Abstract
1. Properties of two-tone inhibition in primary auditory neurones of cats were studied with phase-locked sound stimuli. One sound was a continuous tone at the best frequency of a given neurone, and the other, a tone burst which was changed in amplitude, frequency, and phase relative to the continuous tone.
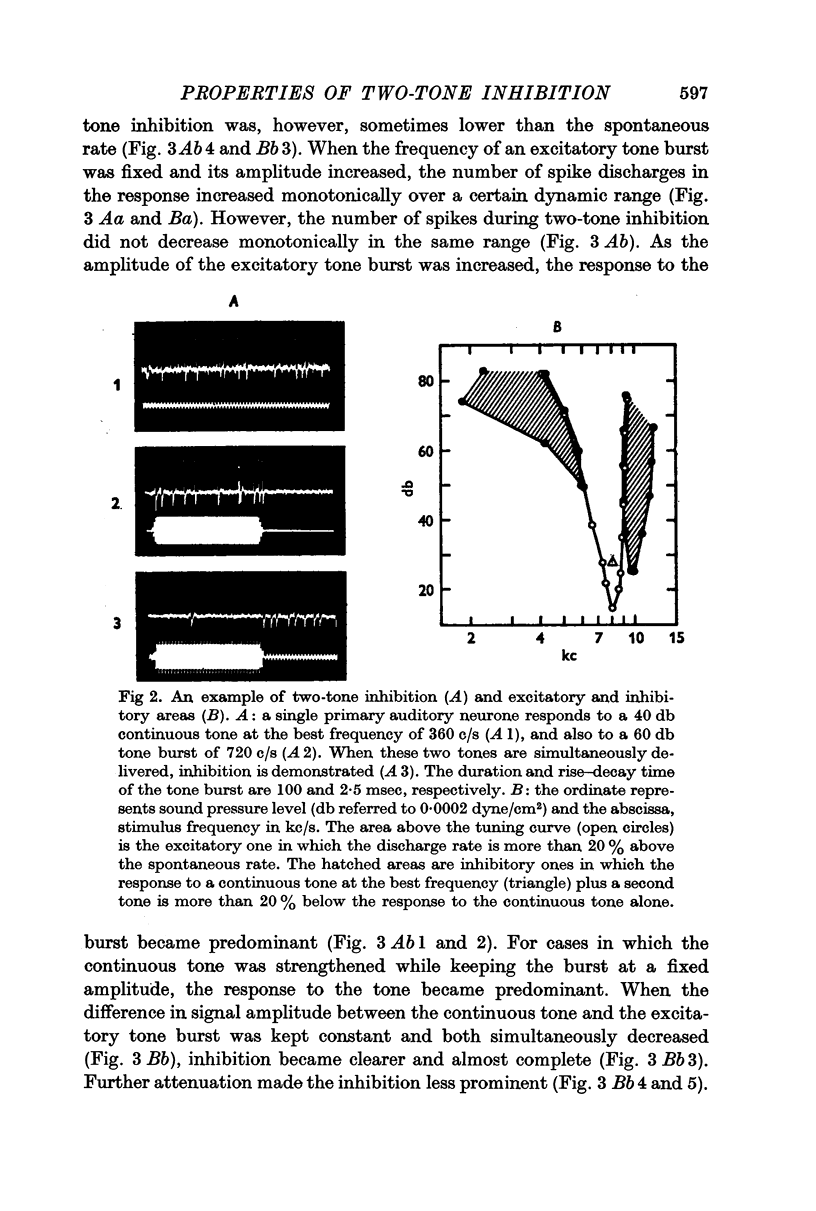
2. The tone burst which caused two-tone inhibition had either an excitatory or no effect when it was delivered alone. Inhibitory areas commonly appeared on both sides of the excitatory area when the best frequency was higher than a few kc/s.
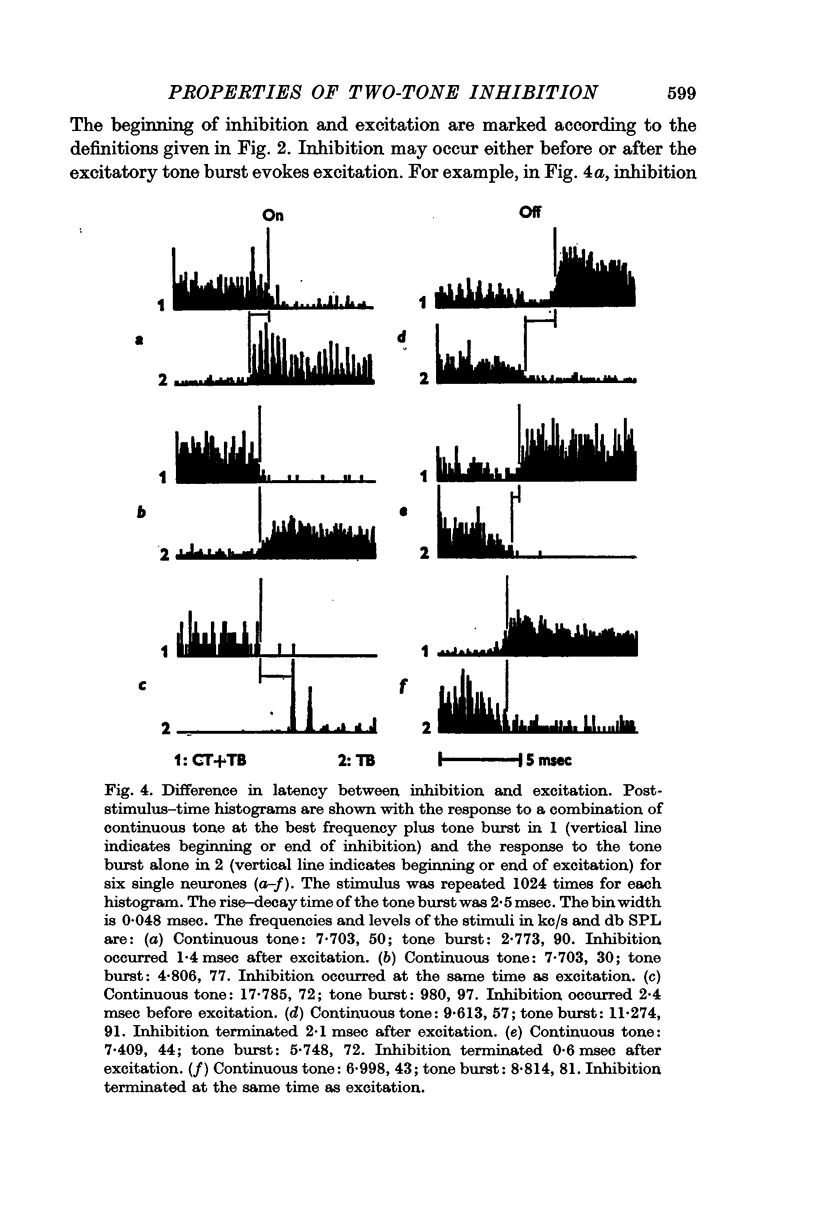
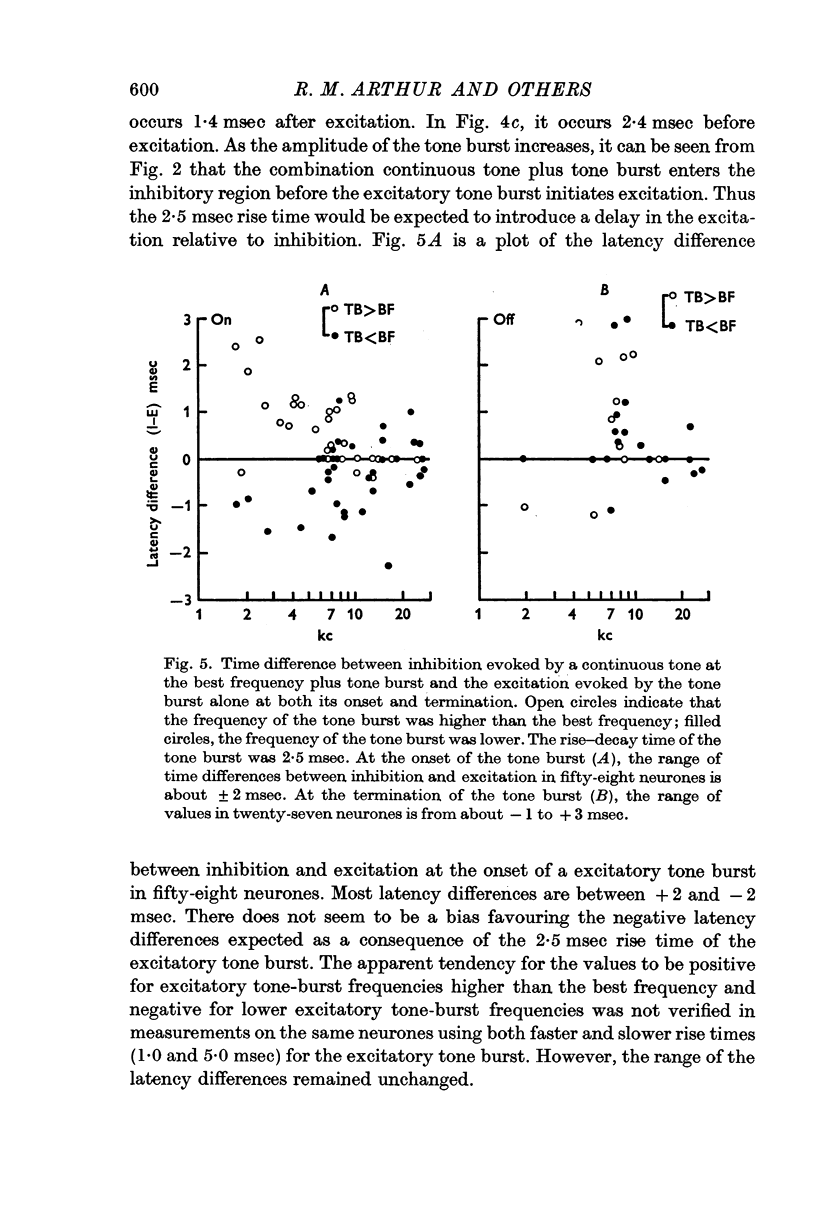
3. Two-tone inhibition began and ceased within a few milliseconds of the onset and termination of the excitation caused by a tone burst. The degree of inhibition was greatest at the beginning of the tone burst and reached a plateau within 500 msec. The discharge rate during inhibition could be lower than the rate for either tone alone or for spontaneous activity. At the termination of inhibition, prominent rebound in the discharge rate was found.
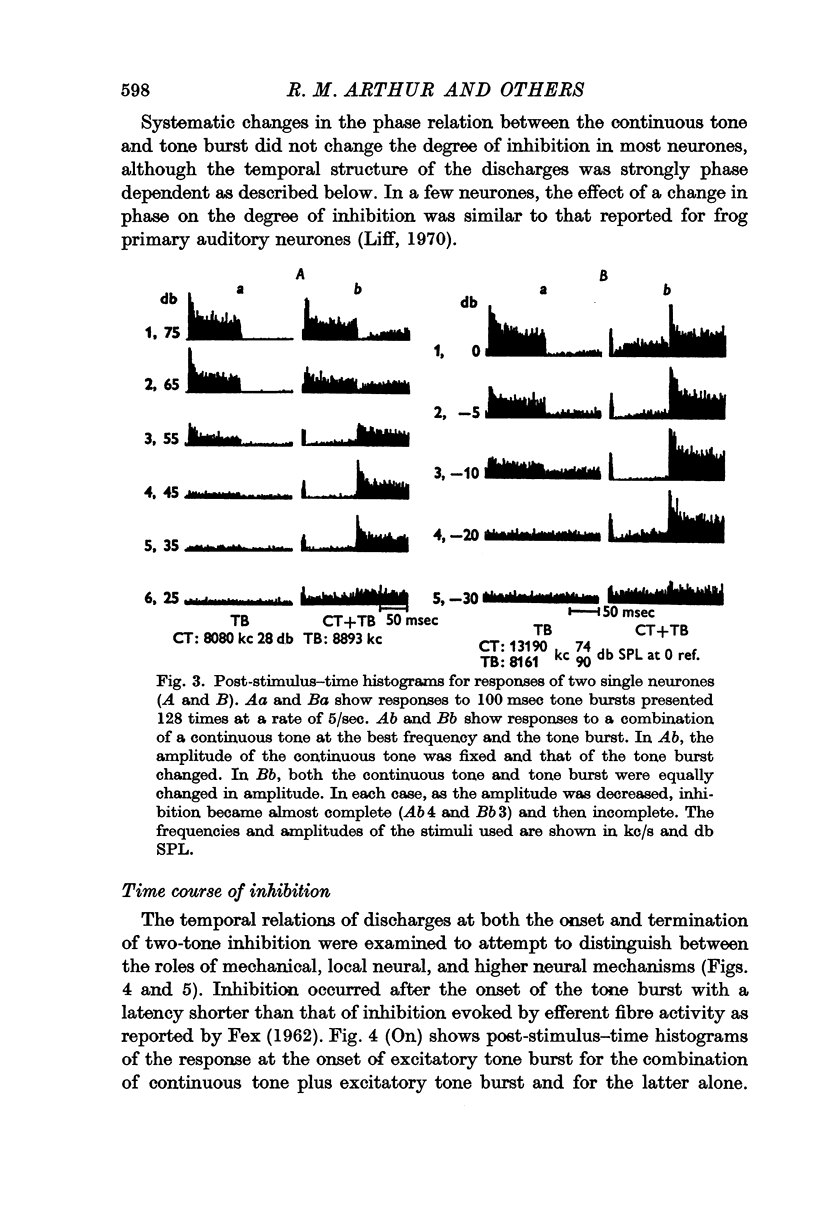
4. With an increase in amplitude of a tone burst, for either a fixed or equally increased continuous tone, the discharge rate during inhibition decreased to a minimum and then began to increase. That is, the degree of inhibition was non-monotonically related to the sound level.
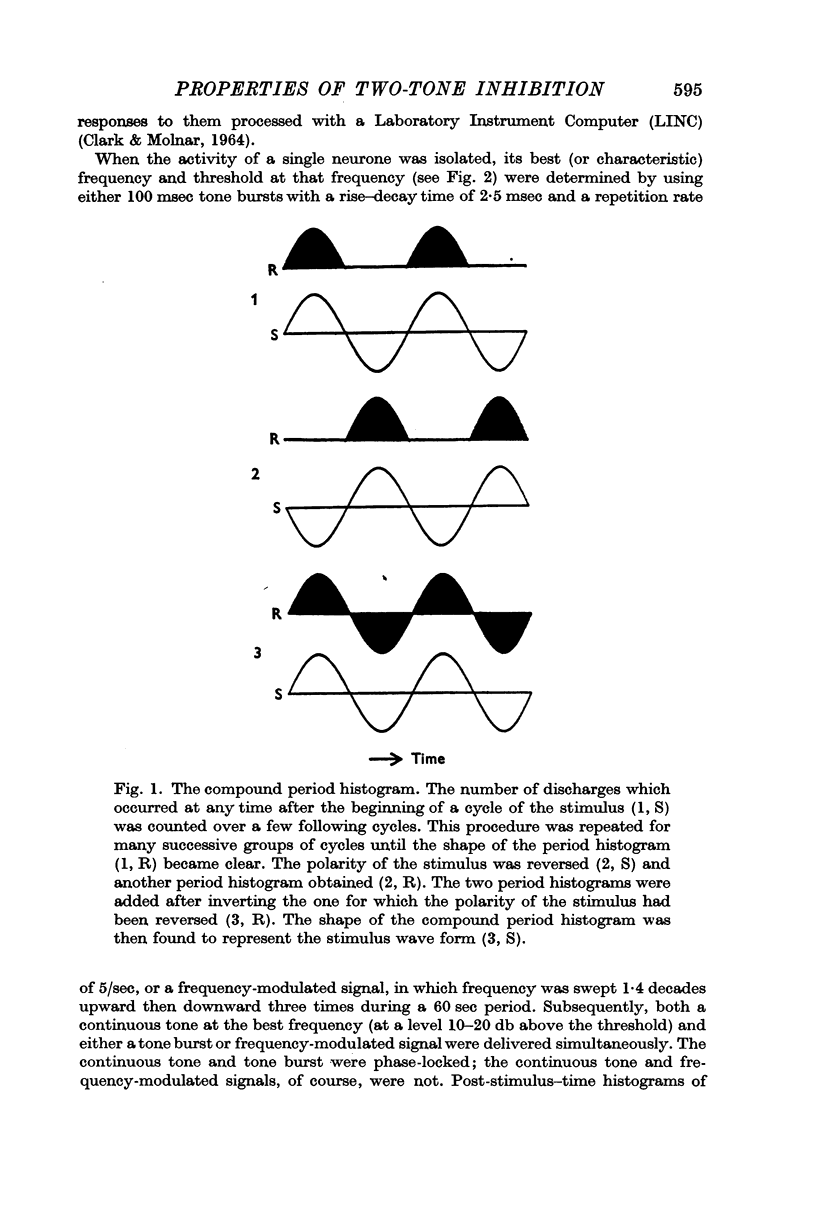
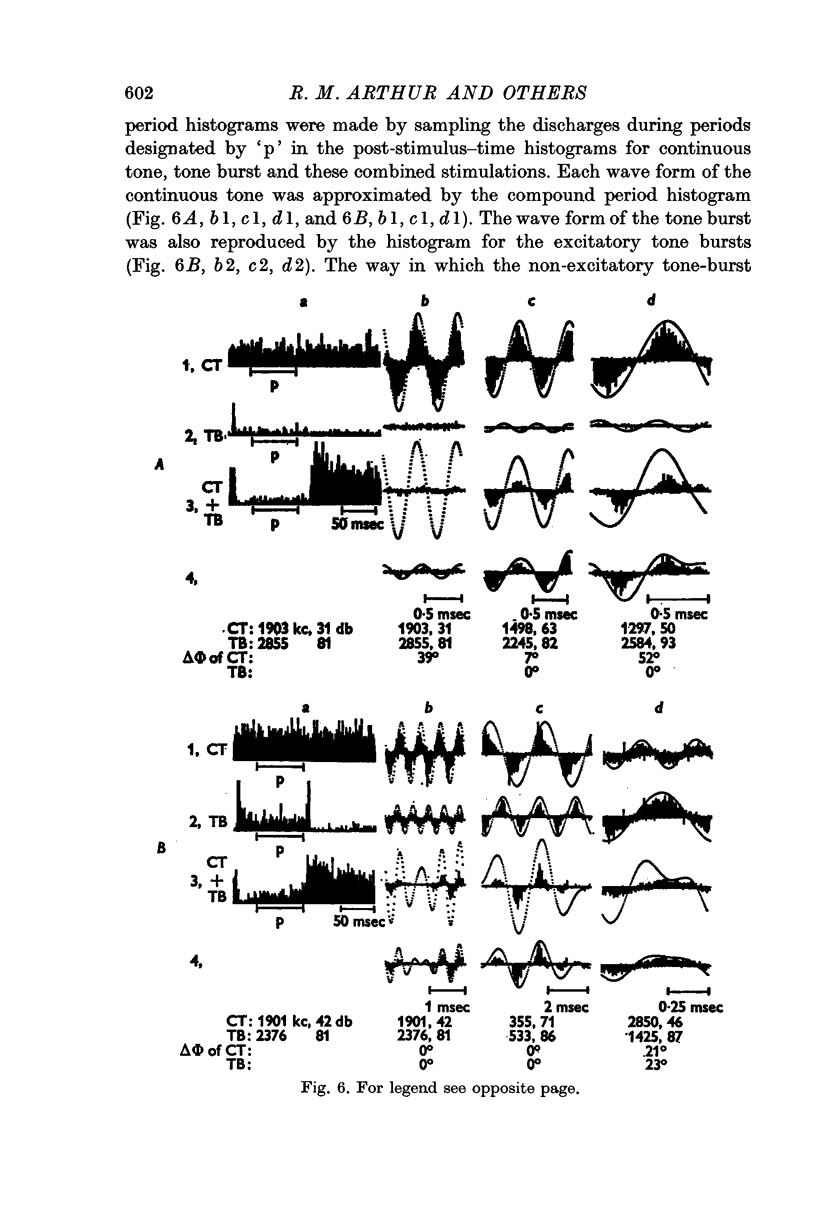
5. Compound period histograms of discharges during inhibition showed that single neurones usually carried information about the combined wave form of the two tones. The information about each tone was, however, modified by the inhibitory phenomenon in both amplitude and phase from that indicated by the compound period histograms for the individual tones.
6. Possible mechanisms and functional significance of two-tone inhibition are discussed.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. F., Anderson D. J., Hind J. E., Rose J. E. Time structure of discharges in single auditory nerve fibers of the squirrel monkey in response to complex periodic sounds. J Neurophysiol. 1969 May;32(3):386–401. doi: 10.1152/jn.1969.32.3.386. [DOI] [PubMed] [Google Scholar]
- CLARK W. A., MOLNAR C. E. THE LINC: A DESCRIPTION OF THE LABORATORY INSTRUMENT COMPUTER. Ann N Y Acad Sci. 1964 Jul 31;115:653–668. [PubMed] [Google Scholar]
- Engebretson A. M., Eldredge D. H. Model for the nonlinear characteristics of cochlear potentials. J Acoust Soc Am. 1968 Aug;44(2):548–554. doi: 10.1121/1.1911119. [DOI] [PubMed] [Google Scholar]
- Goblick T. J., Jr, Pfeiffer R. R. Time-domain measurements of cochlear nonlinearities using combination click stimuli. J Acoust Soc Am. 1969 Oct;46(4):924–938. doi: 10.1121/1.1911812. [DOI] [PubMed] [Google Scholar]
- Hind J. E., Anderson D. J., Brugge J. F., Rose J. E. Coding of information pertaining to paired low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1967 Jul;30(4):794–816. doi: 10.1152/jn.1967.30.4.794. [DOI] [PubMed] [Google Scholar]
- Molnar C. E., Loeffel R. G., Pfeiffer R. R. Distortion compensating, condenser-earphone driver for physiological studies. J Acoust Soc Am. 1968 May;43(5):1177–1178. doi: 10.1121/1.1910953. [DOI] [PubMed] [Google Scholar]
- NOMOTO M., SUGA N., KATSUKI Y. DISCHARGE PATTERN AND INHIBITION OF PRIMARY AUDITORY NERVE FIBERS IN THE MONKEY. J Neurophysiol. 1964 Sep;27:768–787. doi: 10.1152/jn.1964.27.5.768. [DOI] [PubMed] [Google Scholar]
- RATLIFF F., HARTLINE H. K., MILLER W. H. Spatial and temporal aspects of retinal inhibitory interaction. J Opt Soc Am. 1963 Jan;53:110–120. doi: 10.1364/josa.53.000110. [DOI] [PubMed] [Google Scholar]
- RUPERT A., MOUSHEGIAN G., GALAMBOS R. Unit responses to sound from auditory nerve of the cat. J Neurophysiol. 1963 May;26:449–465. doi: 10.1152/jn.1963.26.3.449. [DOI] [PubMed] [Google Scholar]
- SMITH C. A., DEMPSEY E. W. Electron microscopy of the organ of Corti. Am J Anat. 1957 May;100(3):337–367. doi: 10.1002/aja.1001000304. [DOI] [PubMed] [Google Scholar]
- Sachs M. B., Kiang N. Y. Two-tone inhibition in auditory-nerve fibers. J Acoust Soc Am. 1968 May;43(5):1120–1128. doi: 10.1121/1.1910947. [DOI] [PubMed] [Google Scholar]
- Sachs M. B. Stimulus-response relation for auditory-noise fibers: two-tone stimuli. J Acoust Soc Am. 1969 Apr;45(4):1025–1036. doi: 10.1121/1.1911493. [DOI] [PubMed] [Google Scholar]
- Suga N. Analysis of frequency-modulated and complex sounds by single auditory neurones of bats. J Physiol. 1968 Sep;198(1):51–80. doi: 10.1113/jphysiol.1968.sp008593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Analysis of frequency-modulated sounds by auditory neurones of echo-locating bats. J Physiol. 1965 Jul;179(1):26–53. doi: 10.1113/jphysiol.1965.sp007648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Responses of cortical auditory neurones to frequency modulated sounds in echo-locating bats. Nature. 1965 May 29;206(987):890–891. doi: 10.1038/206890a0. [DOI] [PubMed] [Google Scholar]


