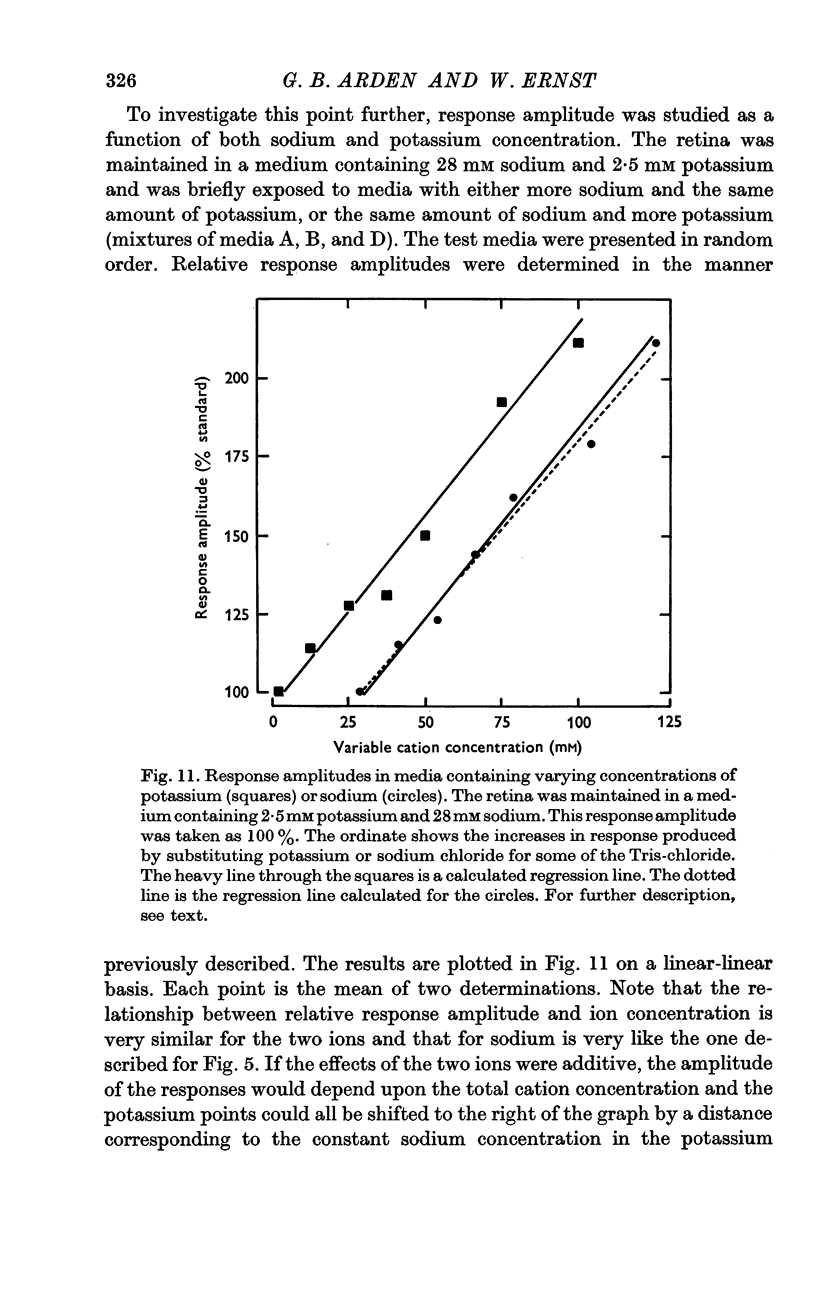
2. There is an approximately linear relationship between response amplitude and sodium concentration of the bathing medium.
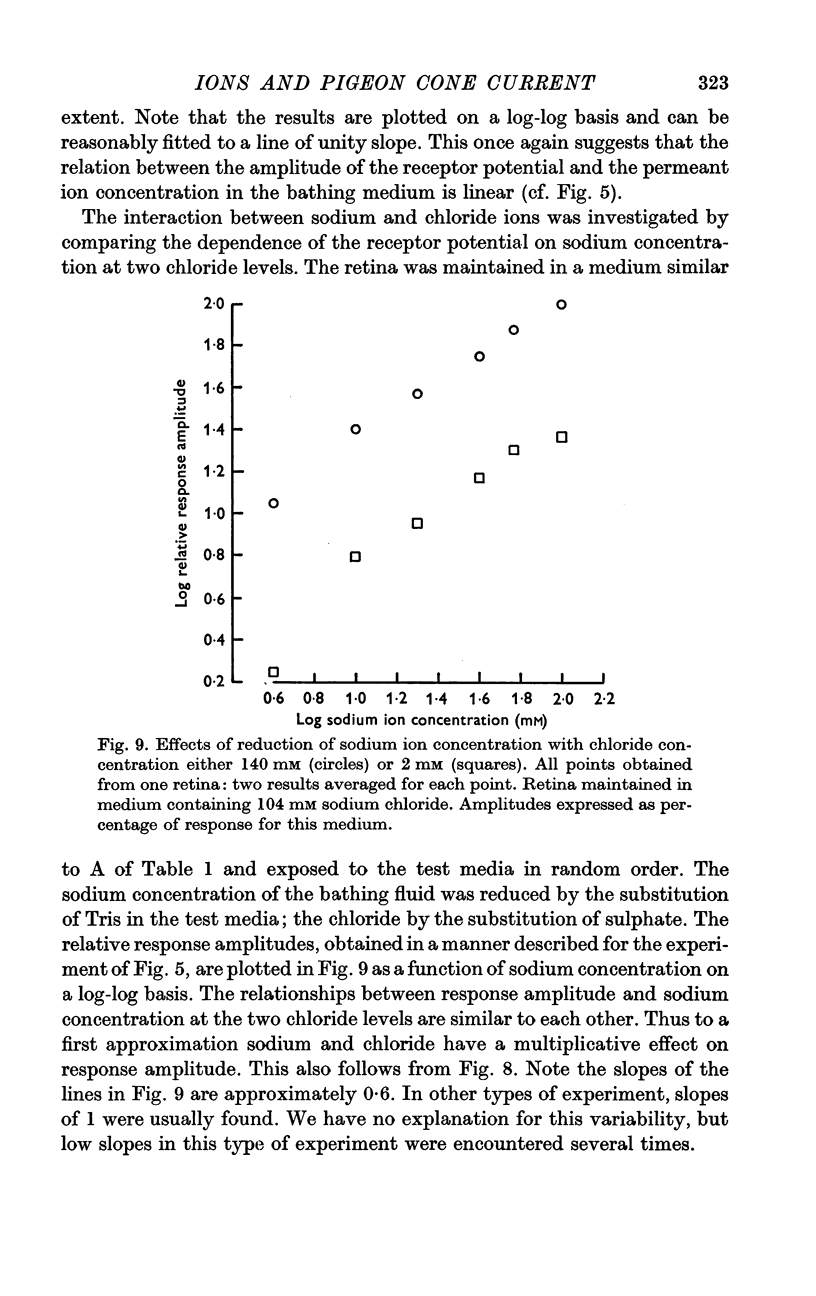
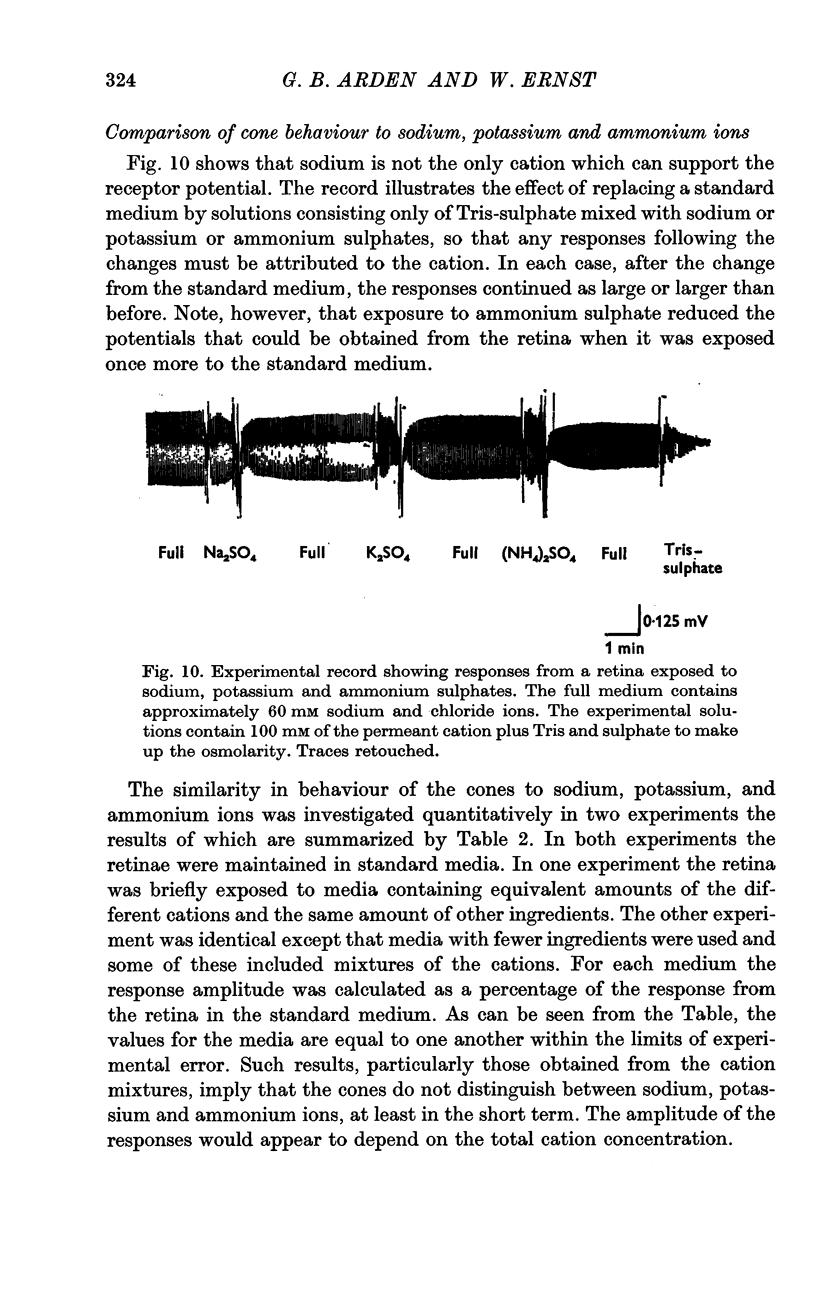
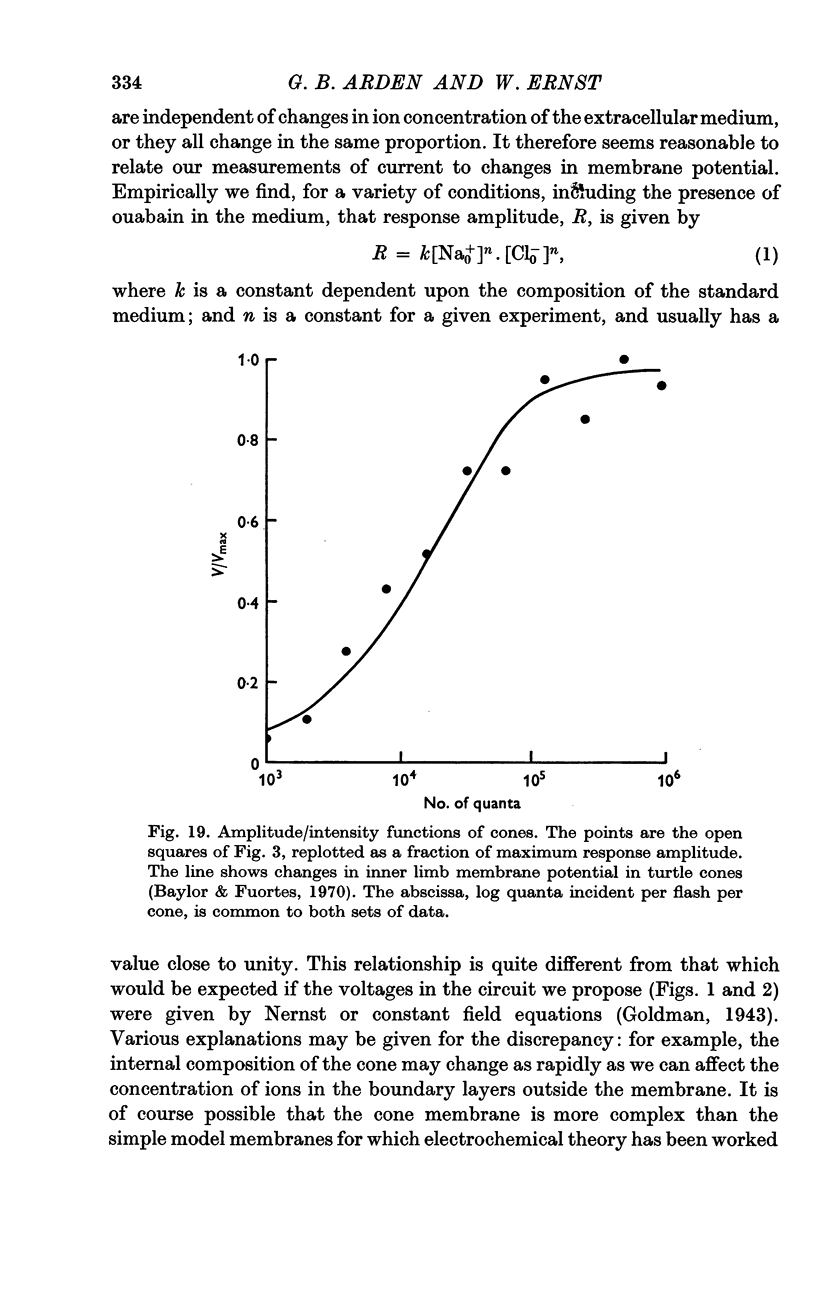
3. A decrease of chloride ions produces results equivalent to a decrease of sodium ions. The response amplitude is a power function of the product of the sodium and chloride concentrations. For most experiments, the exponent is ca. 1.
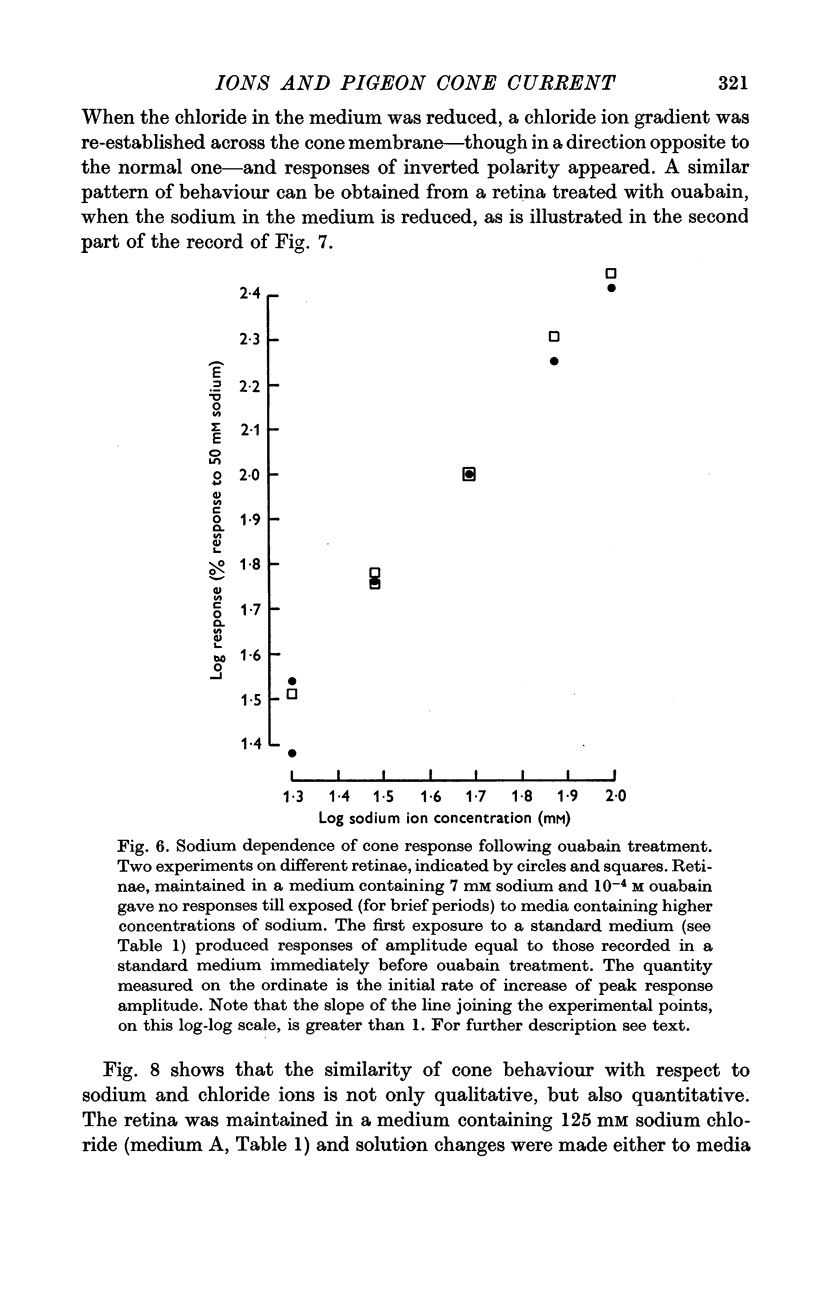
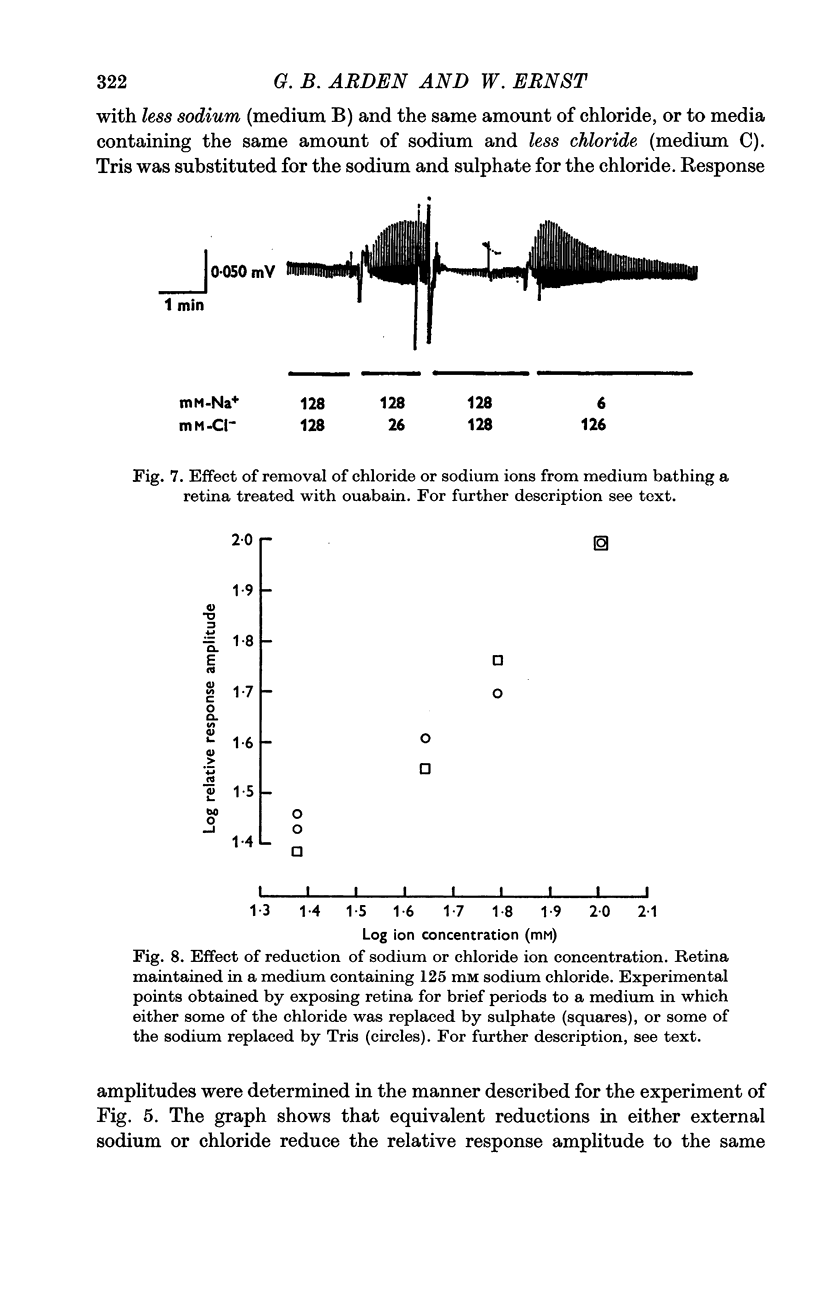
4. After treatment of the retina with ouabain, responses vanish, but can be temporarily restored, in normal, or inverted polarity, by appropriate changes in sodium or chloride concentration.
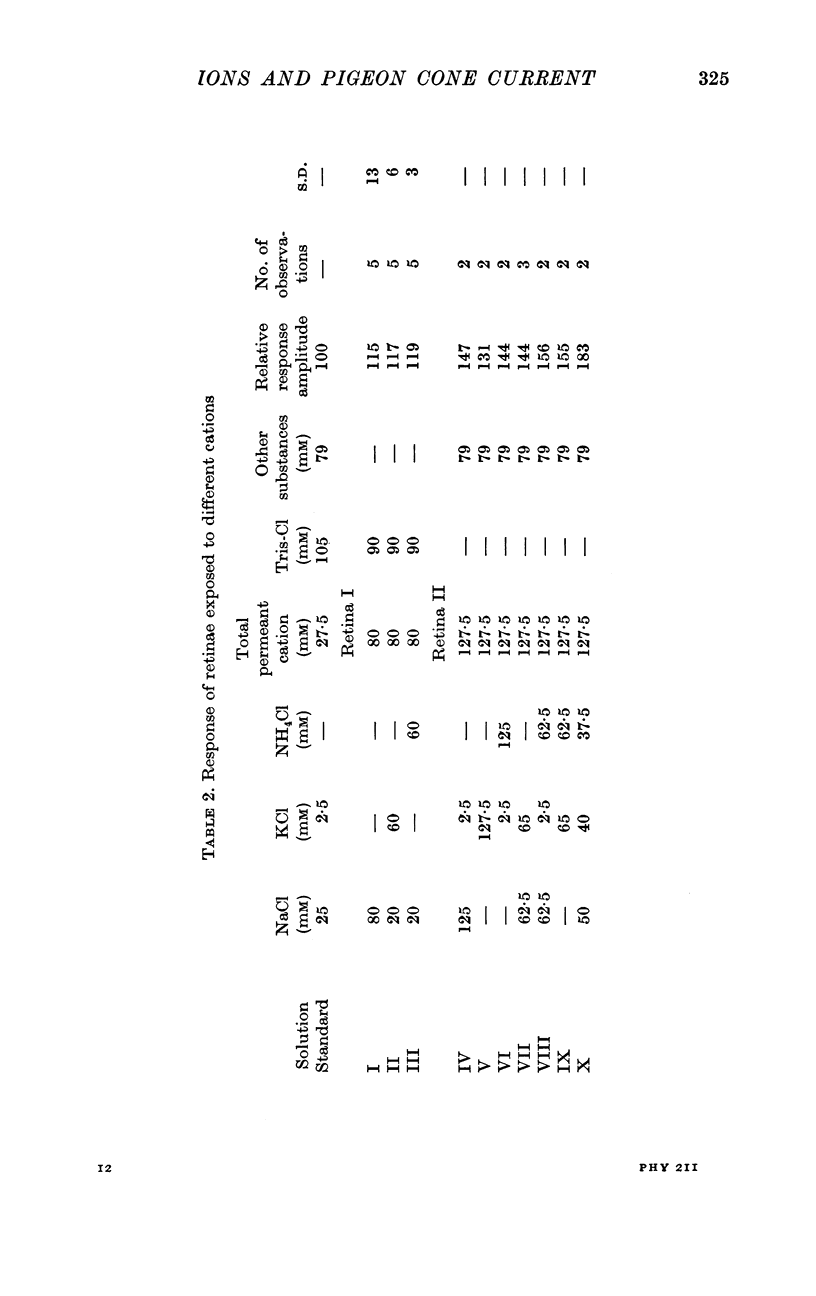
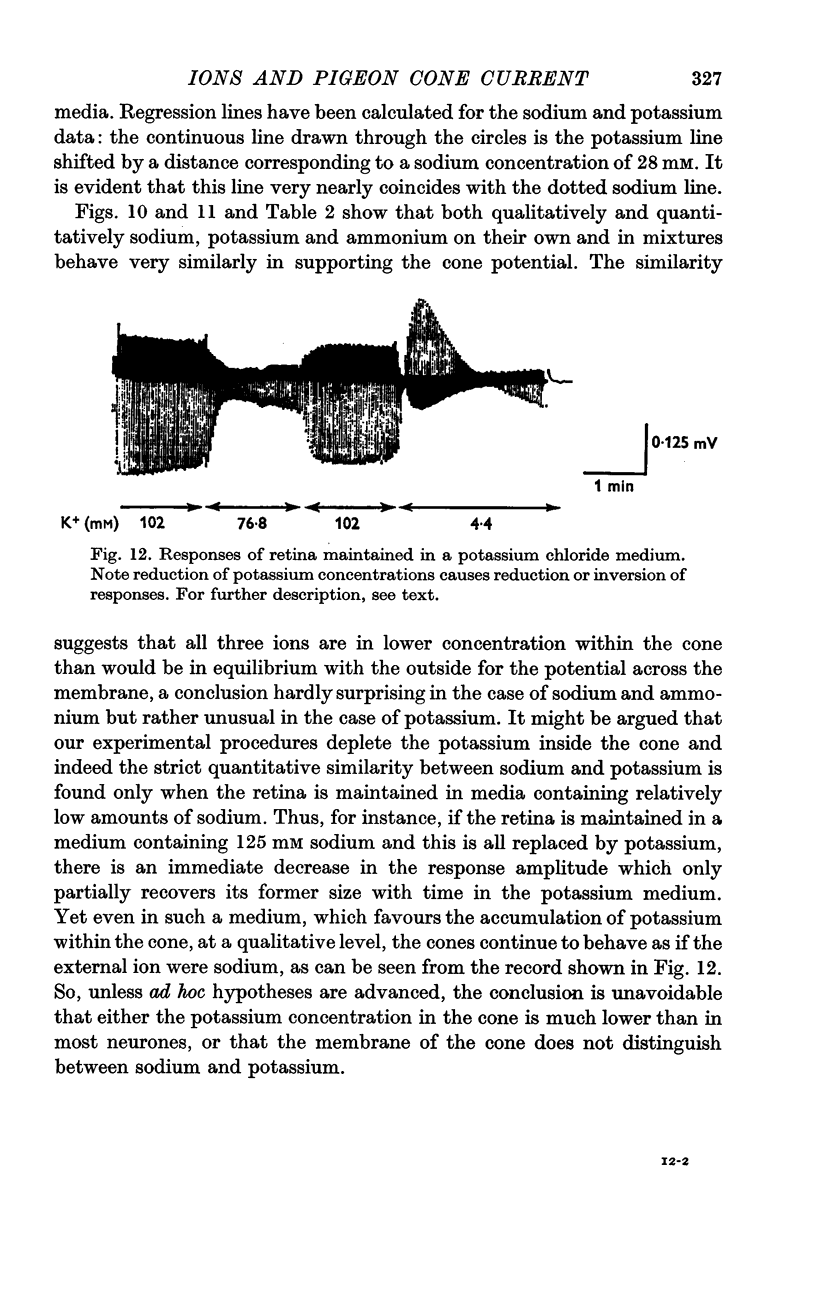
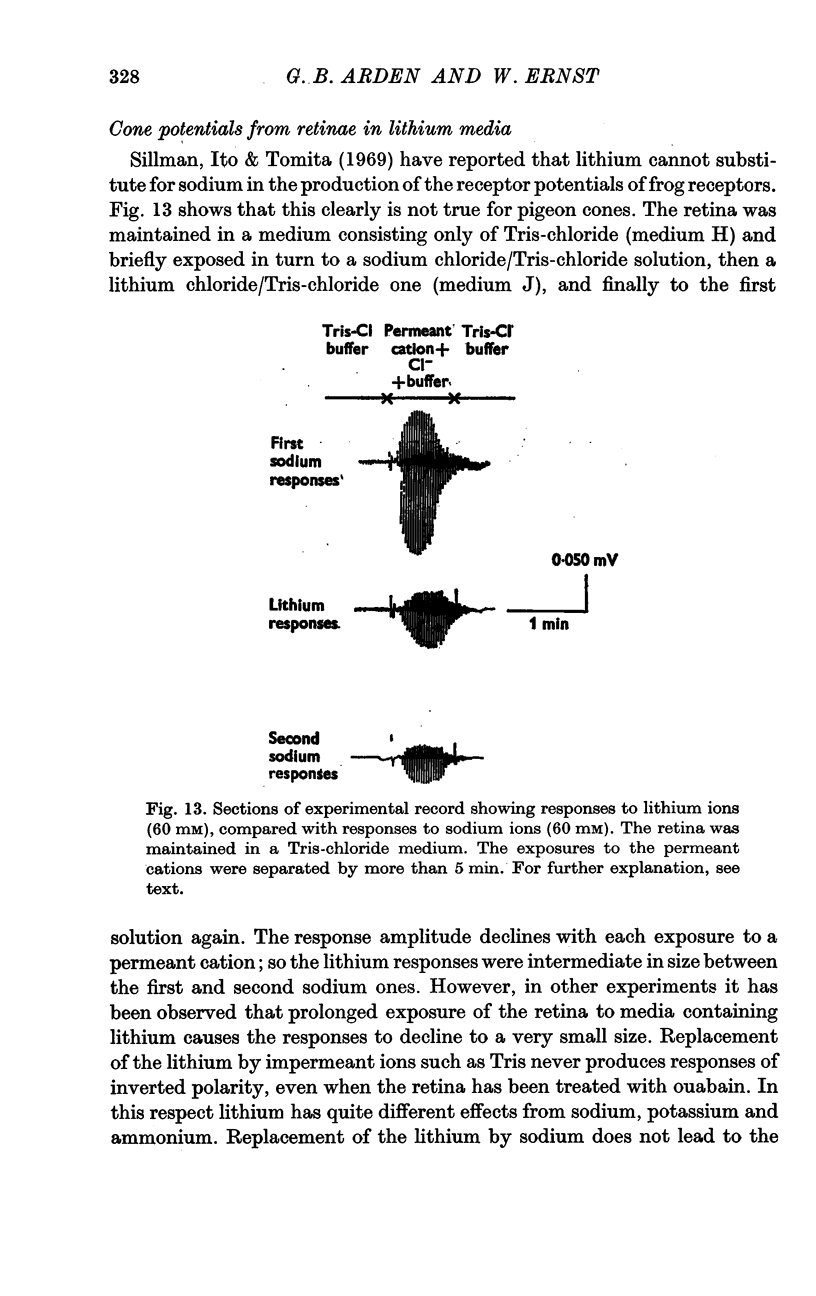
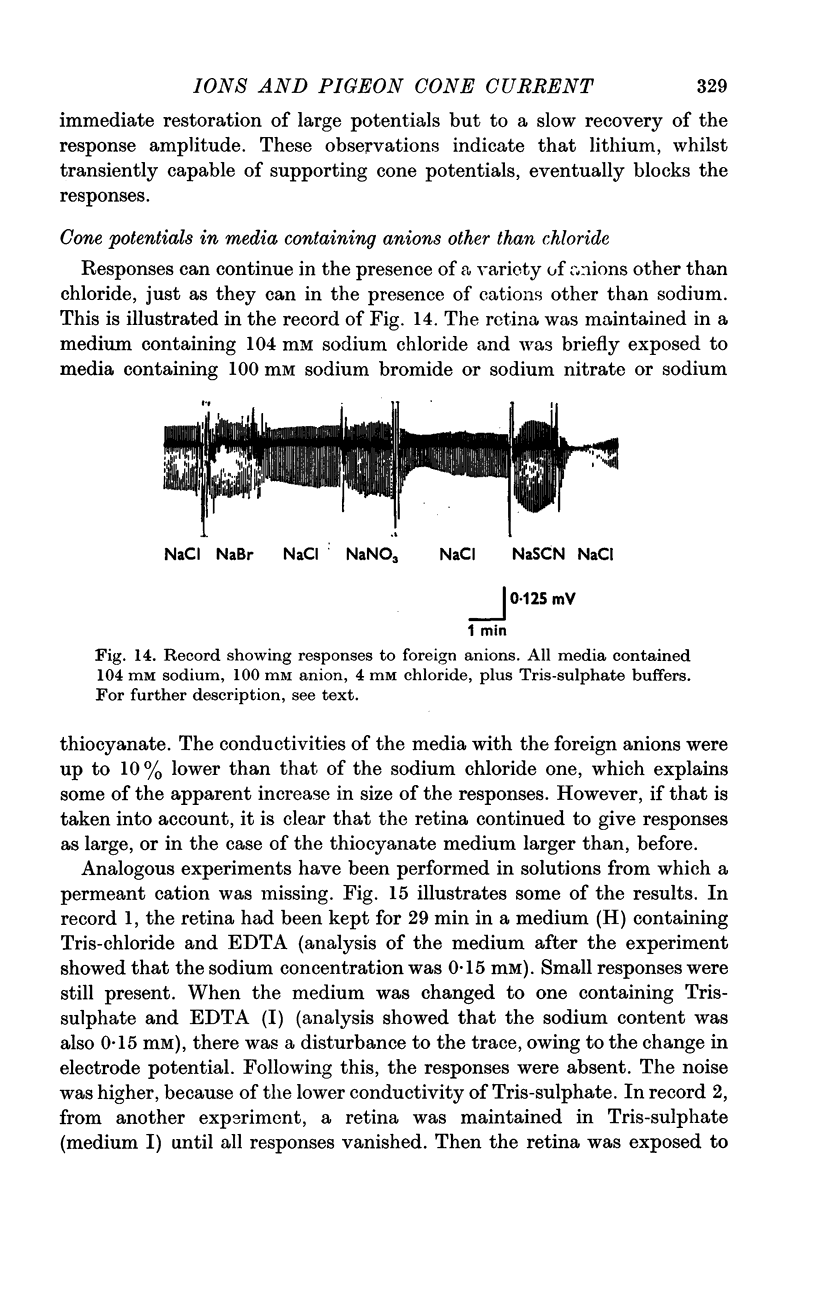
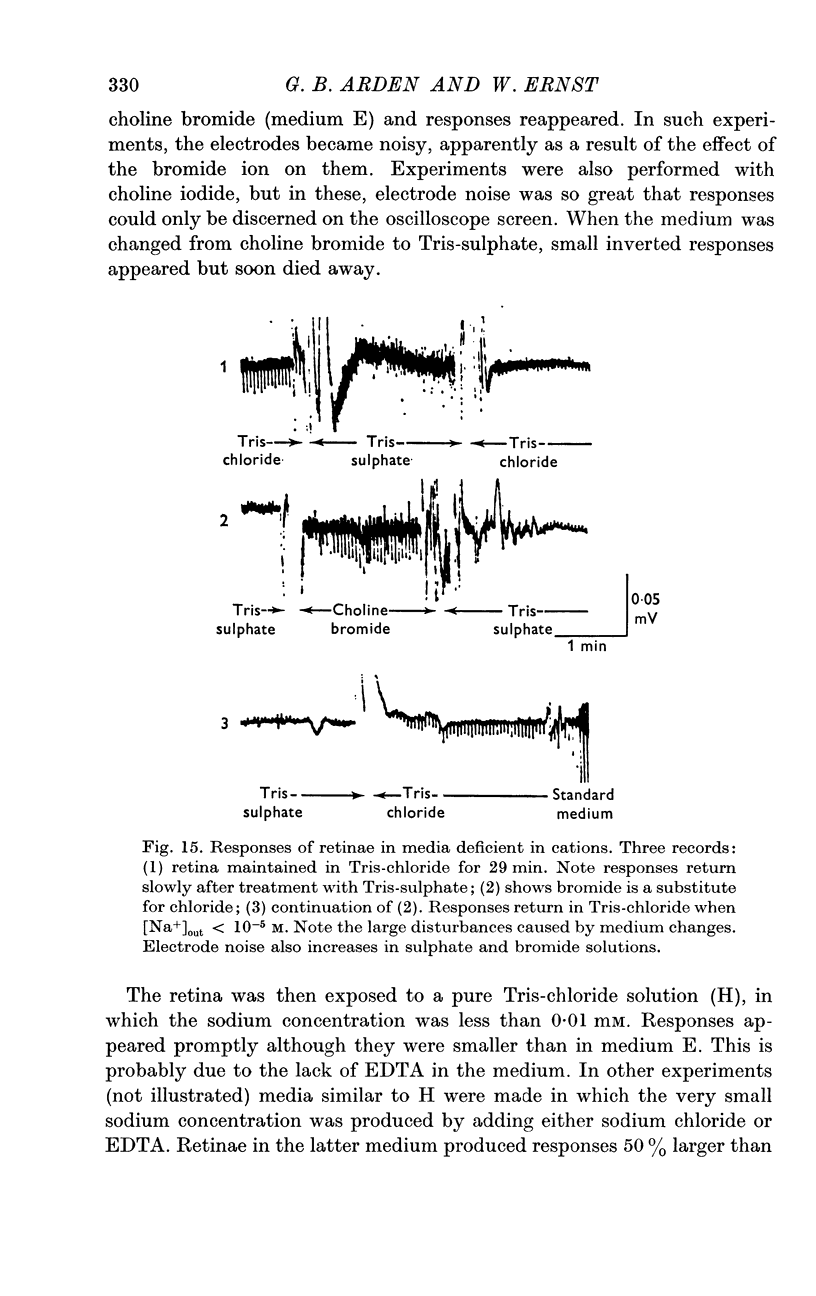
5. Ammonium and potassium ions can substitute quantitatively for sodium ions. Lithium ions initially carry response currents, but later block all responses. Bromide, nitrate and thiocyanate can substitute for chloride ions.
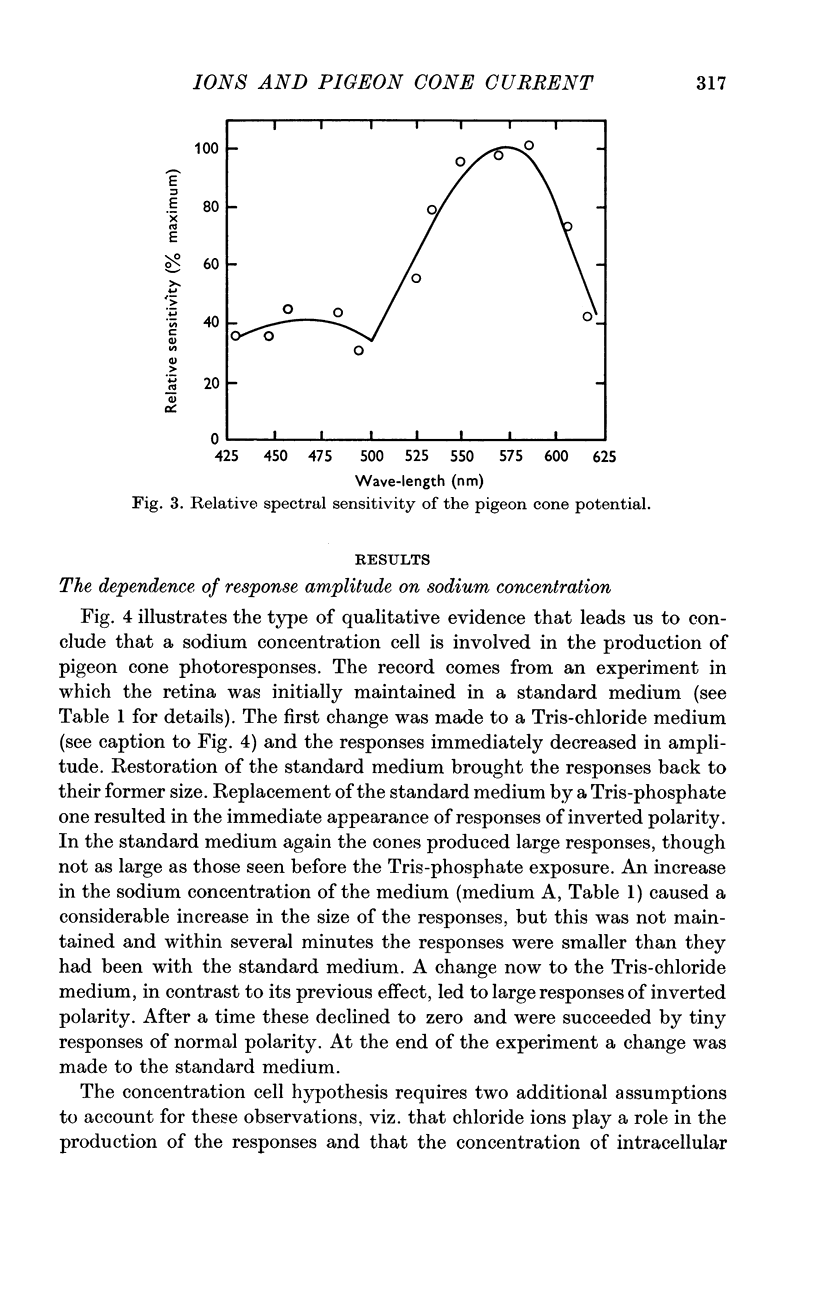
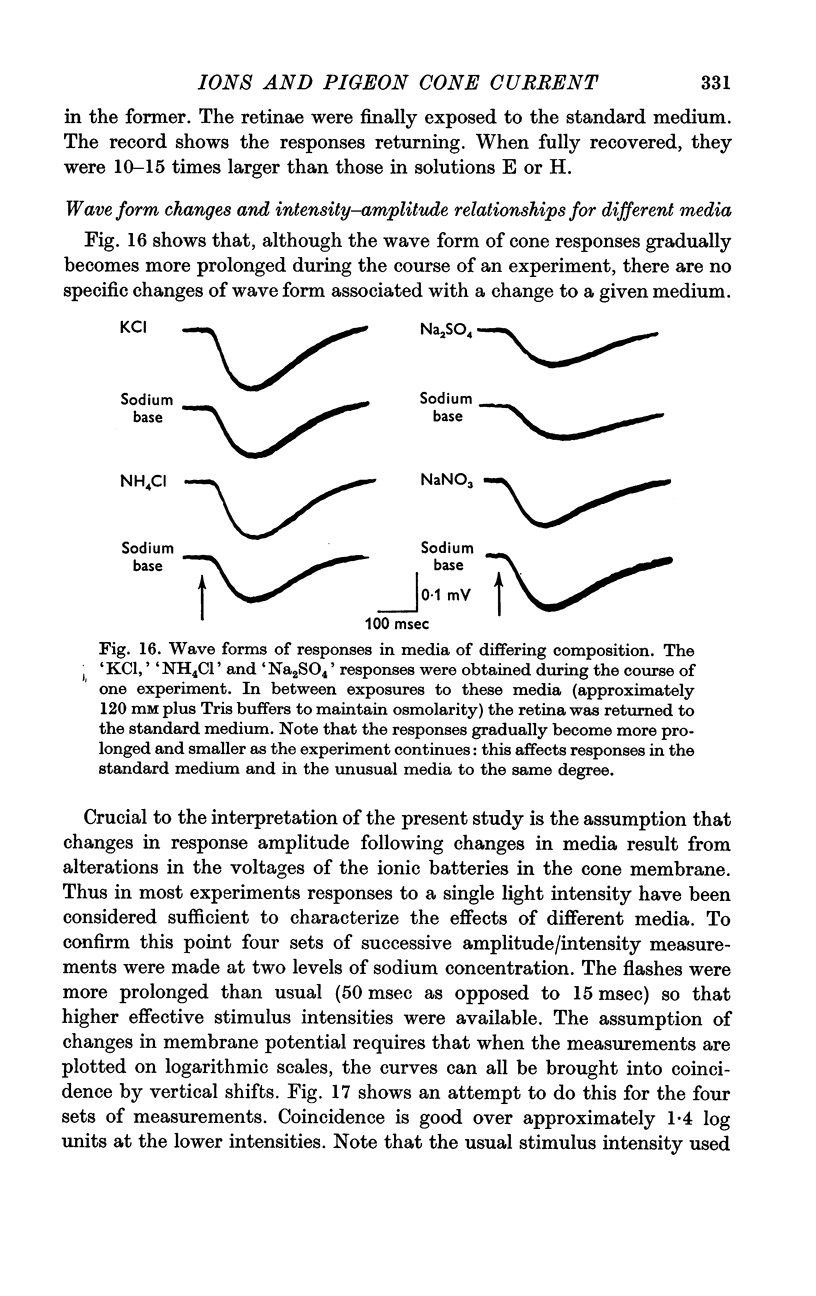
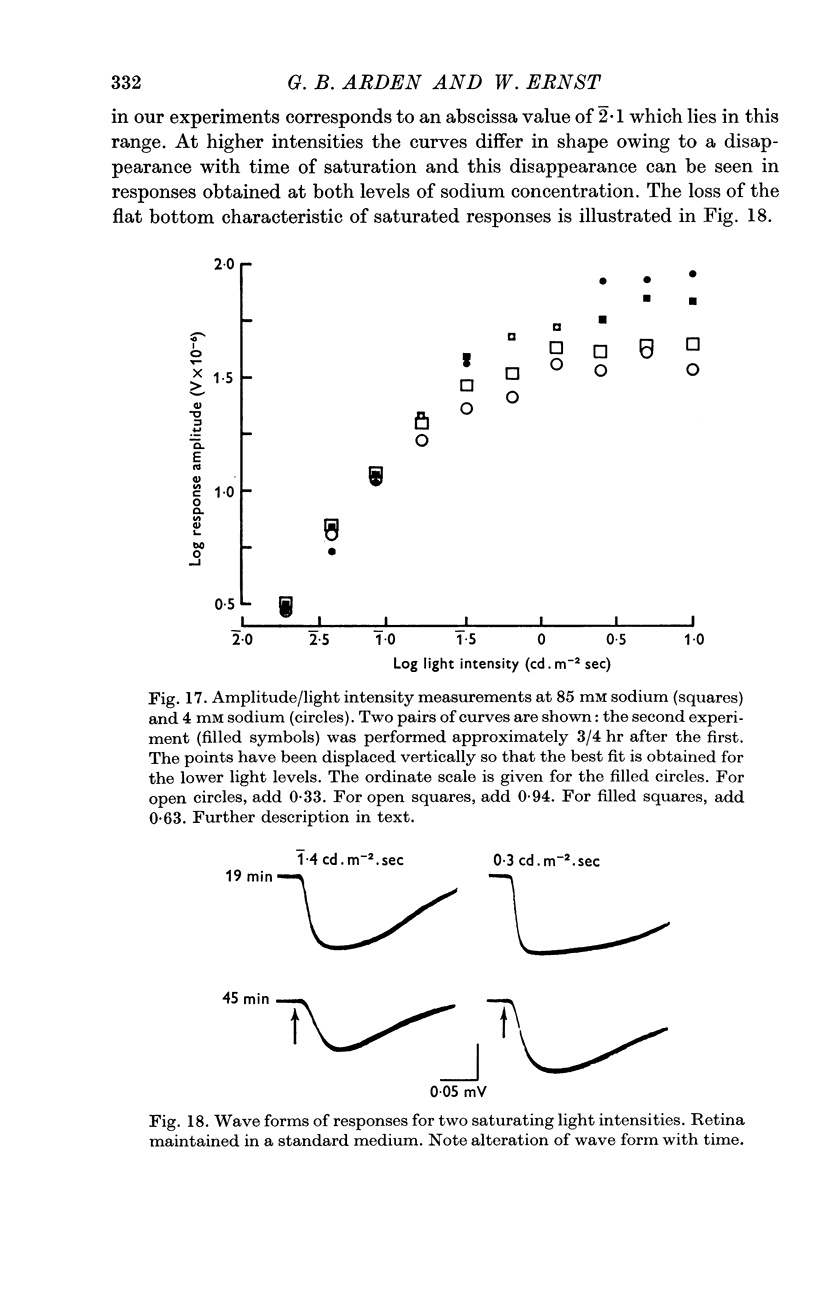
6. The response wave form, and the amplitude/light intensity relationship are independent of ions.
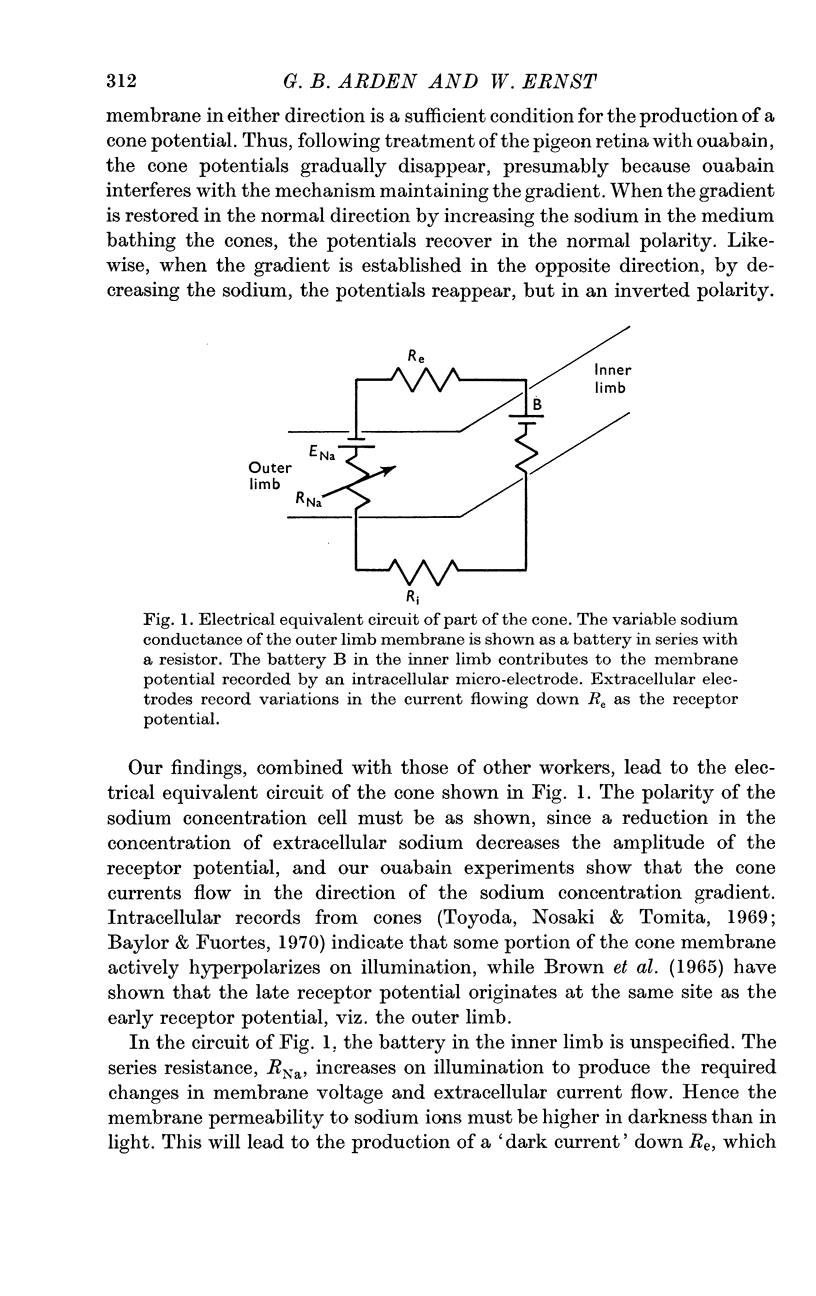
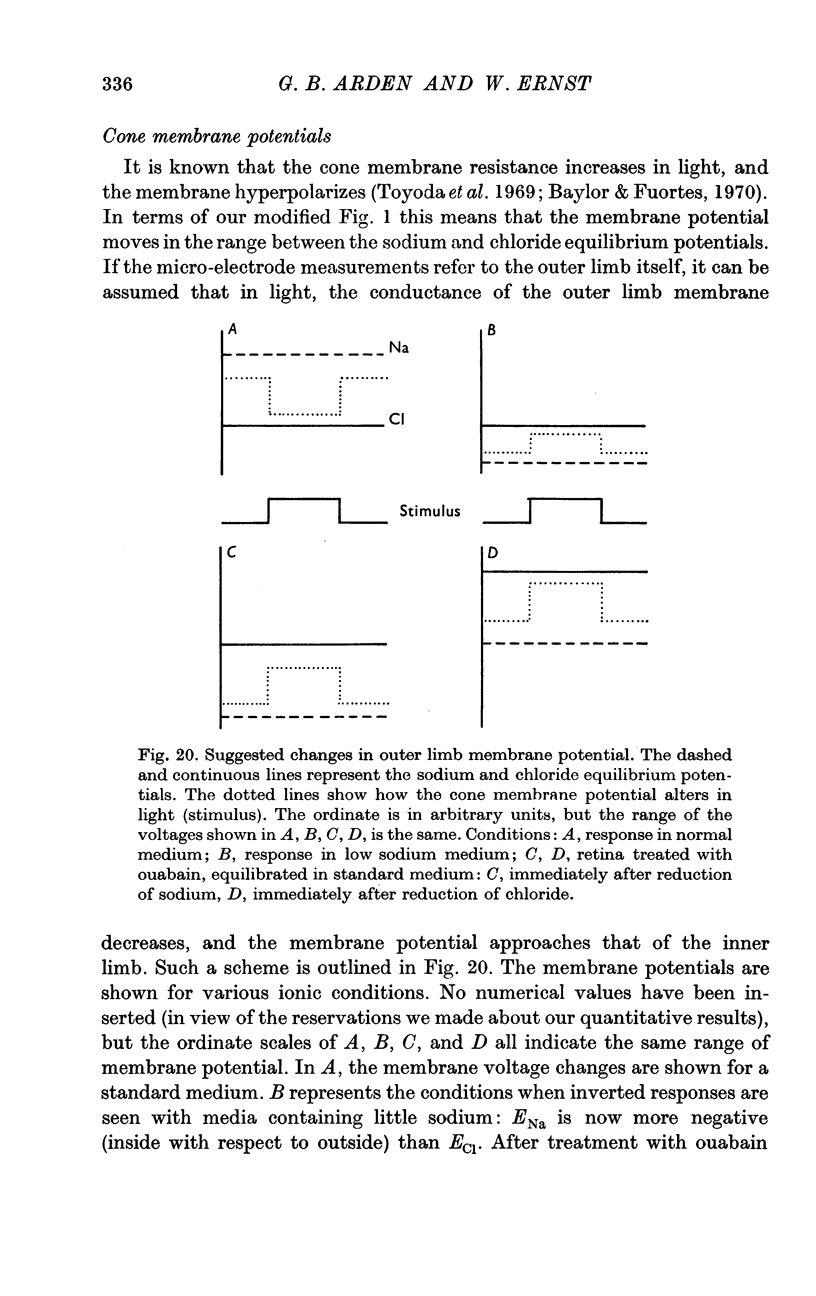
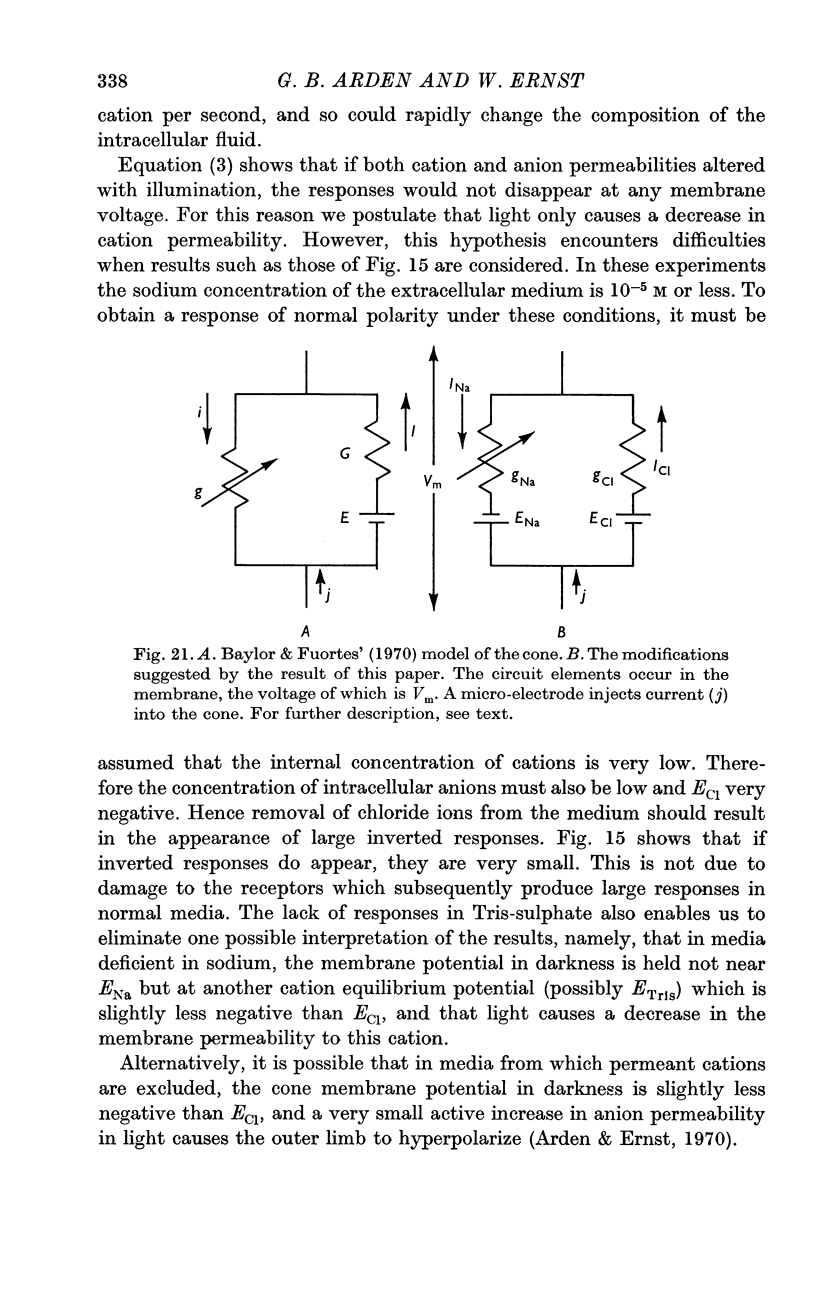
7. Models of the photoreceptor are discussed. It is concluded that the membrane potential of the cone in light is chiefly determined by the distribution of anions across the membrane, and in darkness there is an increase in outer limb cation permeability.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden G. B., Ernst W. Different current generators in rods and cones. J Physiol. 1969 Sep;204(1):35P–36P. [PubMed] [Google Scholar]
- Arden G. B., Ernst W. Mechanism of current production found in pigeon cones but not in pigeon or rat rods. Nature. 1969 Aug 2;223(5205):528–531. doi: 10.1038/223528a0. [DOI] [PubMed] [Google Scholar]
- BRINDLEY G. S. The passive electrical properties of the frog's retina, choroid and sclera for radial fields and currents. J Physiol. 1956 Nov 28;134(2):339–352. doi: 10.1113/jphysiol.1956.sp005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. T., Watanabe K., Murakami M. The early and late receptor potentials of monkey cones and rods. Cold Spring Harb Symp Quant Biol. 1965;30:457–482. doi: 10.1101/sqb.1965.030.01.045. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature. 1969 Jul 12;223(5202):201–204. doi: 10.1038/223201a0. [DOI] [PubMed] [Google Scholar]
- Sillman A. J., Ito H., Tomita T. Studies on the mass receptor potential of the isolated frog retina. II. On the basis of the ionic mechanism. Vision Res. 1969 Dec;9(12):1443–1451. doi: 10.1016/0042-6989(69)90060-1. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]



