Abstract
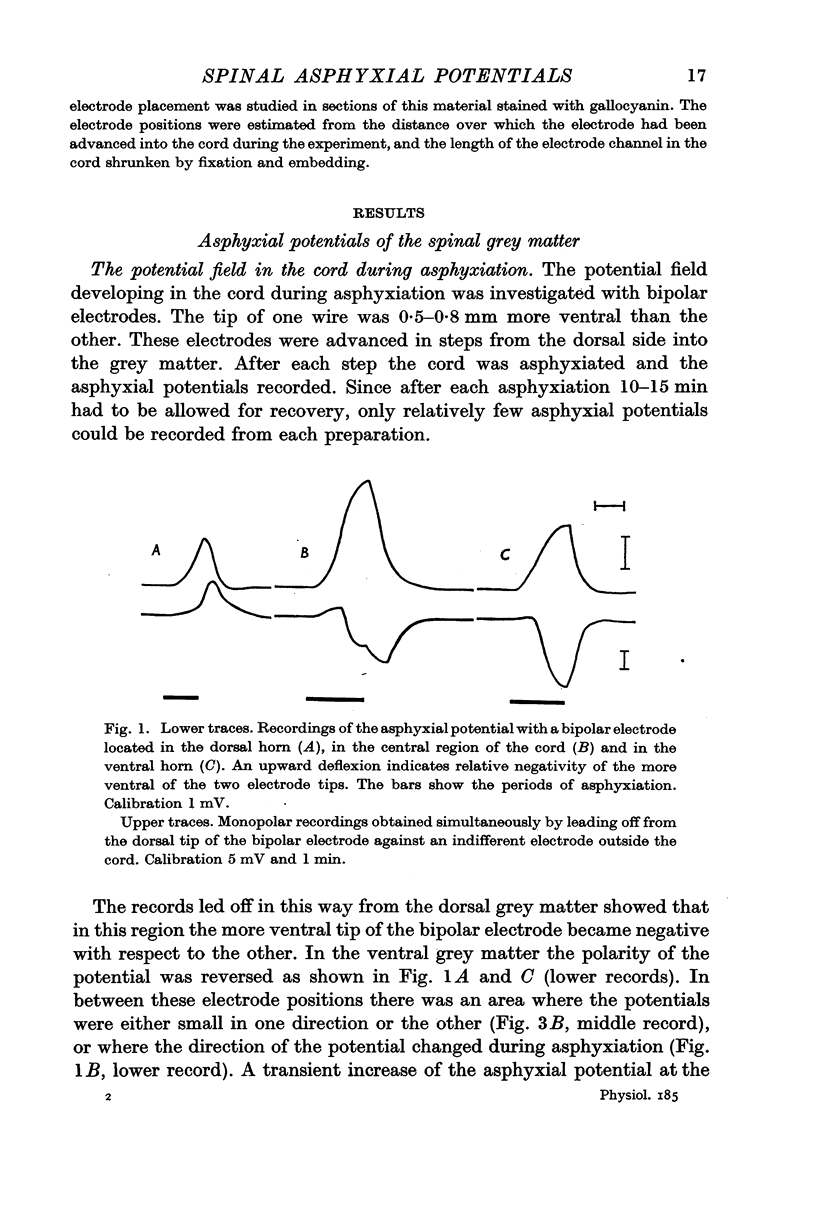
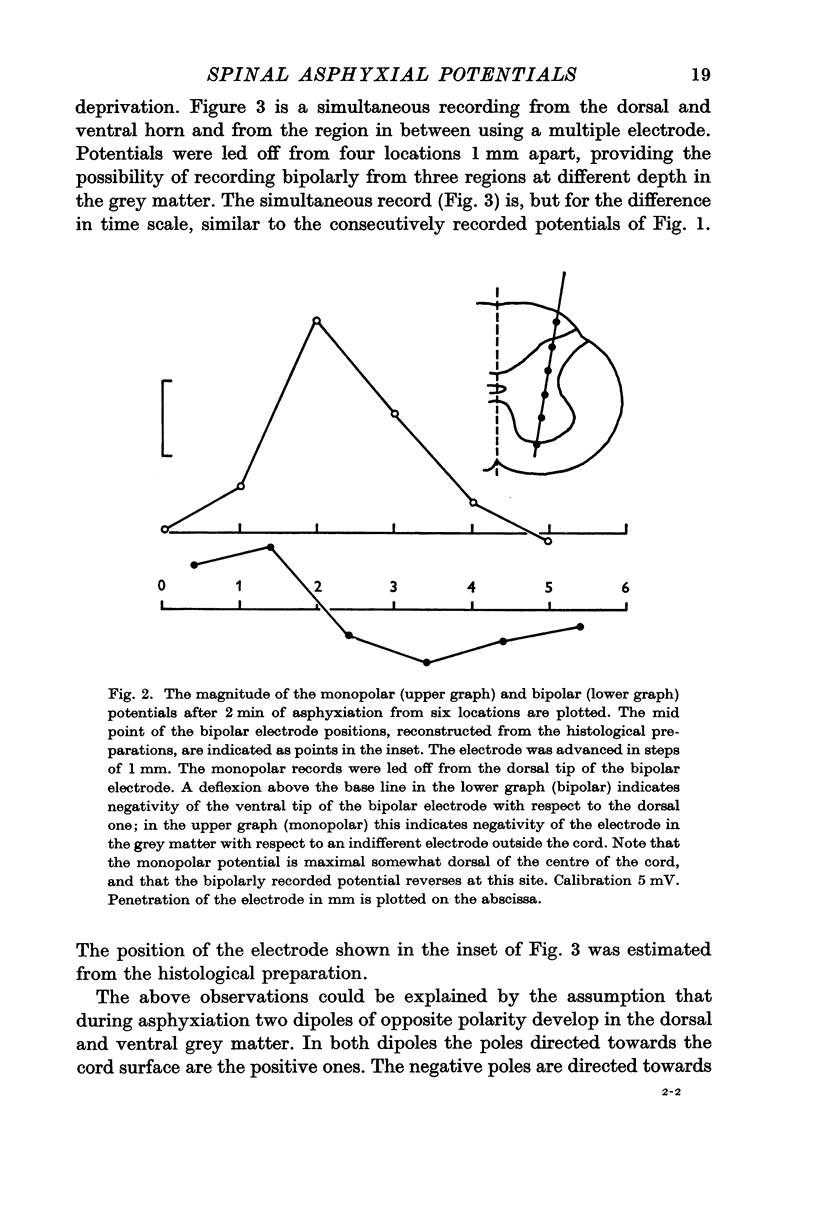
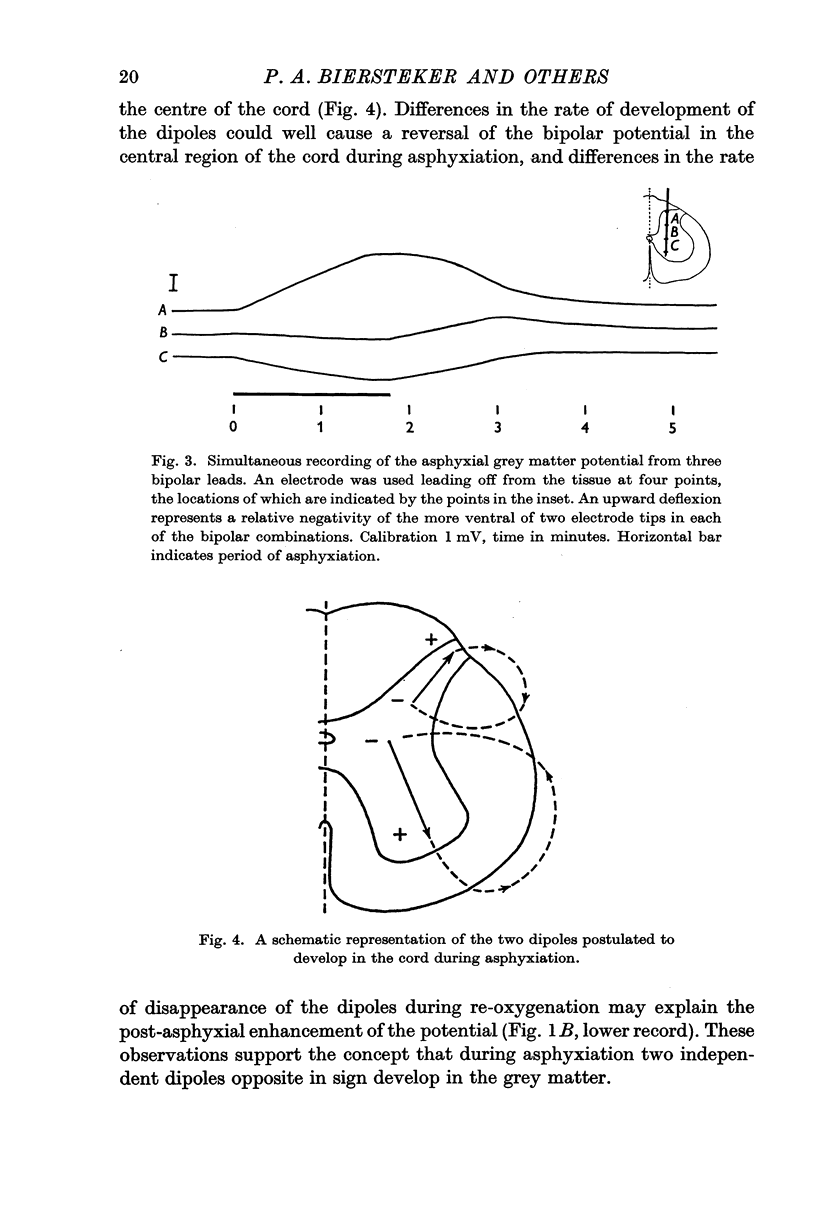
1. Asphyxial potentials of short latency (from a few to about 10 sec) were recorded with mono- and bipolar electrodes from the cat's spinal cord. Monopolarly, a zone of maximum negativity was found somewhat dorsal of the central canal in the dorsal horn. With bipolar leads potentials of opposite polarity were observed in the dorsal and ventral horns. In the dorsal horn the more ventral electrode tip became negative with respect to the more dorsal one, in the ventral horn the more ventral tip became more positive. In the centre of the cord where the monopolar potential showed a maximum the bipolar potentials were small in either direction, or reversed during asphyxiation.
2. These observations can be explained by the development of two independent dipoles of opposite polarity, located in the dorsal and ventral horn respectively, oriented with their negative poles towards the centre of the cord.
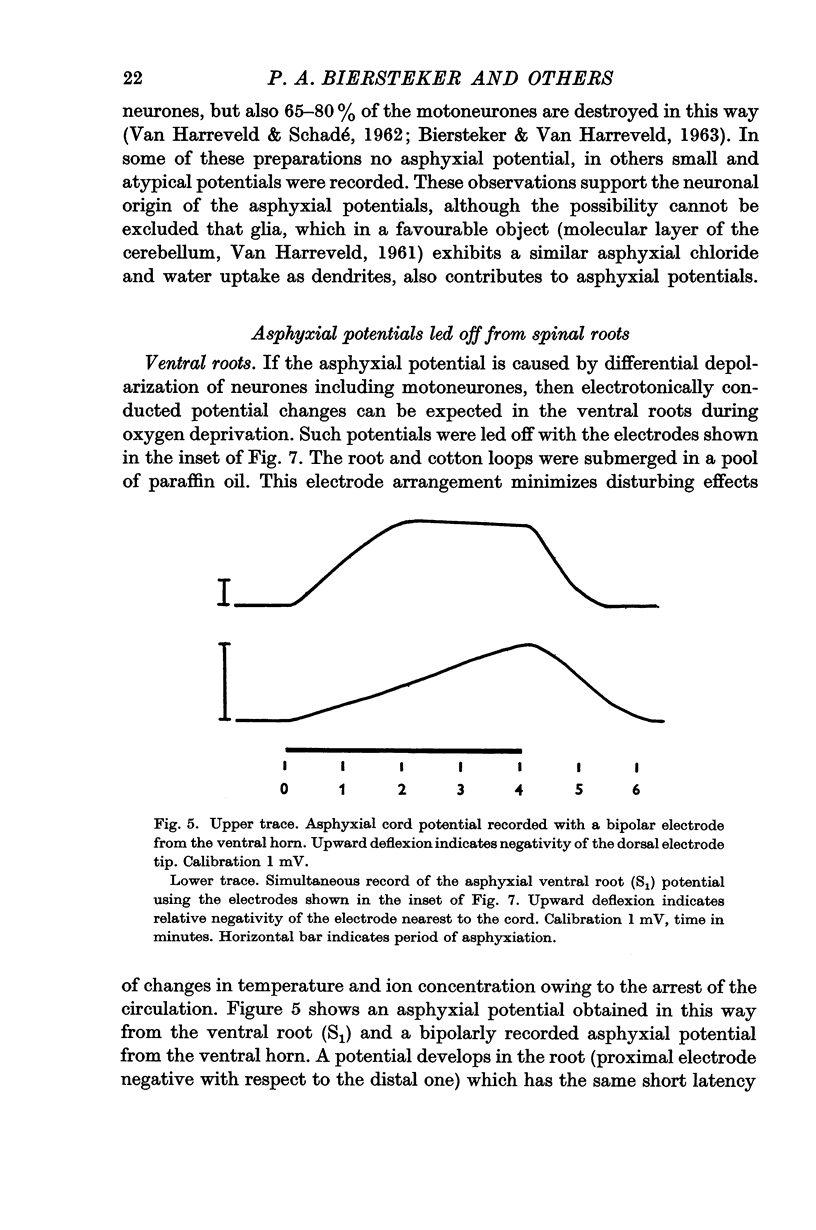
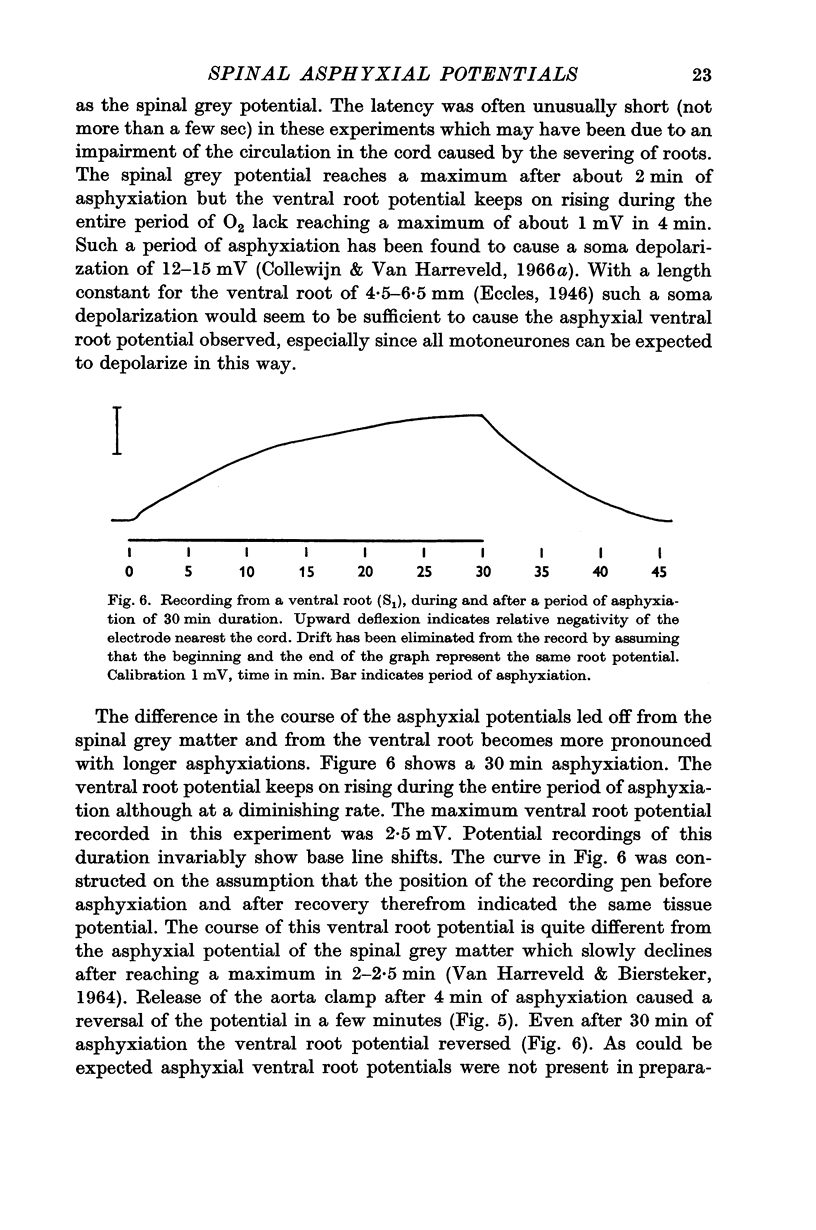
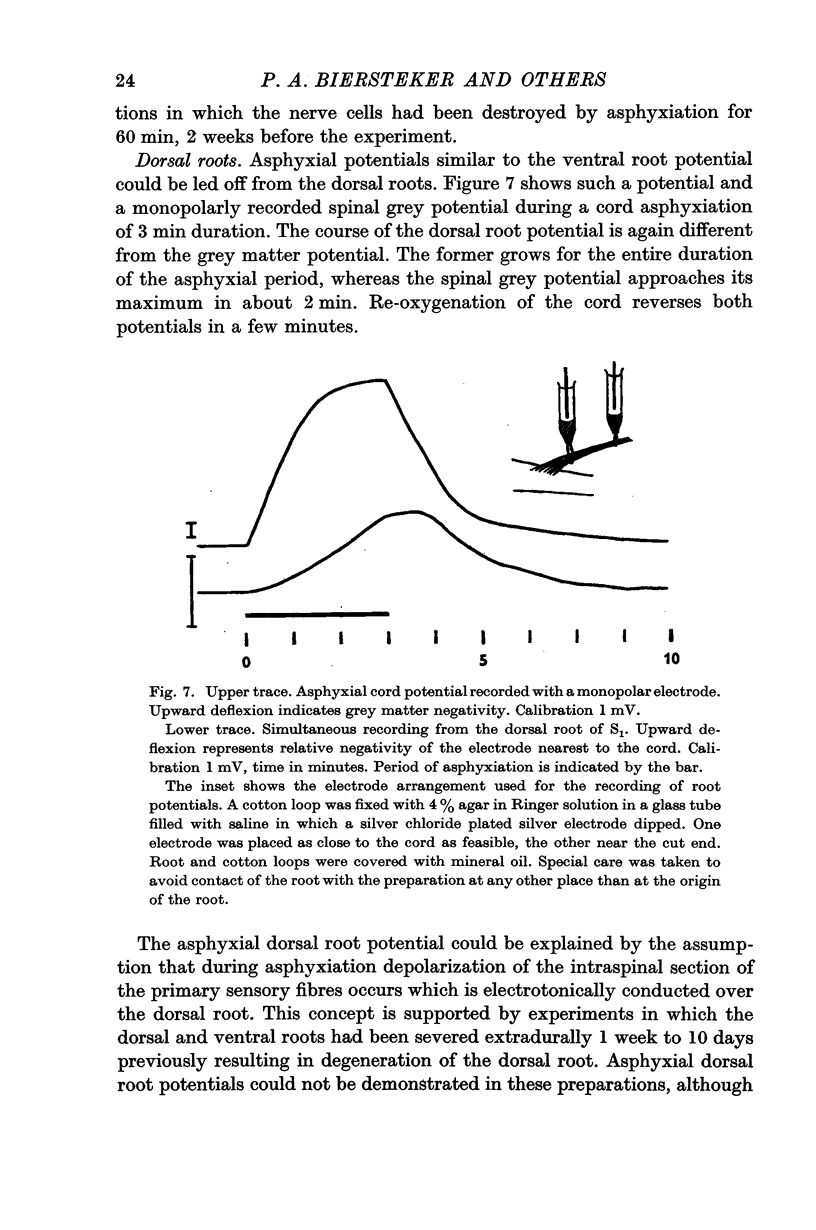
3. In the ventral as well as in the dorsal root a negativity of a proximal electrode with respect to a more distal one developed during asphyxiation after the same latency as the asphyxial cord potentials. The asphyxial root potentials continued to grow during periods of asphyxiation as long as 30 min, and recovered promptly upon re-oxygenation.
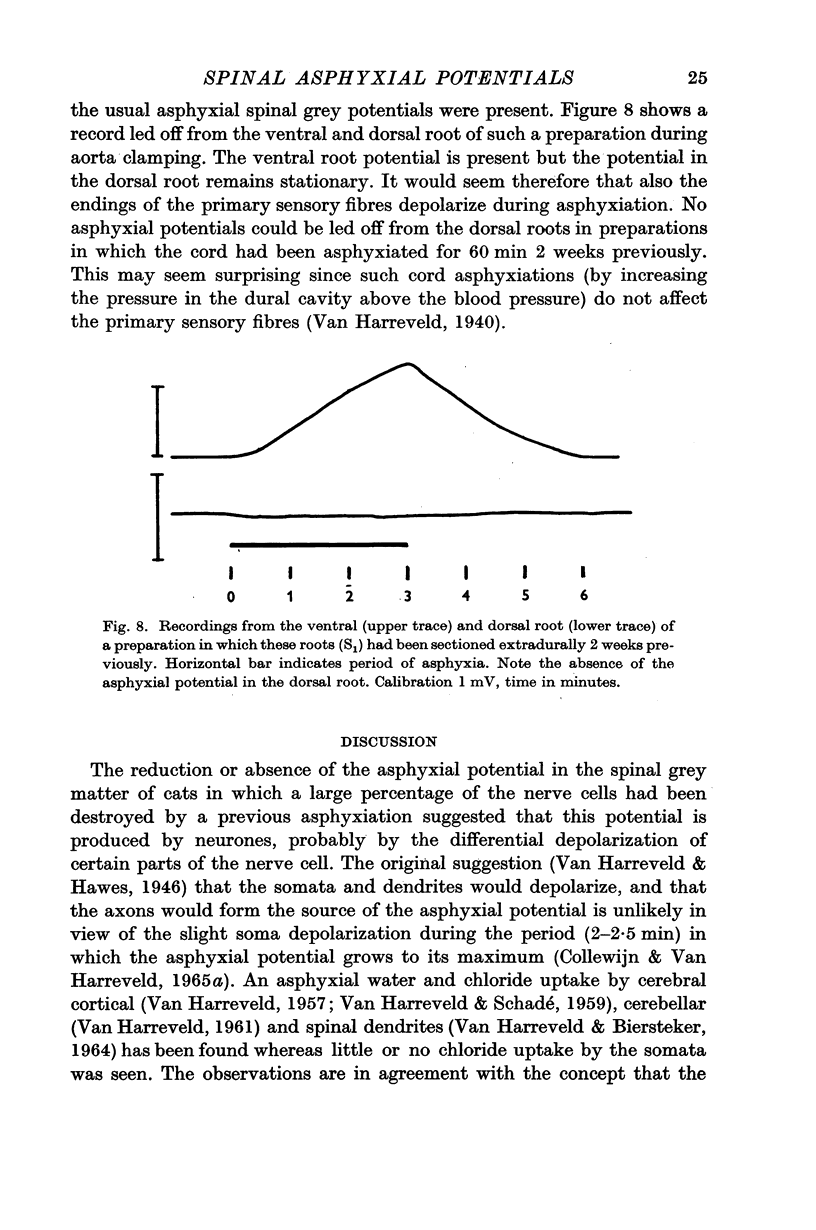
4. Ventral and dorsal root potentials were abolished by asphyxiation of the cord for a period of 60 min, 2 weeks previously. This procedure destroys practically all the neurones, but not the dorsal root fibres. The dorsal root potential, but not the ventral one, was abolished by extradural sectioning of the roots, 2 weeks previously.
5. The asphyxial ventral and dorsal root potentials were interpreted as the result of depolarization of the intraspinal part of the motoneurones and primary afferent endings respectively, conducted electrotonically along the roots. The short latency of these potentials suggests that an early depolarization of motoneurone, and of the primary afferent end knobs occurs. The latter, which may have some relation to presynaptic inhibition, explains the early failure of synaptic conduction during acute asphyxiation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADRIAN R. H. The effect of internal and external potassium concentration on the membrane potential of frog muscle. J Physiol. 1956 Sep 27;133(3):631–658. doi: 10.1113/jphysiol.1956.sp005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIERSTEKER P. A., VAN HARREVELD A. The nature of the rigidity caused by spinal cord asphyxiation. J Physiol. 1963 Apr;166:382–394. doi: 10.1113/jphysiol.1963.sp007110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H., Van Harreveld A. Intracellular recording from cat spinal motoneurones during acute asphyxia. J Physiol. 1966 Jul;185(1):1–14. doi: 10.1113/jphysiol.1966.sp007968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H., Van Harreveld A. Intracellular recording from spinal motoneurones in cats with post-asphyxial rigidity. J Physiol. 1966 Jul;185(1):30–41. doi: 10.1113/jphysiol.1966.sp007970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KOSTYUK P. G., SCHMIDT R. F. Central pathways responsible for depolarization of primary afferent fibres. J Physiol. 1962 May;161:237–257. doi: 10.1113/jphysiol.1962.sp006884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEAO A. A. P. The slow voltage variation of cortical spreading depression of activity. Electroencephalogr Clin Neurophysiol. 1951 Aug;3(3):315–321. doi: 10.1016/0013-4694(51)90079-x. [DOI] [PubMed] [Google Scholar]
- MARSHALL W. H. Spreading cortical depression of Leao. Physiol Rev. 1959 Apr;39(2):239–279. doi: 10.1152/physrev.1959.39.2.239. [DOI] [PubMed] [Google Scholar]
- OCHS S., VAN HARREVELD A. Cerebral impedance changes after circulatory arrest. Am J Physiol. 1956 Sep;187(1):180–192. doi: 10.1152/ajplegacy.1956.187.1.180. [DOI] [PubMed] [Google Scholar]
- TSCHIRGI R. D., TAYLOR J. L. Slowly changing bioelectric potentials associated with the blood-brain barrier. Am J Physiol. 1958 Oct;195(1):7–22. doi: 10.1152/ajplegacy.1958.195.1.7. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A. Asphyxial changes in the cerebellar cortex. J Cell Comp Physiol. 1961 Apr;57:101–110. doi: 10.1002/jcp.1030570207. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A. Changes in the diameter of apical dendrites during spreading depression. Am J Physiol. 1958 Mar;192(3):457–463. doi: 10.1152/ajplegacy.1958.192.3.457. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A. Changes in volume of cortical neuronal elements during asphyxiation. Am J Physiol. 1957 Nov;191(2):233–242. doi: 10.1152/ajplegacy.1957.191.2.233. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A. Compounds in brain extracts causing spreading depression of cerebral cortical activity and contraction of crustacean muscle. J Neurochem. 1959 Feb;3(4):300–315. doi: 10.1111/j.1471-4159.1959.tb12636.x. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A., OCHS S. Electrical and vascular concomitants of spreading depression. Am J Physiol. 1957 Apr;189(1):159–166. doi: 10.1152/ajplegacy.1957.189.1.159. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A., SCHADE J. P. Chloride movements in cerebral cortex after circulatory arrest and during spreading depression. J Cell Comp Physiol. 1959 Aug;54:65–84. doi: 10.1002/jcp.1030540108. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A., SCHADE J. P. Nerve cell destruction by asphyxiation of the spinal cord. J Neuropathol Exp Neurol. 1962 Jul;21:410–423. doi: 10.1097/00005072-196207000-00009. [DOI] [PubMed] [Google Scholar]
- VAN HARREVELD A. Water and electrolyte distribution in central nervous tissue. Fed Proc. 1962 May-Jun;21:659–664. [PubMed] [Google Scholar]
- VANHARREVELD A., BIERSTEKER P. A. ACUTE ASPHYXIATION OF THE SPINAL CORD AND OF OTHER SECTIONS OF THE NERVOUS SYSTEM. Am J Physiol. 1964 Jan;206:8–14. doi: 10.1152/ajplegacy.1964.206.1.8. [DOI] [PubMed] [Google Scholar]
- VANHARREVELD A., CROWELL J., MALHOTRA S. K. A STUDY OF EXTRACELLULAR SPACE IN CENTRAL NERVOUS TISSUE BY FREEZE-SUBSTITUTION. J Cell Biol. 1965 Apr;25:117–137. doi: 10.1083/jcb.25.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANHARREVELD A. EFFECTS OF SPINAL CORD ASPHYXIATION. Prog Brain Res. 1964;12:280–307. [PubMed] [Google Scholar]
- Van Harreveld A., Spinelli D. Reflex activity in spinal cats with postasphyxial rigidity. Arch Int Physiol Biochim. 1965 Mar;73(2):209–230. doi: 10.3109/13813456509084248. [DOI] [PubMed] [Google Scholar]


